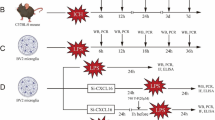
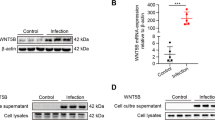
Abstract
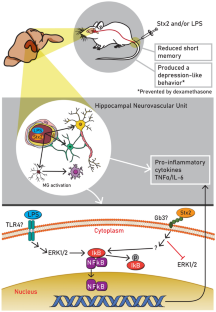
Shiga toxin 2 (Stx2) from enterohemorrhagic Escherichia coli (EHEC) produces hemorrhagic colitis, hemolytic uremic syndrome (HUS), and acute encephalopathy. The mortality rate in HUS increases significantly when the central nervous system (CNS) is involved. Besides, EHEC also releases lipopolysaccharide (LPS). Many reports have described cognitive dysfunctions in HUS patients, the hippocampus being one of the brain areas targeted by EHEC infection. In this context, a translational murine model of encephalopathy was employed to establish the deleterious effects of Stx2 and the contribution of LPS in the hippocampus. The purpose of this work is to elucidate the signaling pathways that may activate the inflammatory processes triggered by Stx2, which produces cognitive alterations at the level of the hippocampus. Results demonstrate that Stx2 produced depression-like behavior, pro-inflammatory cytokine release, and NF-kB activation independent of the ERK1/2 signaling pathway, while co-administration of Stx2 and LPS reduced memory index. On the other hand, LPS activated NF-kB dependent on ERK1/2 signaling pathway. Cotreatment of Stx2 with LPS aggravated the pathologic state, while dexamethasone treatment succeeded in preventing behavioral alterations. Our present work suggests that the use of drugs such as corticosteroids or NF-kB signaling inhibitors may serve as neuroprotectors from EHEC infection.





Similar content being viewed by others
References
Alconcher LF, Balestracci A, Coccia PA, Suarez ADC, Ramirez FB, Monteverde ML et al (2021) Hemolytic uremic syndrome associated with Shiga toxin-producing Escherichia coli infection in Argentina: update of serotypes and genotypes and their relationship with severity of the disease. Pediatr Nephrol 36(9):2811–2817. https://doi.org/10.1007/s00467-021-04988-y
Anisman H, Zacharko RM (1990) Multiple neurochemical and behavioral consequences of stressors: implications for depression. Pharmacol Ther 46(1):119–136. https://doi.org/10.1016/0163-7258(90)90039-5
Aparicio-Nava L, Marquez-Garcia LA, Meneses A (2019) Effects of 5-HT5A receptor blockade on amnesia or forgetting. Behav Brain Res 357–358:98–103. https://doi.org/10.1016/j.bbr.2018.01.009
Arango Duque G, Descoteaux A (2014) Macrophage cytokines: involvement in immunity and infectious diseases. Front Immunol 5:491. https://doi.org/10.3389/fimmu.2014.00491
Attiq A, Yao LJ, Afzal S, Khan MA (2021) The triumvirate of NF-kappaB, inflammation and cytokine storm in COVID-19. Int Immunopharmacol 101(Pt B):108255. https://doi.org/10.1016/j.intimp.2021.108255
Berdasco C, Duhalde Vega M, Rosato-Siri MV, Goldstein J (2019a) Environmental Cues Modulate Microglial Cell Behavior Upon Shiga Toxin 2 From Enterohemorrhagic Escherichia coli Exposure. Front Cell Infect Microbiol 9:442. https://doi.org/10.3389/fcimb.2019.00442
Berdasco C, Pinto A, Calabro V, Arenas D, Cangelosi A, Geoghegan P, Evelson P, Goldstein J (2019b) Shiga toxin 2 from enterohemorrhagic Escherichia coli induces reactive glial cells and neurovascular disarrangements including edema and lipid peroxidation in the murine brain hippocampus. J Biomed Sci 26(1):16. https://doi.org/10.1186/s12929-019-0509-x
