Abstract
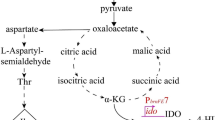
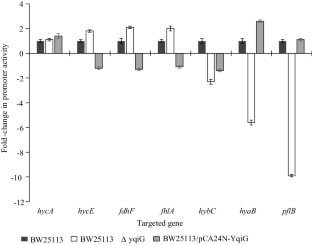
Pseudogenes in the Escherichia coli genome are assumed to be non-functional. In this study, Keio collection BW25113∆yqiG and YqiG-producing strain (BW25113/pCA24N-YqiG) were used to evaluate the importance of pseudogene yqiG in hydrogen metabolism. Our results show pseudogene protein YqiG was identified as an essential protein in the production of biohydrogen from glucose. The mutant yqiG decreased biohydrogen production from 37 µmol mg−1 protein to 6 µmol mg−1 protein compared to the wild-type strain, and glucose consumption was reduced by 80%. Through transcriptional analysis, we found that the yqiG mutation represses pflB transcription tenfold; pflB encodes pyruvate-formate lyase, one of the key enzymes in the anaerobic metabolism of E. coli. Moreover, production of YqiG stimulated glycolysis and increased biohydrogen productivity 1.5-fold compared to that of the wild-type strain. Thus, YqiG is important for the central glycolysis reaction and is able to influence hydrogen metabolism activity in E. coli.


Similar content being viewed by others
References
Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H (2006) Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol 2:2006–2008
Bagramyan K, Trchounian A (2003) Structural and functional features of formate hydrogen lyase, an enzyme of mixed-acid fermentation from Escherichia coli. Biochemistry 68:1159–1170
Balakirev ES, Ayala FJ (2003) Pseudogenes: are they “junk” or functional DNA? Annu Rev Genet 37:123–151
Barker CS, Pruss BM, Matsumura P (2004) Increased motility of Escherichia coli by insertion sequence element integration into the regulatory region of the flhD operon. J Bacteriol 186:7529–7537
Beyer L, Doberenz C, Falke D, Hunger D, Suppmann B, Sawers RG (2013) Coordination of FocA and pyruvate formate-lyase synthesis in Escherichia coli demonstrates preferential translocation of formate over other mixed-acid fermentation products. J Bacteriol 195:1428–1435
Bjarnadottir H, Jonsson JJ (2005) A rapid real-time qRT-PCR assay for ovine beta-actin mRNA. J Biotechnol 117:173–182
Campos-Bermudez VA, Bologna FP, Andreo CS, Drincovich MF (2010) Functional dissection of Escherichia coli phosphotransacetylase structural domains and analysis of key compounds involved in activity regulation. FEBS J 277:1957–1966
Datsenko KA, Wanner BL (2000) One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA 97:6640–6645
Fan Z, Yuan L, Chatterjee R (2009) Increased hydrogen production by genetic engineering of Escherichia coli. PLoS One 4:32–44
Forzi L, Sawers RG (2007) Maturation of [NiFe]-hydrogenases in Escherichia coli. BioMetals 20:565–578
Ghimire A, Trably E, Frunzo L, Pirozzi F, Lens PNL, Esposito G, Cazier EA, Escudié R (2018) Effect of total solids content on biohydrogen production and lactic acid accumulation during dark fermentation of organic waste biomass. Bioresour Technol 248:180–186
Griffiths AJF, Wessler SR, Varroll SB, Doebley J (2012) Introduction to genetic analysis, 10th edn. W. H. Freeman and Company, New York
Haggblom MM, Apetroaie C, Andersson MA, Salkinoja-Salonen MS (2002) Quantitative analysis of cereulide, the emetic toxin of Bacillus cereus, produced under various conditions. Appl Environ Microbiol 68:2479–2483
Hallenbeck PC, Ghosh D (2009) Advances in fermentative biohydrogen production: the way forward? Trends Biotechnol 27:287–297
Jonsson LJ, Alriksson B, Nilvebrant NO (2013) Bioconversion of lignocellulose: inhibitors and detoxification. Biotechnol Biofuels 6:16
Kitagawa M, Ara T, Arifuzzaman M, Ioka-Nakamichi T, Inamoto E, Toyonaga H, Mori H (2005) Complete set of ORF clones of Escherichia coli ASKA library (a complete set of E. coli K-12 ORF archive): unique resources for biological research. DNA Res 12:291–299
Liu Y, Harrison PM, Kunin V, Gerstein M (2004) Comprehensive analysis of pseudogenes in prokaryotes: widespread gene decay and failure of putative horizontally transferred genes. Genome Biol 5(9):R64
Maeda T, Sanchez-Torres V, Wood TK (2008) Metabolic engineering to enhance bacterial hydrogen production. Microb Biotechnol 1:30–39
Maeda T, Tran KT, Yamasaki R, Wood TK (2018) Current state and perspectives in hydrogen production by Escherichia coli: roles of hydrogenases in glucose or glycerol metabolism. Appl Microbiol Biotechnol 102:2041–2050
Menon NK, Chatelus CY, Dervartanian M, Wendt JC, Shanmugam KT, Peck HD, Przybyla AE (1994) Cloning, sequencing, and mutational analysis of the hyb operon encoding Escherichia coli hydrogenase 2. J Bacteriol 176:4416–4423
Meza E, Becker J, Bolivar F, Gosset G, Wittmann C (2012) Consequences of phosphoenolpyruvate:sugar phosphotranferase system and pyruvate kinase isozymes inactivation in central carbon metabolism flux distribution in Escherichia coli. Microb Cell Fact 1:127–140
