Abstract
Background and Objectives
Bayesian forecasting (BF) methods for tobramycin dose individualisation has not seen widespread clinical adoption, despite being endorsed by clinical practice guidelines. Several freeware and commercial programmes using BF methods are available to support personalised dosing. This study evaluated exposure estimates, dose recommendations, and predictive performance compared with current clinical practice.
Methods
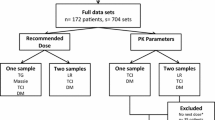
Data from 105 patients (50 adults and 55 children) with cystic fibrosis who received intravenous tobramycin treatment and had paired concentration–time measurements were analysed using (1) log-linear regression analysis, and (2) three BF programmes: TDMx, InsightRX, and DoseMe. Exposure estimates and dose recommendations were compared using the Wilcoxon signed-rank test and Bland–Altman analysis. Predictive performance of BF programmes was compared based on bias and imprecision.
Results
Median estimated tobramycin exposure with current clinical practice was significantly lower (87.8 vs. 92.5, 94.0 and 90.3 mg h l−1; p ≤ 0.01), hence median subsequent dose recommendations were significantly higher (10.1 vs. 9.4, 9.4 and 9.2 mg kg−1; p ≤ 0.01) compared with BF programmes. Furthermore, median relative dose-adjustment differences were higher in adults (> 10%) compared with children (4.4–7.8%), and differences in individual dose recommendations were > 20% on 19.1–27.4% of occasions. BF programmes showed low bias (< 7%) and imprecision (< 20%), and none of the programmes made consistently significantly different recommendations compared with each other.
Conclusions
On average, the predictions made by the BF programmes were similar, however substantial individual differences were observed for some patients. This suggests the need for detailed investigations of true tobramycin exposure.


Similar content being viewed by others
References
Ratjen F, Brockhaus F, Angyalosi G. Aminoglycoside therapy against Pseudomonas aeruginosa in cystic fibrosis: a review. J Cyst Fibros. 2009;8(6):361–9.
Craig WA, Ebert SC. Killing and regrowth of bacteria in vitro: a review. Scand J Infect Dis. 1990;Supplementum 74:63–70.
Llanos-Paez C, Hennig S, Staatz C. Population pharmacokinetic modelling, Monte Carlo simulation and semi-mechanistic pharmacodynamic modelling as tools to personalize gentamicin therapy. J Antimicrob Chemother. 2017;72(3):639–67.
Kashuba ADM, Nafziger AN, Drusano GL, Bertino JS. Optimizing aminoglycoside therapy for nosocomial pneumonia caused by gram-negative bacteria. Antimicrob Agents Chemother. 1999;43(3):623–9.
Antibiotic Expert Group. Therapeutic Guidelines: Antibiotic. Version 15. Melbourne, VIC: Therapeutic Guidelines Ltd; 2014.
Bragonier R, Brown NM. The pharmacokinetics and toxicity of once-daily tobramycin therapy in children with cystic fibrosis. J Antimicrob Chemother. 1998;42(1):103–6.
Glass S, Plant ND, Spencer DA. The effects of intravenous tobramycin on renal tubular function in children with cystic fibrosis. J Cyst Fibros. 2005;4(4):221–5.
Rybak MJ, Abate BJ, Kang SL, Ruffing MJ, Lerner SA, Drusano GL. Prospective evaluation of the effect of an aminoglycoside dosing regimen on rates of observed nephrotoxicity and ototoxicity. Antimicrob Agents Chemother. 1999;43(7):1549–55.
Barras MA, Serisier D, Hennig S, Jess K, Norris RL. Bayesian estimation of tobramycin exposure in cystic fibrosis. Antimicrob Agents Chemother. 2016;60(11):6698–702.
Wong C, Kumar SS, Graham GG, Begg EJ, Chin PK, Brett J, et al. Comparing dose prediction software used to manage gentamicin dosing. Intern Med J. 2013;43(5):519–25.
Avent ML, Rogers BA, Cheng AC, Paterson DL. Current use of aminoglycosides: indications, pharmacokinetics and monitoring for toxicity. Intern Med J. 2011;41(6):441–9.
Hennig S, Holthouse F, Staatz CE. Comparing dosage adjustment methods for once-daily tobramycin in paediatric and adolescent patients with cystic fibrosis. Clin Pharmacokinet. 2015;54(4):409–21.
Avent ML, Teoh J, Lees J, Eckert KA, Kirkpatrick CM. Comparing 3 methods of monitoring gentamicin concentrations in patients with febrile neutropenia. Ther Drug Monit. 2011;33(5):592–601.
Paviour S, Hennig S, Staatz CE. Usage and monitoring of intravenous tobramycin in cystic fibrosis in Australia and the UK. J Pharm Pract Res. 2016;46(1):15–21.
Pai MP, Neely M, Rodvold KA, Lodise TP. Innovative approaches to optimizing the delivery of vancomycin in individual patients. Adv Drug Deliv Rev. 2014;77:50–7.
Begg E, Barclay M, Duffull S. A suggested approach to once-daily aminoglycoside dosing. Br J Clin Pharmacol. 1995;39(6):605–9.
Thomson AH, Whiting B. Bayesian parameter estimation and population pharmacokinetics. Clin Pharmacokinet. 1992;22(6):447–67.
Sheiner LB, Beal S, Rosenberg B, Marathe VV. Forecasting individual pharmacokinetics. Clin Pharmacol Ther. 1979;26(3):294–305.
Hennig S, Standing JF, Staatz CE, Thomson AH. Population pharmacokinetics of tobramycin in patients with and without cystic fibrosis. Clin Pharmacokinet. 2013;52(4):289–301.