Cameron P, Smith SJ, Giembycz MA, Rotondo D, Plevin R (2003) Verotoxin activates mitogen-activated protein kinase in human peripheral blood monocytes: role in apoptosis and proinflammatory cytokine release. Br J Pharmacol 140(7):1320–1330. https://doi.org/10.1038/sj.bjp.0705560
Campbell S, Macqueen G (2004) The role of the hippocampus in the pathophysiology of major depression. J Psychiatry Neurosci 29(6):417–426
Cavaillon JM (1994) Cytokines and macrophages. Biomed Pharmacother 48(10):445–453. https://doi.org/10.1016/0753-3322(94)90005-1
Celi AB, Goldstein J, Rosato-Siri MV, Pinto A (2022) Role of globotriaosylceramide in physiology and pathology. Front Mol Biosci 9:813637. https://doi.org/10.3389/fmolb.2022.813637
Chauveau F, De Job E, Poly-Thomasson B, Cavroy R, Thomasson J, Fromage D et al (2019) Procognitive impact of ciproxifan (a histaminergic H3 receptor antagonist) on contextual memory retrieval after acute stress. CNS Neurosci Ther 25(8):832–841. https://doi.org/10.1111/cns.13113
Cherry JD, Olschowka JA, O’Banion MK (2014) Neuroinflammation and M2 microglia: the good, the bad, and the inflamed. J Neuroinflammation 11:98. https://doi.org/10.1186/1742-2094-11-98
Crews FT, Vetreno RP (2016) Mechanisms of neuroimmune gene induction in alcoholism. Psychopharmacology 233(9):1543–1557. https://doi.org/10.1007/s00213-015-3906-1
Denes A, Vidyasagar R, Feng J, Narvainen J, McColl BW, Kauppinen RA, Allan SM (2007) Proliferating resident microglia after focal cerebral ischaemia in mice. J Cereb Blood Flow Metab 27(12):1941–1953. https://doi.org/10.1038/sj.jcbfm.9600495
Deng Z, Sui G, Rosa PM, Zhao W (2012) Radiation-induced c-Jun activation depends on MEK1-ERK1/2 signaling pathway in microglial cells. PLoS ONE 7(5):e36739. https://doi.org/10.1371/journal.pone.0036739
Derecki N, Norris G, Kipnis J (2014) Microglial sholl analysis. Nat Protoc Exch. https://doi.org/10.1038/protex.2014.029
Di Filippo M, de Iure A, Giampa C, Chiasserini D, Tozzi A, Orvietani PL et al (2016) Persistent activation of microglia and NADPH oxidase drive hippocampal dysfunction in experimental multiple sclerosis. Sci Rep 6:23855. https://doi.org/10.1038/srep23855
Fakhouri F, Zuber J, Fremeaux-Bacchi V, Loirat C (2017) Haemolytic uraemic syndrome. Lancet 390(10095):681–696. https://doi.org/10.1016/S0140-6736(17)30062-4
Fields J, Cisneros IE, Borgmann K, Ghorpade A (2013) Extracellular regulated kinase 1/2 signaling is a critical regulator of interleukin-1beta-mediated astrocyte tissue inhibitor of metalloproteinase-1 expression. PLoS ONE 8(2):e56891. https://doi.org/10.1371/journal.pone.0056891
Fiore RS, Bayer VE, Pelech SL, Posada J, Cooper JA, Baraban JM (1993) p42 mitogen-activated protein kinase in brain: prominent localization in neuronal cell bodies and dendrites. Neuroscience 55(2):463–472. https://doi.org/10.1016/0306-4522(93)90516-i
Foster GH, Tesh VL (2002) Shiga toxin 1-induced activation of c-Jun NH(2)-terminal kinase and p38 in the human monocytic cell line THP-1: possible involvement in the production of TNF-alpha. J Leukoc Biol 71(1):107–114
