Abstract
Objectives
Platelet-rich fibrin (PRF) has gained tremendous momentum in recent years as a natural autologous growth factor derived from blood capable of stimulating tissue regeneration. Owing to its widespread use, many companies have commercialized various centrifugation devices with various proposed protocols. The aim of the present study was to compare 3 different commercially available centrifuges at both high and low g-force protocols.
Materials and methods
PRF was produced on three commercially available centrifuges including the IntraSpin Device (IntraLock), the Duo Quattro (Process for PRF), and Salvin (Salvin Dental). Two separate protocols were tested on each machine including the original leukocyte and platelet-rich fibrin (L-PRF) protocol (~ 700 RCF max (~ 400 RCF clot) for 12 min) as well as the advanced platelet-rich fibrin (A-PRF+) protocol (~ 200 g RCF max (~ 130 g RCF clot) for 8 min). Each of the tested groups was compared for cell numbers, growth factor release, scanning electron microscopy (SEM) for morphological differences, and clot size (both weight and length/width).
Results
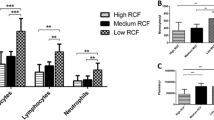
The present study found that PRF clots produced utilizing the low-speed centrifugation speeds (~ 200 g for 8 min) produce clots that (1) contained a higher concentration of evenly distributed platelets, (2) secreted higher concentrations of growth factors over a 10 day period, and (3) were smaller in size. This was irrespective of the centrifugation device utilized and consistently observed on all 3 devices. The greatest impact was found between the protocols utilized (up to a 200%). Interestingly, it was further revealed that the centrifugation tubes used had a much greater impact on the final size outcome of PRF clots when compared to centrifugation devices. It was found that, in general, the Process for PRF tubes produced significantly greater-sized clots when compared to other commercially available tubes. The Salvin Dental tubes also produced significantly greater PRF clots when compared to the IntraLock tubes on each of the tested centrifugation devices.
Conclusions
The present study demonstrated the reproducibility of a scientific concept (reduction in RCF produces PRF clots with more evenly distributed cells and growth factors) utilizing different devices. Furthermore, (and until now overlooked), it was revealed for the first time that the centrifugation tubes are central to the quality production of PRF. Future research investigating tube characteristics thus becomes critically important for the future optimization of PRF.
Clinical relevance
This is the first study to reveal the marked impact of centrifugation tubes on the final production of PRF. Future study thus becomes markedly important to further optimize the quality of PRF-based matrices. It was further found that little variability existed between the centrifugation devices if optimized centrifugation protocols (lower centrifugation speeds) were utilized.










Similar content being viewed by others
References
Miron RJ, Zucchelli G, Pikos MA, Salama M, Lee S, Guillemette V, Fujioka-Kobayashi M, Bishara M, Zhang Y, Wang HL, Chandad F, Nacopoulos C, Simonpieri A, Aalam AA, Felice P, Sammartino G, Ghanaati S, Hernandez MA, Choukroun J (2017) Use of platelet-rich fibrin in regenerative dentistry: a systematic review. Clin Oral Investig 21:1913–1927. https://doi.org/10.1007/s00784-017-2133-z
Marx RE (2004) Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg 62:489–496
Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR (1998) Platelet-rich plasma: growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 85:638–646
Cai YZ, Zhang C, Lin XJ (2015) Efficacy of platelet-rich plasma in arthroscopic repair of full-thickness rotator cuff tears: a meta-analysis. J Shoulder Elbow Surg 24(12):1852–1859. https://doi.org/10.1016/j.jse.2015.07.035.Epub 2015 Oct 9. Review
