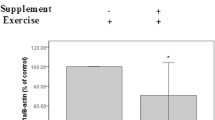
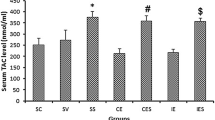
Abstract
To prevent oxidative tissue damage induced by strenuous exercise in the liver and kidney superoxide dismutase derivative (SM-SOD), which circulated bound to albumin with a half-life of 6 h, was injected intraperitoneally into rats. Exhausting treadmill running caused a significant increase in the activities of xanthine oxidase (XO), and glutathione peroxidase (GPX) in addition to concentrations of thiobarbituric acid-reactive substances (TBARS) in hepatic tissue immediately after running. There was a definite increase in the immunoreactive content of mitochondrial superoxide dismutase (Mn-SOD) 1 day after the running. Meanwhile, the TBARS concentration in the kidney was markedly elevated 3 days after running. The activities of GPX, and catalase in the kidney increased significantly immediately and on days 1 and 3 following the test. The immunoreactive content of Mn-SOD also increased 1 day after running. The exercise induced no significant changes in immunoreactive Cu, Zn-SOD content in either tissue. The administration of SM-SOD provided effective protection against lipid peroxidation, and significantly attenuated the alterations in XO and all the anti-oxidant enzymes, measured. In summary, the present data would suggest that exhausting exercise may induce XO-derived oxidative damage in the liver, while the increase in lipid peroxidation in the kidney might be the result of washout-dependent accumulation of peroxidised metabolites. We found that the administration of SM-SOD provided excellent protection against exercise-induced oxidative stress in both liver and kidney.
Similar content being viewed by others
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Alessio HM, Goldfarb AH (1988) Lipid peroxidation and scavenger enzymes during exercise: adaptative response to training. J Appl Physiol 64:1333–1336
Åstrand PO, Rodahl K (1986) Textbook of work physiology, 3rd edn. McGraw-Hill, New York, pp 152–153
Baker GL, Corry RJ, Autor AP (1985) Oxygen free radical induced damage in kidneys subjected to warm ischemia and reperfusion. Ann Surg 202:628–641
Barnard ML, Snyder SJ, Engerson TD, Turrens JF (1993) Antioxidant enzyme status of ischemic and postischemic liver and ischemic kidney in rats. Free Radic Biol Med 15:227–232
Cannon JG, Kluger MJ (1983) Endogenous pyrogen activity in human plasma after exercise. Science (Washington) 220:617–619
Cannon JG, Fielding RA, Fiatarone MA, Orencole SF, Dinarello CA, Evans WJ (1989) Increased interleukin 1β in human skeletal muscle after exercise. Am J Physiol 257:R451-R455
Das KD, Russel JC, Jones RM (1993) Reduction of cold injury by superoxide dismutase and catalase. Free Radic Res Commun 12–13:653–662
Davies KJA, Quintanilha AT, Brooks GA, Packer L (1982) Free radicals and tissue damage produced by exercise. Biochem Biophys Res Commun 107:1198–1205
Dedrick AL, Zaharko DS, Lutz RJ (1973) Transport and binding of methotrexate in vivo. J Pharm Sci 62:882–890
Fridovich I (1970) Quantitative aspects of the production of superoxide anion radical by milk xanthine oxidase. J Biol Chem 245:4053–4057
Fried R, Fried LW (1974) Xanthine oxidase (xanthine dehydrogenase). In: Bergmeyer HU (ed) Methods of enzymatic analysis. Academic Press, New York, pp 664–649
Grisham MB, Hernandez LA, Granger DN (1986) Xanthine oxidase and neutrophil infiltration in intestinal ischemia. Am J Physiol 251:G567-G574
Halliwell B, Gutteridge JMC (1986) Oxygen free radicals and iron relation to biology and medicone: some problems and concepts. Arch Biochem Biophys 246:501–514
Hellsten Y, Ahlborg G, Jensen-Urstad M, Sjödin B (1988) Indication of in vivo xanthine oxidase activity in human skeletal muscle during exercise. Acta Physiol Scand 134:159–160
Inoue M (1989) Topological aspects of oxygen toxicity in ischemia/ reperfusion-induced tissue injury: analysis by H+-sensitive SOD derivatives. In: Hayaishi O, Niki E, Kondo M, Yoshikawa T (eds) Medical, biochemical and chemical aspects of free radicals. Elsevier, Amsterdam, pp 1119–1126
Inoue M, Ebashi I, Watanabe N, Morino Y (1988) Synthesis of a superoxide dismutase derivative that circulates bound to albumin and accumulates in tissues whose pH is decreased. Biochemistry 28:6619–6624
Jenkins RR (1988) Free radical chemistry: relationship to exercise. Sports Med 5:156–170