Foster GH, Armstrong CS, Sakiri R, Tesh VL (2000) Shiga toxin-induced tumor necrosis factor alpha expression: requirement for toxin enzymatic activity and monocyte protein kinase C and protein tyrosine kinases. Infect Immun 68(9):5183–5189. https://doi.org/10.1128/IAI.68.9.5183-5189.2000
Garg AX, Suri RS, Barrowman N, Rehman F, Matsell D, Rosas-Arellano MP, Salvadori M, Haynes RB, Clark WF (2003) Long-term renal prognosis of diarrhea-associated hemolytic uremic syndrome: a systematic review, meta-analysis, and meta-regression. JAMA 290(10):1360–1370. https://doi.org/10.1001/jama.290.10.1360
Ghose R (2019) Nature of the pre-chemistry ensemble in mitogen-activated protein kinases. J Mol Biol 431(2):145–157. https://doi.org/10.1016/j.jmb.2018.12.007
Goldstein J, Nunez-Goluboay K, Pinto A (2020) Therapeutic strategies to protect the central nervous system against shiga toxin from enterohemorrhagic Escherichia coli. Curr Neuropharmacol. https://doi.org/10.2174/1570159X18666200220143001
Hall G, Kurosawa S, Stearns-Kurosawa DJ (2017) Shiga toxin therapeutics: beyond neutralization. Toxins (basel) 9(9):291. https://doi.org/10.3390/toxins9090291
Heindl S, Gesierich B, Benakis C, Llovera G, Duering M, Liesz A (2018) Automated morphological analysis of microglia after stroke. Front Cell Neurosci 12:106. https://doi.org/10.3389/fncel.2018.00106
Hosaka T, Nakamagoe K, Tamaoka A (2017) Hemolytic uremic syndrome-associated encephalopathy successfully treated with corticosteroids. Intern Med 56(21):2937–2941. https://doi.org/10.2169/internalmedicine.8341-16
Ikeda M, Gunji Y, Yamasaki S, Takeda Y (2000) Shiga toxin activates p38 MAP kinase through cellular Ca(2+) increase in vero cells. FEBS Lett 485(1):94–98. https://doi.org/10.1016/s0014-5793(00)02204-3
Ito D, Imai Y, Ohsawa K, Nakajima K, Fukuuchi Y, Kohsaka S (1998) Microglia-specific localisation of a novel calcium binding protein, Iba1. Brain Res Mol Brain Res 57(1):1–9. https://doi.org/10.1016/s0169-328x(98)00040-0
Jeon SW, Kim YK (2017) Inflammation-induced depression: Its pathophysiology and therapeutic implications. J Neuroimmunol 313:92–98. https://doi.org/10.1016/j.jneuroim.2017.10.016
Kaltschmidt B, Kaltschmidt C (2009) NF-kappaB in the nervous system. Cold Spring Harb Perspect Biol 1(3):a001271. https://doi.org/10.1101/cshperspect.a001271
Kanazawa H, Ohsawa K, Sasaki Y, Kohsaka S, Imai Y (2002) Macrophage/microglia-specific protein Iba1 enhances membrane ruffling and Rac activation via phospholipase C-gamma -dependent pathway. J Biol Chem 277(22):20026–20032. https://doi.org/10.1074/jbc.M109218200
Karin M (1999) How NF-kappaB is activated: the role of the IkappaB kinase (IKK) complex. Oncogene 18(49):6867–6874. https://doi.org/10.1038/sj.onc.1203219
Karmali MA, Steele BT, Petric M, Lim C (1983) Sporadic cases of haemolytic-uraemic syndrome associated with faecal cytotoxin and cytotoxin-producing Escherichia coli in stools. Lancet 1(8325):619–620. https://doi.org/10.1016/s0140-6736(83)91795-6
Karmali MA, Petric M, Lim C, Fleming PC, Arbus GS, Lior H (1985) The association between idiopathic hemolytic uremic syndrome and infection by verotoxin-producing Escherichia coli. J Infect Dis 151(5):775–782. https://doi.org/10.1093/infdis/151.5.775
Kessler RC (1997) The effects of stressful life events on depression. Annu Rev Psychol 48:191–214. https://doi.org/10.1146/annurev.psych.48.1.191