Meheux CJ, McCulloch PC, Lintner DM, Varner KE, Harris JD (2016) Efficacy of intra-articular platelet-rich plasma injections in knee osteoarthritis: a systematic review. Arthroscopy 32(3):495–505. https://doi.org/10.1016/j.arthro.2015.08.005
Singh B, Goldberg LJ (2016) Autologous platelet-rich plasma for the treatment of pattern hair loss. Am J Clin Dermatol 17:359–367. https://doi.org/10.1007/s40257-016-0196-2
Castro AB, Meschi N, Temmerman A, Pinto N, Lambrechts P, Teughels W, Quirynen MJJocp (2017) Regenerative potential of leucocyte-and platelet-rich fibrin. Part B: sinus floor elevation, alveolar ridge preservation and implant therapy. J Clin Periodontol 44(2):225–234. https://doi.org/10.1111/jcpe.12658
Castro AB, Meschi N, Temmerman A, Pinto N, Lambrechts P, Teughels W, Quirynen MJJocp (2017) Regenerative potential of leucocyte-and platelet-rich fibrin. Part A: intra-bony defects, furcation defects and periodontal plastic surgery. J Clin Periodontol 44(1):67–82. https://doi.org/10.1111/jcpe.12643
Anfossi G, Trovati M, Mularoni E, Massucco P, Calcamuggi G, Emanuelli G (1989) Influence of propranolol on platelet aggregation and thromboxane B2 production from platelet-rich plasma and whole blood. Prostaglandins Leukot Essent Fat Acids 36:1–7
Fijnheer R, Pietersz RN, de Korte D, Gouwerok CW, Dekker WJ, Reesink HW, Roos D (1990) Platelet activation during preparation of platelet concentrates: a comparison of the platelet-rich plasma and the buffy coat methods. Transfusion 30:634–638
Chow TW, McIntire LV, Peterson DM (1983) Importance of plasma fibronectin in determining PFP and PRP clot mechanical properties. Thromb Res 29:243–248
Delaini F, Poggi A, Donati MB (1982) Enhanced affinity for arachidonic acid in platelet-rich plasma from rats with Adriamycin-induced nephrotic syndrome. Thromb Haemost 48:260–262
Ehrenfest DMD, Rasmusson L and Albrektsson TJTib (2009) Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte-and platelet-rich fibrin (L-PRF) 27:158–167
Kobayashi E, Fluckiger L, Fujioka-Kobayashi M, Sawada K, Sculean A, Schaller B, Miron RJ (2016) Comparative release of growth factors from PRP, PRF, and advanced-PRF. Clin Oral Investig 20:2353–2360. https://doi.org/10.1007/s00784-016-1719-1
Fujioka-Kobayashi M, Miron RJ, Hernandez M, Kandalam U, Zhang Y, Choukroun J (2017) Optimized platelet-rich fibrin with the low-speed concept: growth factor release, biocompatibility, and cellular response. J Periodontol 88:112–121. https://doi.org/10.1902/jop.2016.160443
Kubesch A, Barbeck M, Al-Maawi S, Orlowska A, Booms PF, Sader RA, Miron RJ, Kirkpatrick CJ, Choukroun J and Ghanaati S (2018) A low-speed centrifugation concept leads to cell accumulation and vascularization of solid platelet-rich fibrin: an experimental study in vivo. Platelets:1–12. doi: https://doi.org/10.1080/09537104.2018.1445835
Wend S, Kubesch A, Orlowska A, Al-Maawi S, Zender N, Dias A, Miron RJ, Sader R, Booms P, Kirkpatrick CJ, Choukroun J, Ghanaati S (2017) Reduction of the relative centrifugal force influences cell number and growth factor release within injectable PRF-based matrices. J Mater Sci Mater Med 28:188. https://doi.org/10.1007/s10856-017-5992-6
Dohan Ehrenfest DM, Pinto NR, Pereda A, Jimenez P, Corso MD, Kang BS, Nally M, Lanata N, Wang HL, Quirynen M (2018) The impact of the centrifuge characteristics and centrifugation protocols on the cells, growth factors, and fibrin architecture of a leukocyte- and platelet-rich fibrin (L-PRF) clot and membrane. Platelets 29:171–184. https://doi.org/10.1080/09537104.2017.1293812
Miron RJ, Fujioka-Kobayashi M, Hernandez M, Kandalam U, Zhang Y, Ghanaati S, Choukroun J (2017) Injectable platelet rich fibrin (i-PRF): opportunities in regenerative dentistry? Clin Oral Investig 21:2619–2627. https://doi.org/10.1007/s00784-017-2063-9
Nunes CR, Roedersheimer M, Simske S, Luttges M (1995) Effect of microgravity, temperature, and concentration on fibrin and collagen assembly. Microgravity Sci Technol 8(2):125–130
