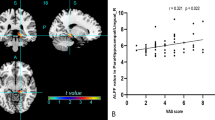
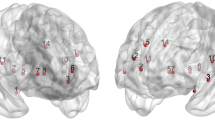
Abstract
Background and Objective
There are indicates that raphe nuclei may be involved in the occurrence of chronic pain in Parkinson’s disease (PD). In the study, we investigated the functional connectivity pattern of raphe nuclei in Parkinson’s disease with chronic pain (PDP) to uncover its possible pathophysiology.
Methods
Fifteen PDP, who suffered from pain, lasted longer than 3 months, sixteen Parkinson’s disease patients with no pain (nPDP) and eighteen matched normal health controls (NCs) were recruited. All subjects completed the King’s Parkinson’s Pain Scale (KPPS) besides Parkinson-related scale and demographics. We performed a seed-based resting-state analysis of functional magnetic resonance imaging to explore whole-brain functional connectivity of the raphe nuclei. Multiple regression model was used to explore the related factors of pain including disease duration, disease severity, Hamilton Depression Rating Scale, age, sex, levodopa equivalent dose and the strength of network functional connectivity.
Results
Compared with the nPDP, the PDP group showed stronger functional connectivity between raphe nuclei and pain-related brain regions, including parietal lobe, insular lobe, cingulum cortex and prefrontal cortex, and the functional connectivity values of those areas were significantly positively correlated with KPPS independent of the clinical variables. Compared with NCs, the combined PD groups showed decreased functional connectivity including prefrontal cortex and cingulum cortex.
Conclusions
Abnormal functional connectivity model of raphe nuclei may be partly involved in pathophysiological mechanism of pain in PD.



Similar content being viewed by others
References
Broen MP, Braaksma MM, Patijn J, Weber WE (2012) Prevalence of pain in Parkinson’s disease: a systematic review using the modified QUADAS tool. Mov Disord 27(4):480–484
Negre-Pages L, Regragui W, Bouhassira D, Grandjean H, Rascol O, DoPaMi PSG (2008) Chronic pain in Parkinson’s disease: the cross-sectional French DoPaMiP survey. Mov Disord 23(10):1361–1369
Chaudhuri KR, Schapira AH (2009) Non-motor symptoms of parkinson’s disease: dopaminergic pathophysiology and treatment. Lancet Neurol 8(5):464–474
Fil A, Cano-de-la-Cuerda R, Munoz-Hellin E, Vela L, Ramiro-Gonzalez M, Fernandez-de-Las-Penas C (2013) Pain in Parkinson disease: a review of the literature. Parkinsonism Relat Disord 19(3):285–94 discussion
Schapira AHV, Chaudhuri KR, Jenner P (2017) Non-motor features of Parkinson disease. Nat Rev Neurosci 18(7):435–450
Dellapina E, Gerdelat-Mas A, Ory-Magne F, Pourcel L, Brefel-Courbon C (2011) Apomorphine effect on pain threshold in parkinson’s disease: a clinical and positron emission tomography study. Mov Disord 26(1):153–157
Tong Q, Zhang L, Yuan Y, Jiang S, Zhang R, Xu Q et al (2015) Reduced plasma serotonin and 5-hydroxyindoleacetic acid levels in Parkinson’s disease are associated with nonmotor symptoms. Parkinsonism Relat Disord 21(8):882–887
Djaldetti R, Yust-Katz S, Kolianov V, Melamed E, Dabby R (2007) The effect of duloxetine on primary pain symptoms in Parkinson disease. Clin Neuropharmacol 30(4):201–205
Bellingham GA, Peng PW (2010) Duloxetine: a review of its pharmacology and use in chronic pain management. Reg Anesth Pain Med 35(3):294–303
Braak H, Del Tredici K, Rüb U, de Vos RA, Jansen Steur EN, Braak E (2003) Staging of brain pathology related to sporadic parkinson’s disease. Neurobiol Aging 24(2):197–211