Kim S, Lee MS, Lee B, Gwon WG, Joung EJ, Yoon NY et al (2014) Anti-inflammatory effects of sargachromenol-rich ethanolic extract of Myagropsis myagroides on lipopolysaccharide-stimulated BV-2 cells. BMC Complement Altern Med 14:231. https://doi.org/10.1186/1472-6882-14-231
Kioka N, Minami K, Tamura A, Yoshikawa N (2012) Chemokine expression in human astrocytes in response to shiga toxin 2. Int J Inflam 2012:135803. https://doi.org/10.1155/2012/135803
Kuc KA, Gregersen BM, Gannon KS, Dodart JC (2006) Holeboard discrimination learning in mice. Genes Brain Behav 5(4):355–363. https://doi.org/10.1111/j.1601-183X.2005.00168.x
Lemon N, Manahan-Vaughan D (2006) Dopamine D1/D5 receptors gate the acquisition of novel information through hippocampal long-term potentiation and long-term depression. J Neurosci 26(29):7723–7729. https://doi.org/10.1523/JNEUROSCI.1454-06.2006
Lisman JE, Otmakhova NA (2001) Storage, recall, and novelty detection of sequences by the hippocampus: elaborating on the SOCRATIC model to account for normal and aberrant effects of dopamine. Hippocampus 11(5):551–568. https://doi.org/10.1002/hipo.1071
MacQueen G, Frodl T (2011) The hippocampus in major depression: evidence for the convergence of the bench and bedside in psychiatric research? Mol Psychiatry 16(3):252–264. https://doi.org/10.1038/mp.2010.80
Magnus T, Rother J, Simova O, Meier-Cillien M, Repenthin J, Moller F, Gbadamosi J, Panzer U, Wengenroth M, Hagel C, Kluge S, Stahl RK, Wegscheider K, Urban P, Eckert B, Glatzel M, Fiehler J, Gerloff C (2012) The neurological syndrome in adults during the 2011 northern German E. coli serotype O104:H4 outbreak. Brain 135(Pt 6):1850–1859. https://doi.org/10.1093/brain/aws090
Martinez FO, Gordon S (2014) The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep 6:13. https://doi.org/10.12703/P6-1313
Matthies J, Hunseler C, Ehren R, Volland R, Korber F, Hoppe B, Weber LT, Habbig S (2016) Extrarenal manifestations in shigatoxin-associated haemolytic uremic syndrome. Klin Padiatr 228(4):181–188. https://doi.org/10.1055/s-0042-108444
Meyza KZ, Boguszewski PM, Nikolaev E, Zagrodzka J (2009) Diverse sensitivity of RHA/Verh and RLA/Verh rats to emotional and spatial aspects of a novel environment as a result of a distinct pattern of neuronal activation in the fear/anxiety circuit. Behav Genet 39(1):48–61. https://doi.org/10.1007/s10519-008-9234-z
Nakamura A, Johns EJ, Imaizumi A, Yanagawa Y, Kohsaka T (2001) Activation of beta (2)-adrenoceptor prevents shiga toxin 2-induced TNF-alpha gene transcription. J Am Soc Nephrol 12(11):2288–2299
Nasehi M, Sharifi S, Zarrindast MR (2012) Involvement of the cholinergic system of CA1 on harmane-induced amnesia in the step-down passive avoidance test. J Psychopharmacol 26(8):1151–1161. https://doi.org/10.1177/0269881111421972
Nimmerjahn A, Kirchhoff F, Helmchen F (2005) Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308(5726):1314–1318. https://doi.org/10.1126/science.1110647
Obata F, Obrig T (2010) Distribution of Gb (3) immunoreactivity in the mouse central nervous system. Toxins (basel) 2(8):1997–2006. https://doi.org/10.3390/toxins2081997
Obata F, Obrig T (2014) Role of Shiga/Vero toxins in pathogenesis. Microbiol Spectr. https://doi.org/10.1128/microbiolspec.EHEC-0005-2013