Ji LL, Lennon DLF, Kochan RG, Nagle FJ, Lardy HA (1986) Enzymatic adaptation to physical training underβ-blockade in the rat: evidence of aβ2-adrenergic mechanism in skeltal muscle. J Clin Invest 78:771–778
Ji LL, Stratman FW, Lardy HA (1988) Antioxidant enzyme system in rat liver and skeletal muscle. Arch Biochem Biophys 263:150–160
Kanter MM, Lesmes GR, Kamisky LA, Ham-Saeger JL, Nequin D (1988) Serum creatine kinase and lactate dehydrogenase changes following an eighty kilometer race. Eur J Appl Physiol 57:60–63
Kanter MM, Nolte LA, Holosszy JO (1993) Effects of an antioxidant vitamin mixture on lipid peroxidation at rest and postexercise. J Appl Physiol 74:965–969
Kayashima S, Ohno H, Fujioka T, Taniguchi N, Nagata N (1995) Leucocytosis as a marker of organ damage induced by chronic strenuous physical exercise. Eur J Appl Physiol 70:413–420
Kuwamoto S, Inoue M, Tashiro S, Morino Y, Miyauchi Y (1990) Inhibition of ischemia and reflow-induced liver injury by an SOD derivative that circulates bound to albumin. Arch Biochem Biophys 277:160–165
Lowry OH, Rosenbourgh NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Leeuwenburgh C, Ji LL (1995) Glutathione depletion in rested and exercised mice: biochemical consequences and adaptation. Arch Biochem Biophys 316:941–949
Matsuda Y, Higashiyama S, Kijima Y, Suzuki K, Akiyama M, Kawano K, Kawata S, Tarui S, Deutsch HF, Taniguchi N (1990) Preparation, subunit structure and sulfhydryl reactivity of human manganase superoxide dismutase. Eur J Biochem 194:713–720
McCord JM (1985) Oxygen-derived free radicals in postischemic tissue injury. New Engl J Med 312:159–163
McCord JM (1987) Oxygen-derived radicals: a link between reperfusion injury and inflammation. Fed Proc 46:2402–2406
McKelvey GT, Höllwarth ME, Granger DN, Engerson TD, Landker U, Jones HP (1988) Mechanisms of conversion of xanthine dehydrogenase to xanthine oxidase in ischemic rat liver and kidney. Am J Physiol :G753–G760
Metzger J, Dore SP, Lauterburg BH (1988) Oxidant stress during reperfusion of ischemic liver: no evidence for role of xanthine oxidase. Hepatology 8:580–584
Minor T, Isselhard W (1993) Role of the hepatovasculature in free radical mediated reperfusion damage of the liver. Eur Surg Res 25:287–293
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Ohno H, Suzuki K, Fujii J, Yamashita H, Kizaki T, Oh-ishi S, Taniguchi N (1994) Superoxide dismutases in exercise and disease. In: Sen CK, Packer L, Hänninen O (eds) Exercise and oxygen toxicity. Elsevier, Amsterdam, pp 127–161
Osswald HH, Schmitz HJ, Kemper R (1977) Tissue content of adenosine, inosine and hypoxanthine in the rat kidney after ischemia and postischemic recirculation. Pflügers Arch 371:45–49
Paller MS, Hoidal JR, Ferris TF (1984) Oxygen free radicals in ischemic acute renal failure in the rat. J Clin Invest 74:1156–1164
Radák Z, Asano K, Inoue M, Kizaki T, Oh-ishi S, Suzuki K, Taniguchi N, Ohno H (1995) Superoxide dismutase derivative reduces oxidative damage in skeletal muscle of rats during exhaustive exercise. J Appl Physiol 79:129–135
Saitoh D, Kadota T, Senoh A, Takahara T, Okada Y, Mimura K, Yamashita H, Ohno H, Inoue M (1993) Superoxide dismutase with prolonged in vivo half-life inhibits intravascular hemolysis and renal injury in burned rats. Am J Emerg Med 11:355–359
Sjödin B, Hellsten Westing Y, Apple FS (1990) Biochemical mechanism for oxygen free radical formation during exercise. Sports Med 10:236–150
Suzuki K, Nakata T, Seo HG, Miyazawa N, Sugiyama T, Taniguchi N (1991) Differential expression of Mn and Cu, Zn-superoxide dismutases in various tissues of LEC rats. In: Mori M, Yoshida MC, Takeichi N, Taniguchi N (eds) The LEC rats. Springer, Berlin Heidelberg New York, pp 142–148
Tappel AL (1978) Glutathione peroxidase and hydroperoxidase. Methods Enzymol 52:506–513
Taniguchi N (1992) Clinical significances of superoxide dismutase: changes in aging, diabetes, ischemia, and cancer. Adv Clin Chem 29:1–59
Yokoyama Y, Beckman JS, Beckman TK, Wheat JK, Cash TG, Freeman BA, Parks DA (1990) Circulating xanthine oxidase: potential mediator of ischemic injury. Am J Physiol 258:G564-G570
Yu BP (1994) Cellular defense against damage from reactive oxygen species. Physiol Rev 74:139–155
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Radák, Z., Asano, K., Inoue, M. et al. Superoxide dismutase derivative prevents oxidative damage in liver and kidney of rats induced by exhausting exercise. Europ. J. Appl. Physiol. 72, 189–194 (1996). https://doi.org/10.1007/BF00838637
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00838637