Scherder E, Wolters E, Polman C, Sergeant J, Swaab D (2005) Pain in Parkinson’s disease and multiple sclerosis: its relation to the medial and lateral pain systems. Neurosci Biobehav Rev 29(7):1047–1056
Wang CT, Mao CJ, Zhang XQ, Zhang CY, Lv DJ, Yang YP et al (2017) Attenuation of hyperalgesia responses via the modulation of 5-hydroxytryptamine signalings in the rostral ventromedial medulla and spinal cord in a 6-hydroxydopamine-induced rat model of Parkinson’s disease. Mol Pain 13:1744806917691525
Zhou L, Liu MZ, Li Q, Deng J, Mu D, Sun YG (2017) Organization of Functional Long-Range Circuits Controlling the Activity of Serotonergic Neurons in the Dorsal Raphe Nucleus. Cell Rep 20(8):1991–1993
Phan KL, Wager T, Taylor SF, Liberzon I (2002) Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage 16(2):331–348
Chen T, Wang XL, Qu J, Wang W, Zhang T, Yanagawa Y et al (2013) Neurokinin-1 receptor-expressing neurons that contain serotonin and gamma-aminobutyric acid in the rat rostroventromedial medulla are involved in pain processing. J Pain 14(8):778–792
Taylor BK (2009) Spinal inhibitory neurotransmission in neuropathic pain. Curr Pain Headache Rep 13(3):208–214
Ferraro S, Nigri A, Bruzzone MG, Brivio L, ProiettiCecchini A, Verri M et al (2018) Defective functional connectivity between posterior hypothalamus and regions of the diencephalic-mesencephalic junction in chronic cluster headache. Cephalalgia 38(13):1910–1918
Lee MJ, Park BY, Cho S, Kim ST, Park H, Chung CS (2019) Increased connectivity of pain matrix in chronic migraine: a resting-state functional MRI study. J Headache Pain 20(1):29
Biswal Bharat, ZerrinYetkin F, Haughton VM, Hyde JS (2005) Functional connectivity in the motor cortex of resting human brain using echo-planar mri. Magn Reson Med 34(4):537–541
Rogers BP, Morgan VL, Newton AT, Gore JC (2007) Assessing functional connectivity in the human brain by fMRI. Magn Reson Imaging 25(10):1347–1357
Polli A, Weis L, Biundo R, Thacker M, Turolla A, Koutsikos K et al (2016) Anatomical and functional correlates of persistent pain in Parkinson’s disease. Mov Disord 31(12):1854–1864
Ferraro S, Grazzi L, Mandelli ML, Aquino D, Di Fiore D, Usai S, Bruzzone MG (2012) Pain processing in medication overuse headache: a functional magnetic resonance imaging (fmri) study. Pain Med 13(2):255–262
Hutchison WD, Davis KD, Lozano AM, Tasker RR, Dostrovsky JO (1999) Pain-related neurons in the human cingulate cortex [2]. Nat Neurosci 2(5):403–405
Otti A, Guendel H, Afra Wohlschläger, Zimmer C, Noll-Hussong M (2013) Frequency shifts in the anterior default mode network and the salience network in chronic pain disorder. BMC Psychiatry 13(1):84
Vierck CJ, Cannon RL, Fry G, Maixner W, Whitsel BL (1997) Characteristics of temporal summation of second pain sensations elicited by brief contact of glabrous skin by a preheated thermode. J Neurophysiol 78(2):992–1002
Garry EM, Fleetwood-Walker SM (2004) Organizing pains. Trends Neurosci 27(6):292–294
Chaudhuri KR, Rizos A, Trenkwalder C, Rascol O, Pal S, Martino D et al (2015) King’s Parkinson’s disease pain scale, the first scale for pain in PD: An international validation. Mov Disord 30(12):1623–1631
Garcia RG, Lin RL, Lee J, Kim J, Barbieri R, Sclocco R et al (2017) Modulation of brainstem activity and connectivity by respiratory-gated auricular vagal afferent nerve stimulation in migraine patients. Pain 158(8):1461–1472
Faulkner P, Ghahremani DG, Tyndale RF, Hellemann G, London ED (2018) Functional Connectivity of the Raphe Nuclei: Link to Tobacco Withdrawal in Smokers. Int J Neuropsychopharmacol 21(9):800–808