Orihuela R, McPherson CA, Harry GJ (2016) Microglial M1/M2 polarization and metabolic states. Br J Pharmacol 173(4):649–665. https://doi.org/10.1111/bph.13139
Park GH, Jeon SJ, Ryu JR, Choi MS, Han SH, Yang SI et al (2009) Essential role of mitogen-activated protein kinase pathways in protease activated receptor 2-mediated nitric-oxide production from rat primary astrocytes. Nitric Oxide 21(2):110–119. https://doi.org/10.1016/j.niox.2009.05.007
Park J, Min JS, Kim B, Chae UB, Yun JW, Choi MS et al (2015) Mitochondrial ROS govern the LPS-induced pro-inflammatory response in microglia cells by regulating MAPK and NF-kappaB pathways. Neurosci Lett 584:191–196. https://doi.org/10.1016/j.neulet.2014.10.016
Pinto A, Jacobsen M, Geoghegan PA, Cangelosi A, Cejudo ML, Tironi-Farinati C, Goldstein J (2013) Dexamethasone rescues neurovascular unit integrity from cell damage caused by systemic administration of shiga toxin 2 and lipopolysaccharide in mice motor cortex. PLoS ONE 8(7):e70020. https://doi.org/10.1371/journal.pone.0070020
Pinto A, Cangelosi A, Geoghegan PA, Goldstein J (2017) Dexamethasone prevents motor deficits and neurovascular damage produced by shiga toxin 2 and lipopolysaccharide in the mouse striatum. Neuroscience 344:25–38. https://doi.org/10.1016/j.neuroscience.2016.12.036
Pomilio C, Pavia P, Gorojod RM, Vinuesa A, Alaimo A, Galvan V, Kotler ML, Beauquis J, Saravia F (2016) Glial alterations from early to late stages in a model of Alzheimer’s disease: evidence of autophagy involvement in Abeta internalization. Hippocampus 26(2):194–210. https://doi.org/10.1002/hipo.22503
Qiu Z, Lu P, Wang K, Zhao X, Li Q, Wen J et al (2020) Dexmedetomidine inhibits neuroinflammation by altering microglial M1/M2 polarization through MAPK/ERK pathway. Neurochem Res 45(2):345–353. https://doi.org/10.1007/s11064-019-02922-1
Roddy DW, Farrell C, Doolin K, Roman E, Tozzi L, Frodl T et al (2019) The hippocampus in depression: more than the sum of its parts? Advanced hippocampal substructure segmentation in depression. Biol Psychiatry 85(6):487–497. https://doi.org/10.1016/j.biopsych.2018.08.021
Sabroe I, Jones EC, Usher LR, Whyte MK, Dower SK (2002) Toll-like receptor (TLR)2 and TLR4 in human peripheral blood granulocytes: a critical role for monocytes in leukocyte lipopolysaccharide responses. J Immunol 168(9):4701–4710. https://doi.org/10.4049/jimmunol.168.9.4701
Schnare M, Barton GM, Holt AC, Takeda K, Akira S, Medzhitov R (2001) Toll-like receptors control activation of adaptive immune responses. Nat Immunol 2(10):947–950. https://doi.org/10.1038/ni712
Schuppner R, Maehlmann J, Dirks M, Worthmann H, Tryc AB, Sandorski K, Bahlmann E, Kielstein JT, Giesemann AM, Lanfermann H, Weissenborn K (2016) Neurological sequelae in adults after E coli O104: H4 infection-induced hemolytic-uremic syndrome. Medicine (baltimore) 95(6):e2337. https://doi.org/10.1097/MD.0000000000002337
Shabab T, Khanabdali R, Moghadamtousi SZ, Kadir HA, Mohan G (2017) Neuroinflammation pathways: a general review. Int J Neurosci 127(7):624–633. https://doi.org/10.1080/00207454.2016.1212854
Singh SS, Rai SN, Birla H, Zahra W, Rathore AS, Singh SP (2020) NF-kappaB-mediated neuroinflammation in parkinson’s disease and potential therapeutic effect of polyphenols. Neurotox Res 37(3):491–507. https://doi.org/10.1007/s12640-019-00147-2
