ABSTRACT
Purpose
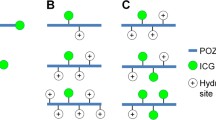
Indocyanine green (ICG), an FDA-approved near infrared (NIR) dye, has potential application as a contrast agent for tumor detection. Because ICG binds strongly to plasma proteins and exhibits aqueous, photo, and thermal instability, its current applications are largely limited to monitoring blood flow. To address these issues, ICG was encapsulated and stabilized within polymeric micelles formed from the thermo-sensitive block copolymer Pluronic F-127, poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide), to increase the stability and circulation time of ICG.
Methods
ICG-loaded Pluronic micelles were prepared at various concentrations of Pluronic and ICG and characterized by determining particle sizes, dye loading efficiency, and the kinetics of dye degradation. Förster resonance energy transfer spectroscopy was employed to monitor the stability of Pluronic micelles in physiological solutions. The plasma clearance kinetics and biodistribution of ICG-loaded micelles was also determined after intravenous delivery to CT-26 colon carcinoma tumor-bearing mice, and NIR whole-body imaging was performed for tumor detection.
Results
The Pluronic F-127 micelles showed efficient ICG loading, small size, stabilized ICG fluorescence, and prolonged circulation in vivo. Solid tumors in mice were specifically visualized after intravenous administration of ICG-loaded micelles.
Conclusions
These materials are therefore promising formulations for noninvasive NIR tumor imaging applications.







Similar content being viewed by others
REFERENCES
Ke S, Wen X, Gurfinkel M, Charnsangavej C, Wallace S, Sevick-Muraca EM, et al. Near-infrared optical imaging of epidermal growth factor receptor in breast cancer xenografts. Cancer Res. 2003;63:7870–5.
Klohs J, Wunder A, Licha K. Near-infrared fluorescent probes for imaging vascular pathophysiology. Basic Res Cardiol. 2008;103:144–51.
Taroni P, Pifferi A, Torricelli A, Comelli D, Cubeddu R. In vivo absorption and scattering spectroscopy of biological tissues. Photochem Photobiol Sci. 2003;2:124–9.
Licha K, Olbrich C. Optical imaging in drug discovery and diagnostic applications. Adv Drug Deliv Rev. 2005;57:1087–108.
Rao J, Dragulescu-Andrasi A, Yao H. Fluorescence imaging in vivo: recent advances. Curr Opin Biotechnol. 2007;18:17–25.
Saxena V, Sadoqi M, Shao J. Degradation kinetics of indocyanine green in aqueous solution. J Pharm Sci. 2003;92:2090–7.
Slakter JS, Yannuzzi LA, Guyer DR, Sorenson JA, Orlock DA. Indocyanine-green angiography. Curr Opin Ophthalmol. 1995;6:25–32.
Caesar J, Shaldon S, Chiandussi L, Guevara L, Sherlock S. The use of indocyanine green in the measurement of hepatic blood flow and as a test of hepatic function. Clin Sci. 1961;21:43–57.
Maarek JM, Holschneider DP, Rubinstein EH. Fluorescence dilution technique for measurement of cardiac output and circulating blood volume in healthy human subjects. Anesthesiology. 2007;106:491–8.
Paumgart G, Probst P, Kraines R, Leevy CM. Kinetics of indocyanine green removal from blood. Ann N Y Acad Sci. 1970;170:134–47.
Intes X, Ripoll J, Chen Y, Nioka S, Yodh AG, Chance B. In vivo continuous-wave optical breast imaging enhanced with Indocyanine Green. Med Phys. 2003;30:1039–47.
Ntziachristos V, Yodh AG, Schnall M, Chance B. Concurrent MRI and diffuse optical tomography of breast after indocyanine green enhancement. Proc Natl Acad Sci U S A. 2000;97:2767–72.
Saxena V, Sadoqi M, Shao J. Enhanced photo-stability, thermal-stability and aqueous-stability of indocyanine green in polymeric nanoparticulate systems. J Photochem Photobiol B. 2004;74:29–38.
Fickweiler S, Szeimies RM, Baumler W, Steinbach P, Karrer S, Goetz AE, et al. Indocyanine green: intracellular uptake and phototherapeutic effects in vitro. J Photochem Photobiol B. 1997;38:178–83.
Kim G, Huang SW, Day KC, O’Donnell M, Agayan RR, Day MA, et al. Indocyanine-green-embedded PEBBLEs as a contrast agent for photoacoustic imaging. J Biomed Opt. 2007;12:044020.