Peyron R, Laurent B, GarcíaLarrea L (2000) Functional imaging of brain responses to pain. A review and meta-analysis (2000). Neurophysiol Clin-Clin Neurophysiol 30(5):263–288
Price DD (2000) Psychological and neural mechanisms of the affective dimension of pain. Science 288(5472):1769–1772
Bodnar R, Heinricher MM (2016) Central Mechanisms of Pain Suppression: Central Mechanisms of Pain Modulation. Neuroscience in the 21st Century. p 3439–64
Gallace A, Bellan V (2018) The parietal cortex and pain perception: a body protection system. Handb Clin Neurol 151:103–117
Dum RP, Levinthal DJ, Strick PL (2009) The spinothalamic system targets motor and sensory areas in the cerebral cortex of monkeys. J Neurosci 29(45):14223–14235
Munn EM, Harte SE, Lagman A, Borszcz GS (2009) Contribution of the periaqueductal gray to the suppression of pain affect produced by administration of morphine into the intralaminar thalamus of rat. J Pain 10(4):426–435
Tang JS, Qu CL, Huo FQ (2009) The thalamic nucleus submedius and ventrolateral orbital cortex are involved in nociceptive modulation: a novel pain modulation pathway. Prog Neurobiol 89(4):383–389
Jasmin L, Burkey AR, Granato A, Ohara PT (2004) Rostral agranular insular cortex and pain areas of the central nervous system: a tract-tracing study in the rat. J Comp Neurol 468(3):425–440
Ploner M, Gross J, Timmermann L, Schnitzler A (2002) Cortical representation of first and second pain sensation in humans. Proc Natl Acad Sci USA 99(19):12444–12448
Yu CX, Li B, Xu YK, Ji TT, Li L, Zhao CJ et al (2017) Altered functional connectivity of the periaqueductal gray in chronic neck and shoulder pain. NeuroReport 28(12):720–725
Zhuo M (2006) Molecular mechanisms of pain in the anterior cingulate cortex. J Neurosci Res 84(5):927–933
Hajós M, Richards CD, Székely AD, Sharp T (1998) An electrophysiological and neuroanatomical study of the medial prefrontal cortical projection to the midbrain raphe nuclei in the rat. Neuroscience 87(1):95–108
Davis KD, Moayedi M (2013) Central mechanisms of pain revealed through functional and structural MRI. J Neuroimmune Pharmacol 8(3):518–534
Jones AK, Qi LY, Fujirawa T, Luthra SK, Ashburner J, Bloomfield P, Cunningham VJ, Itoh M, Fukuda H, Jones T (1991) In vivo distribution of opioid receptors in man in relation to the cortical projections of the medial and lateral pain systems measured with positron emission tomography. Neurosci Lett 126(1):25–28
Wiech K, Ploner M, Tracey I (2008) Neurocognitive aspects of pain perception. Trends Cogn Sci 12(8):306–313
Bar KJ, Kohler S, Cruz F, Schumann A, Zepf FD, Wagner G (2020) Functional consequences of acute tryptophan depletion on raphe nuclei connectivity and network organization in healthy women. Neuroimage 207:116362
Pizzagalli DA (2011) Frontocingulate dysfunction in depression: toward biomarkers of treatment response. Neuropsychopharmacology 36(1):183–206
Funding
This research was supported by the National Natural Science Foundation of China (No. 81871002, 81471334, 81100981) and the National Key Clinical Specialties Construction Program of China.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shen, Y., Wang, J., Peng, J. et al. Abnormal connectivity model of raphe nuclei with sensory-associated cortex in Parkinson’s disease with chronic pain. Neurol Sci 43, 3175–3185 (2022). https://doi.org/10.1007/s10072-022-05864-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-022-05864-9