Sinha P, Verma B, Ganesh S (2021) Trehalose ameliorates seizure susceptibility in lafora disease mouse models by suppressing neuroinflammation and endoplasmic reticulum stress. Mol Neurobiol 58(3):1088–1101. https://doi.org/10.1007/s12035-020-02170-3
Smith WE, Kane AV, Campbell ST, Acheson DW, Cochran BH, Thorpe CM (2003) Shiga toxin 1 triggers a ribotoxic stress response leading to p38 and JNK activation and induction of apoptosis in intestinal epithelial cells. Infect Immun 71(3):1497–1504. https://doi.org/10.1128/iai.71.3.1497-1504.2003
Stricklett PK, Hughes AK, Kohan DE (2005) Inhibition of p38 mitogen-activated protein kinase ameliorates cytokine up-regulated shigatoxin-1 toxicity in human brain microvascular endothelial cells. J Infect Dis 191(3):461–471. https://doi.org/10.1086/427188
Su X, Chen Q, Chen W, Chen T, Li W, Li Y et al (2014) Mycoepoxydiene inhibits activation of BV2 microglia stimulated by lipopolysaccharide through suppressing NF-kappaB, ERK 1/2 and toll-like receptor pathways. Int Immunopharmacol 19(1):88–93. https://doi.org/10.1016/j.intimp.2014.01.004
Sullivan PF, Neale MC, Kendler KS (2000) Genetic epidemiology of major depression: review and meta-analysis. Am J Psychiatry 157(10):1552–1562. https://doi.org/10.1176/appi.ajp.157.10.1552
Sun J, Nan G (2017) The extracellular signal-regulated kinase 1/2 pathway in neurological diseases: a potential therapeutic target (Review). Int J Mol Med 39(6):1338–1346. https://doi.org/10.3892/ijmm.2017.2962
Thorpe CM, Hurley BP, Lincicome LL, Jacewicz MS, Keusch GT, Acheson DW (1999) Shiga toxins stimulate secretion of interleukin-8 from intestinal epithelial cells. Infect Immun 67(11):5985–5993
Torre-Fuentes L, Moreno-Jimenez L, Pytel V, Matias-Guiu JA, Gomez-Pinedo U, Matias-Guiu J (2020) Experimental models of demyelination and remyelination. Neurologia 35(1):32–39. https://doi.org/10.1016/j.nrl.2017.07.002
Torres AG, Amaral MM, Bentancor L, Galli L, Goldstein J, Kruger A, Rojas-Lopez M (2018) Recent advances in shiga toxin-producing Escherichia coli Research in Latin America. Microorganisms 6(4):100. https://doi.org/10.3390/microorganisms6040100
Wappler EA, Szilagyi G, Gal A, Skopal J, Nyakas C, Nagy Z et al (2009) Adopted cognitive tests for gerbils: validation by studying ageing and ischemia. Physiol Behav 97(1):107–114. https://doi.org/10.1016/j.physbeh.2009.02.011
Weinstein JR, Zhang M, Kutlubaev M, Lee R, Bishop C, Andersen H et al (2009) Thrombin-induced regulation of CD95(Fas) expression in the N9 microglial cell line: evidence for involvement of proteinase-activated receptor(1) and extracellular signal-regulated kinase 1/2. Neurochem Res 34(3):445–452. https://doi.org/10.1007/s11064-008-9803-9
Weissenborn K, Donnerstag F, Kielstein JT, Heeren M, Worthmann H, Hecker H, Schmitt R, Schiffer M, Pasedag T, Schuppner R, Tryc AB, Raab P, Hartmann H, Ding XQ, Hafer C, Menne J, Schmidt BM, Bultmann E, Haller H, Dengler R, Lanfermann H, Giesemann AM (2012) Neurologic manifestations of E coli infection-induced hemolytic-uremic syndrome in adults. Neurology 79(14):1466–1473. https://doi.org/10.1212/WNL.0b013e31826d5f26