Saxena V, Sadoqi M, Shao J. Polymeric nanoparticulate delivery system for Indocyanine green: biodistribution in healthy mice. Int J Pharm. 2006;308:200–4.
Choi SH, Lee JH, Choi SM, Park TG. Thermally reversible pluronic/heparin nanocapsules exhibiting 1000-fold volume transition. Langmuir. 2006;22:1758–62.
Cardillo JA, Jorge R, Costa RA, Nunes SM, Lavinsky D, Kuppermann BD, et al. Experimental selective choriocapillaris photothrombosis using a modified indocyanine green formulation. Br J Ophthalmol. 2008;92:276–80.
Licha K, Riefke B, Ntziachristos V, Becker A, Chance B, Semmler W. Hydrophilic cyanine dyes as contrast agents for near-infrared tumor imaging: synthesis, photophysical properties and spectroscopic in vivo characterization. Photochem Photobiol. 2000;72:392–8.
Kirchherr AK, Briel A, Mader K. Stabilization of indocyanine green by encapsulation within micellar systems. Mol Pharm. 2009;6:480–91.
Rodriguez VB, Henry SM, Hoffman AS, Stayton PS, Li X, Pun SH. Encapsulation and stabilization of indocyanine green within poly(styrene-alt-maleic anhydride) block-poly(styrene) micelles for near-infrared imaging. J Biomed Opt. 2008;13:014025.
Soppimath KS, Aminabhavi TM, Kulkarni AR, Rudzinski WE. Biodegradable polymeric nanoparticles as drug delivery devices. J Control Release. 2001;70:1–20.
Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2:751–60.
Blanco E, Kessinger CW, Sumer BD, Gao J. Multifunctional micellar nanomedicine for cancer therapy. Exp Biol Med (Maywood). 2009;234:123–31.
Romberg B, Hennink WE, Storm G. Sheddable coatings for long-circulating nanoparticles. Pharm Res. 2008;25:55–71.
Yu J, Yaseen MA, Anvari B, Wong MS. Synthesis of near-infrared-absorbing nanoparticle-assembled capsules. Chem Mater. 2007;19:1277–84.
Liu H, Farrell S, Uhrich K. Drug release characteristics of unimolecular polymeric micelles. J Control Release. 2000;68:167–74.
Moghimi SM, Porter CJ, Muir IS, Illum L, Davis SS. Non-phagocytic uptake of intravenously injected microspheres in rat spleen: influence of particle size and hydrophilic coating. Biochem Biophys Res Commun. 1991;177:861–6.
Yuan F, Dellian M, Fukumura D, Leunig M, Berk DA, Torchilin VP, et al. Vascular permeability in a human tumor xenograft: molecular size dependence and cutoff size. Cancer Res. 1995;55:3752–6.
Stolnik S, Illum L, Davis SS. Long circulating microparticulate drug carriers. Adv Drug Deliv Rev. 1995;16:195–214.
Bhardwajand R, Blanchard J. Controlled-release delivery system for the alpha-MSH analog melanotan-I using poloxamer 407. J Pharm Sci. 1996;85:915–9.
Bae KH, Lee Y, Park TG. Oil-encapsulating PEO-PPO-PEO/PEG shell cross-linked nanocapsules for target-specific delivery of paclitaxel. Biomacromolecules. 2007;8:650–6.
Batrakova EV, Kabanov AV. Pluronic block copolymers: evolution of drug delivery concept from inert nanocarriers to biological response modifiers. J Control Release. 2008;130:98–106.
Chen H, Kim S, He W, Wang H, Low PS, Park K, et al. Fast release of lipophilic agents from circulating PEG-PDLLA micelles revealed by in vivo forster resonance energy transfer imaging. Langmuir. 2008;24:5213–7.
Rajagopalan R, Uetrecht P, Bugaj JE, Achilefu SA, Dorshow RB. Stabilization of the optical tracer agent indocyanine green using noncovalent interactions. Photochem Photobiol. 2000;71:347–50.
Sauda K, Imasaka T, Ishibashi N. Determination of protein in human-serum by high-performance liquid-chromatography with semiconductor-laser fluorometric detection. Anal Chem. 1986;58:2649–53.
Kabanov AV, Batrakova EV, Alakhov VY. Pluronic block copolymers as novel polymer therapeutics for drug and gene delivery. J Control Release. 2002;82:189–212.