Dohan Ehrenfest DM, Del Corso M, Diss A, Mouhyi J, Charrier J-B (2010) Three-dimensional architecture and cell composition of a Choukroun’s platelet-rich fibrin clot and membrane. J Periodontol 81:546–555
Yajamanya SR, Chatterjee A, Babu CN, Karunanithi D (2016) Fibrin network pattern changes of platelet-rich fibrin in young versus old age group of individuals: a cell block cytology study. J Indian Soc Periodontol 20(2):151–6. https://doi.org/10.4103/0972-124X.176390
Miron RJ and Choukroun J (2017) Platelet rich fibrin in regenerative dentistry: biological background and clinical indications. John Wiley & Sons,
Miron RJ, Dham A, Dham U, Zhang Y, Pikos MA, Sculean A (2018) The effect of age, gender, and time between blood draw and start of centrifugation on the size outcomes of platelet-rich fibrin (PRF) membranes. Clin Oral Investig 23:2179–2185. https://doi.org/10.1007/s00784-018-2673-x
Miron RJ, Chai J, Zheng S, Feng M, Sculean A, Zhang Y. (2019) A novel method for evaluating and quantifying cell types in platelet rich fibrin and an introduction to horizontal centrifugation. J Biomed Mater Res A. https://doi.org/10.1002/jbm.a.36734
Miron R, Choukroun J, Ghanaati SJIJoGF and Dentistry SCi (2018) Controversies related to scientific report describing g-forces from studies on platelet-rich fibrin: necessity for standardization of relative centrifugal force values. 1:80
Ghanaati S, Booms P, Orlowska A, Kubesch A, Lorenz J, Rutkowski J, Landes C, Sader R, Kirkpatrick C, Choukroun J (2014) Advanced platelet-rich fibrin: a new concept for cell-based tissue engineering by means of inflammatory cells. The Journal of oral implantology 40:679–689. https://doi.org/10.1563/aaid-joi-D-14-00138
El Bagdadi K, Kubesch A, Yu X, Al-Maawi S, Orlowska A, Dias A, Booms P, Dohle E, Sader R, Kirkpatrick CJ, Choukroun J, Ghanaati S (2017) Reduction of relative centrifugal forces increases growth factor release within solid platelet-rich-fibrin (PRF)-based matrices: a proof of concept of LSCC (low speed centrifugation concept). Eur J Trauma Emerg Surg 45(3):467–479. https://doi.org/10.1007/s00068-017-0785-7
Choukroun J, Ghanaati S (2018) Reduction of relative centrifugation force within injectable platelet-rich-fibrin (PRF) concentrates advances patients’ own inflammatory cells, platelets and growth factors: the first introduction to the low speed centrifugation concept. Eur J Trauma Emerg Surg 44(1):87–95. https://doi.org/10.1007/s00068-017-0767-9
Dohle E, El Bagdadi K, Sader R, Choukroun J, James Kirkpatrick C, Ghanaati S (2018) Platelet-rich fibrin-based matrices to improve angiogenesis in an in vitro co-culture model for bone tissue engineering. J Tissue Eng Regen Med 12(3):598–610. https://doi.org/10.1002/term.2475
Miron RJ, Choukroun J, Ghanaati S (2018) Necessity for standardization of relative centrifugal force values in studies on platelet rich fibrin; response to letter to the editor. J Periodontol. https://doi.org/10.1002/jper.18-0329
Pinto N, Quirynen M (2018) Letter to the editor regarding Fujioka-Kobayashi et al. 2017 (JOP-16-0443.R1). J Periodontol. https://doi.org/10.1002/jper.18-0175
Funding
This work was supported by the funds of the National Key R&D Program of China (2018YFC1105300 to Yufeng Zhang).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
No ethical approval was required for this study, as human samples were not identified.
Informed consent
For this study, informed consent was provided prior to blood draw to conduct the outlined experiments.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Miron, R.J., Xu, H., Chai, J. et al. Comparison of platelet-rich fibrin (PRF) produced using 3 commercially available centrifuges at both high (~ 700 g) and low (~ 200 g) relative centrifugation forces. Clin Oral Invest 24, 1171–1182 (2020). https://doi.org/10.1007/s00784-019-02981-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-019-02981-2