Wu H, Zheng J, Xu S, Fang Y, Wu Y, Zeng J, Shao A, Shi L, Lu J, Mei S, Wang X, Guo X, Wang Y, Zhao Z, Zhang J (2021) Mer regulates microglial/macrophage M1/M2 polarization and alleviates neuroinflammation following traumatic brain injury. J Neuroinflammation 18(1):2. https://doi.org/10.1186/s12974-020-02041-7
Yankelevitch-Yahav R, Franko M, Huly A, Doron R (2015) The forced swim test as a model of depressive-like behavior. J vis Exp. https://doi.org/10.3791/52587
Zhang H, Peterson JW, Niesel DW, Klimpel GR (1997) Bacterial lipoprotein and lipopolysaccharide act synergistically to induce lethal shock and proinflammatory cytokine production. J Immunol 159(10):4868–4878
Zhou Y, Tao T, Liu G, Gao X, Gao Y, Zhuang Z, Lu Y, Wang H, Li W, Wu L, Zhang D, Hang C (2021) TRAF3 mediates neuronal apoptosis in early brain injury following subarachnoid hemorrhage via targeting TAK1-dependent MAPKs and NF-κB pathways. Cell Death Dis 12(1):10. https://doi.org/10.1038/s41419-020-03278-z
Acknowledgements
The authors are especially grateful to German Nicolás La Iacona for his technical assistance in using the confocal microscope. We are also grateful to María Marta Rancez for her special dedication and technical assistance.
Funding
This work was supported by the Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT) (Grant No. PICT-2016-1175) and Universidad de Buenos Aires (UBACyT) (20020160100135BA), Argentina, National Research Council—CONICET—(PUE 0041) & (PIP 2021-2023) [11220200101293CO] Argentina (JG) and (ANPCyT) 2016-0129 & 2016-0803 (FC).
Author information
Authors and Affiliations
Contributions
JG conceived the project. CB, AP, MB, MG, and JG designed and coordinated the experiments. CB, AP, MB, NALC, ABC, and MN performed the experiments and analyzed the data. CB, AP, MB, and JG wrote this manuscript. PAG, AC, and MN revised this manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Ethical Approval
All animal procedures were reviewed and approved by the Institutional Animal Care and Use Committee of the School of Medicine, Universidad de Buenos Aires, Argentina (Resolution No. 184/2020), in accordance with the EEC guidelines for the care and use of experimental animals (EEC Council 86/609).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10571_2022_1298_MOESM1_ESM.tif
Supplementary file1 (TIF 9520 KB) Supplementary figure 1 A: Change in mouse weight throughout the period of acclimatization and treatment. ANOVA of 1 factor was applied, assuming non-homogeneous variances. There were no significant differences across treatments. n=6 (per group). B: Representative Gb3 immunofluorescence from the CA1 area of the mouse hippocampus. C: Representative TLR4 immunofluorescence from the CA1 area of the mouse hippocampus. Scale bar in C applies for all microglial micrographs. py: pyramidal cell layer of the hippocampus. rad: stratum radiatum of the hippocampus.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Berdasco, C., Pinto, A., Blake, M.G. et al. Cognitive Deficits Found in a Pro-inflammatory State are Independent of ERK1/2 Signaling in the Murine Brain Hippocampus Treated with Shiga Toxin 2 from Enterohemorrhagic Escherichia coli. Cell Mol Neurobiol 43, 2203–2217 (2023). https://doi.org/10.1007/s10571-022-01298-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-022-01298-1