Mohd Yasin NH, Fukuzaki M, Maeda T, Miyazaki T, Che Maail CMH, Ariffin H, Wood TK (2013) Biohydrogen production from oil palm frond juice and sewage sludge by a metabolically engineered Escherichia coli strain. Int J Hydrog Energy 38:10277–10283
Mohd Yusoff MZ, Maeda T, Sanchez-Torres V, Ogawa HI, Shirai Y, Hassan MA, Wood TK (2012) Uncharacterized Escherichia coli proteins YdjA and YhjY are related to biohydrogen production. Int J Hydrog Energy 37:17778–17787
Mohd Yusoff MZ, Hashiguchi Y, Maeda T, Wood TK (2013) Four products from Escherichia coli pseudogenes increase hydrogen production. Biochem Biophys Res Commun 439:576–579
Muñoz M, Ponce E (2003) Pyruvate kinase: current status of regulatory and functional properties. Comp Biochem Physiol B Biochem Mol Biol 135:197–218
Nakashima N, Akita H, Hoshino T (2014) Establishment of a novel gene expression method, BICES (biomass-inducible chromosome-based expression system), and its application to the production of 2,3-butanediol and acetoin. Metab Eng 25:204–214
Noguchi K, Riggins DP, Eldahan KC, Kitko RD, Slonczewski JL (2010) Hydrogenase-3 contributes to anaerobic acid resistance of Escherichia coli. PLoS ONE 5:e10132
Nuccio S-P, Bäumler AJ (2007) Evolution of the chaperone/usher assembly pathway: fimbrial classification goes Greek. Microbiol Mol Biol Rev 71:551–575
Pandelia ME, Fourmond V, Tron-Infossi P, Lojou E, Bertrand P, Leger C, Giudici-Orticoni MT, Lubitz W (2010) Membrane-bound hydrogenase I from the hyperthermophilic bacterium Aquifex aeolicus: enzyme activation, redox intermediates and oxygen tolerance. J Am Chem Soc 132:6991–7004
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:2002–2007
Pink RC, Wicks K, Caley DP, Punch EK, Jacobs L, Francisco Carter DR (2011) Pseudogenes: pseudo-functional or key regulators in health disease? RNA 17:792–798
Rachman MA, Furutani Y, Nakashimada Y, Kakizono T, Nishio N (1997) Enhanced hydrogen production in altered mixed acid fermentation of glucose by Enterobacter aerogenes. J Ferment Bioeng 83:358–363
Rouchka EC, Cha IE (2009) Current trends in pseudogene detection and characterization. Curr Bioinform 4:112–119
Sanchez-Torres V, Maeda T, Wood TK (2009) Protein engineering of the transcriptional activator FhlA to enhance hydrogen production in Escherichia coli. Appl Environ Microbiol 75:5639–5646
Seol E, Ainala SK, Sekar BS, Park S (2014) Metabolic engineering of Escherichia coli strains for co-production of hydrogen and ethanol from glucose. Int J Hydrog Energy 39:19323–19330
Sinha P, Roy S, Das D (2015) Role of formate hydrogen lyase complex in hydrogen production in facultative anaerobes. Int J Hydrog Energy 40:8806–8815
Tran KT, Maeda T, Wood TK (2014) Metabolic engineering of Escherichia coli to enhance hydrogen production from glycerol. Appl Microbiol Biotechnol 98:4757–4770
Zhou J, Rudd KE (2013) EcoGene 3.0. Nucleic Acids Res 41:613–624
Acknowledgements
We express our gratitude to the National Institute of Genetics (Japan) for providing the Keio mutant and ASKA strains. The authors would like to thank the Ministry of Higher Education, Malaysia for providing Fundamental Research Grant Scheme (02-01-14-1401FR), the School of Graduate Studies, Universiti Putra Malaysia and Japan Student Services Organisation for the scholarship to Muhammad Azman Zakaria during this study.
Author information
Authors and Affiliations
Contributions
Study conception and design: MZMY and TM. Experiment, analysis and interpretation of data: MAZ and MZMY. Draft and revise the manuscript: MZMY, TM and MRZ. Coordinate the experiments and critical revision: MAH and TKW.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there have no conflict of interest in relation to this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
13205_2018_1461_MOESM1_ESM.pptx
Fig. S1 H2 productivity from different strains in complex glucose medium. BW25113 (plain white column), BW25113ΔyqiG (black column) and BW25113/pCA24N-YqiG (grey column). Fig. S2 Protein purification using Ni-NTA column. Overexpressed strain visualised by 10% of SDS-PAGE. Arrows indicate expected YqiG protein. Lane 1: Cell lysate, lane 2: Flow through (after bind to Ni-NTA resin), lane 3: After washing with wash buffer and 10 mM imidazole, lane 4: First washing with wash buffer and 20 mM imidazole, lane 5: Second washing with wash buffer and 20 mM imidazole, lane 6 and lane 7: Eluent wash buffer with 130 mM imidazole, and lane 8: protein marker, XL ladder. (PPTX 106 KB)
Rights and permissions
About this article
Cite this article
Zakaria, M.A., Mohd Yusoff, M.Z., Zakaria, M.R. et al. Pseudogene product YqiG is important for pflB expression and biohydrogen production in Escherichia coli BW25113. 3 Biotech 8, 435 (2018). https://doi.org/10.1007/s13205-018-1461-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-018-1461-2