Malmsten M, Lindman B. Self-assembly in aqueous block copolymer solutions. Macromolecules. 1992;25:5440–5.
Gomes AJ, Lunardi LO, Marchetti JM, Lunardi CN, Tedesco AC. Indocyanine green nanoparticles useful for photomedicine. Photomed Laser Surg. 2006;24:514–21.
Devoisselle JM, Soulie-Begu S, Mordon S, Desmettre T, Maillols H. Fluorescence properties of indocyanine green: I.in-vitro study with micelles and liposomes. In: Thompson RB, editor. SPIE, Vol. 2980, Advances in Fluorescence Sensing Technology III, San Jose, CA, USA; 1997, p. 530–537.
Altinoglu EI, Russin TJ, Kaiser JM, Barth BM, Eklund PC, Kester M, et al. Near-infrared emitting fluorophore-doped calcium phosphate nanoparticles for in vivo imaging of human breast cancer. ACS Nano. 2008;2:2075–84.
Chen H, Kim S, Li L, Wang S, Park K, Cheng JX. Release of hydrophobic molecules from polymer micelles into cell membranes revealed by Forster resonance energy transfer imaging. Proc Natl Acad Sci U S A. 2008;105:6596–601.
Yaseen MA, Yu J, Jung BS, Wong MS, Anvari B. Biodistribution of encapsulated indocyanine green in healthy mice. Mol Pharm. 2009;6:1321–32.
Kozlov MY, Melik-Nubarov NS, Batrakova EV, Kabanov AV. Relationship between pluronic block copolymer structure, critical micellization concentration and partitioning coefficients of low molecular mass solutes. Macromolecules. 2000;33:3305–13.
le Masne Q, de Chermont C, Chaneac JS, Pelle F, Maitrejean S, Jolivet JP, et al. Nanoprobes with near-infrared persistent luminescence for in vivo imaging. Proc Natl Acad Sci U S A. 2007;104:9266–71.
Batrakova EV, Li S, Li YL, Alakhov VY, Elmquist WF, Kabanov AV. Distribution kinetics of a micelle-forming block copolymer Pluronic P85. J Control Release. 2004;100:389–97.
Ishida T, Atobe K, Wang X, Kiwada H. Accelerated blood clearance of PEGylated liposomes upon repeated injections: effect of doxorubicin-encapsulation and high-dose first injection. J Control Release. 2006;115:251–8.
Ishida T, Ichihara M, Wang X, Yamamoto K, Kimura J, Majima E, et al. Injection of PEGylated liposomes in rats elicits PEG-specific IgM, which is responsible for rapid elimination of a second dose of PEGylated liposomes. J Control Release. 2006;112:15–25.
Koide H, Asai T, Hatanaka K, Urakami T, Ishii T, Kenjo E, et al. Particle size-dependent triggering of accelerated blood clearance phenomenon. Int J Pharm. 2008;362:197–200.
Tanaka E, Choi HS, Humblet V, Ohnishi S, Laurence RG, Frangioni JV. Real-time intraoperative assessment of the extrahepatic bile ducts in rats and pigs using invisible near-infrared fluorescent light. Surgery. 2008;144:39–48.
Desmettre T, Devoisselle JM, Mordon S. Fluorescence properties and metabolic features of indocyanine green (ICG) as related to angiography. Surv Ophthalmol. 2000;45:15–27.
Alexandridis P, Holzwarth JF, Hatton TA. Micellization of Poly(Ethylene Oxide)-Poly(Propylene Oxide)-Poly(Ethylene Oxide) Triblock Copolymers in Aqueous-Solutions—Thermodynamics of Copolymer Association. Macromolecules. 1994;27:2414–25.
ACKNOWLEDGEMENTS
This work was supported by the funds from the Washington Technology Center and Omeros Corporation (Pun and Li), and in part by the National Institutes of Health (Grant No. R01 CA120480-Li). Xenogen Spectrum imaging was conducted through the Center for Intracellular Delivery of Biologics, funded by Washington State Life Sciences Discovery Fund Grant 2496490.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Kim, T.H., Chen, Y., Mount, C.W. et al. Evaluation of Temperature-Sensitive, Indocyanine Green-Encapsulating Micelles for Noninvasive Near-Infrared Tumor Imaging. Pharm Res 27, 1900–1913 (2010). https://doi.org/10.1007/s11095-010-0190-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-010-0190-y