Pottel H, Hoste L, Dubourg L, Ebert N, Schaeffner E, Eriksen BO, et al. An estimated glomerular filtration rate equation for the full age spectrum. Nephrol Dial Transplant. 2016;31(5):798–806.
Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41.
Sandroff BM, Schwartz CE, DeLuca J. Measurement and maintenance of reserve in multiple sclerosis. J Neurol. 2016;263(11):2158–69.
Wicha S, Kees M, Solms A, Minichmayr I, Kratzer A, Kloft C. TDMx: a novel web-based open-access support tool for optimising antimicrobial dosing regimens in clinical routine. Int J Antimicrob Agents. 2015;45:442–4.
Sheiner LB, Beal SL. Some suggestions for measuring predictive performance. J Pharmacokinet Biopharm. 1981;9(4):503–12.
Principles of aminoglycoside use. In: eTG complete. Therapeutic Guidelines Ltd (eTG March 2017 edition). 2014. https://tgldcdp-tg-org-au.ezproxy.library.uq.edu.au/viewTopic?topicfile=aminoglycoside-use-principles#toc_d1e1496. Accessed 23 May 2017.
Schentag JJ, Lasezkay G, Cumbo TJ, Plaut ME, Jusko WJ. Accumulation pharmacokinetics of tobramycin. Antimicrob Agents Chemother. 1978;13(4):649–56.
Bloomfield C, Staatz CE, Unwin S, Hennig S. Assessing predictive performance of published population pharmacokinetic models of intravenous tobramycin in pediatric patients. Antimicrob Agents Chemother. 2016;60(6):3407–14.
Nezic L, Derungs A, Bruggisser M, Tschudin-Sutter S, Krahenbuhl S, Haschke M. Therapeutic drug monitoring of once daily aminoglycoside dosing: comparison of two methods and investigation of the optimal blood sampling strategy. Eur J Clin Pharmacol. 2014;70(7):829–37.
Harun SN, Wainwright C, Klein K, Hennig S. A systematic review of studies examining the rate of lung function decline in patients with cystic fibrosis. Paediatr Respir Rev. 2016;20:55–66.
Darwich AS, Ogungbenro K, Vinks AA, Powell JR, Reny JL, Marsousi N, et al. Why has model-informed precision dosing not yet become common clinical reality? Lessons from the past and a roadmap for the future. Clin Pharmacol Ther. 2017;101(5):646–56.
Duffull SB, Kirkpatrick CMJ, Begg EJ. Comparison of two Bayesian approaches to dose-individualization for once-daily aminoglycoside regimens. Br J Clin Pharmacol. 1997;43(2):125–35.
Acknowledgements
The authors would like to thank Elouise Jendra for help with the data collection, and Prof. Dr. Sebastian G. Wicha (TDMx), Dr. Ron Keizer (InsightRX) and the team of DoseMe for their ongoing software support.
Author information
Authors and Affiliations
Contributions
MB collected and analysed the data, and drafted the manuscript; IS, SvH and SS supported data collection, and undertook manuscript review; and SH conceived the study, supported data analysis, and wrote the manuscript.
Corresponding author
Ethics declarations
Funding
No sources of funding were used in the preparation of this article.
Conflict of interest
Marc Burgard, Indy Sandaradura, Sebastiaan J. van Hal, Sonya Stacey and Stefanie Hennig have no conflicts of interest to declare.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study, formal consent is not required.
Electronic supplementary material
Below is the link to the electronic supplementary material.
40262_2017_610_MOESM1_ESM.tiff
Fig. S1 (a) Estimated daily area under the concentration-time curve (AUC0-24; n= 459) for 105 patients obtained using the LLR method (LLR, light green), TDMx (TDMx, light blue), InsightRX (IRX, orange) and DoseMe (DM, red). The black horizontal line represents the average target exposure for both hospitals of 100 mg∙h∙l-1. (b) Difference in predicted daily AUC0-24 obtained using TDMx (TDMx-LLR, light green), InsightRX (IRX-LLR, light blue) and DoseMe (DM-LLR, red) compared to the LLR method for the i th individual on the j th episode. The horizontal line represents a difference of zero between the predicted daily AUC0-24 given the LLR method and either of the BF programs. Three outliers have been excluded to increase readability. (c) Subsequent dose recommendation using LLR method (LLR, light green), TDMx (TDMx, light blue), InsightRX (IRX, orange) and DoseMe (DM, red). The black horizontal line represents current guideline initial dose recommendation of 10 mg/kg. (d) Relative difference in subsequent dose recommendation comparing the LLR method to TDMx (TDMx-LLR, light blue), InsightRX (IRX-LLR, orange) and DoseMe (DM-LLR, red) for a target drug exposure of 100 mg∙h∙l-1. The black horizontal line indicates no relative difference between the two methods compared. The boxes span the range between the first and third quartiles, with the median marked as a horizontal line. Whiskers represent the points within one-and-a-half interquartile ranges of the first and third quartile, which are represented at each end of the boxes. The individual dots represent outliers which lay more than one-and-a-half interquartile ranges outside of the interquartile range of the box plot. n total number of contributing concentration-time sets. (TIFF 2744 kb)
Rights and permissions
About this article
Cite this article
Burgard, M., Sandaradura, I., van Hal, S.J. et al. Evaluation of Tobramycin Exposure Predictions in Three Bayesian Forecasting Programmes Compared with Current Clinical Practice in Children and Adults with Cystic Fibrosis. Clin Pharmacokinet 57, 1017–1027 (2018). https://doi.org/10.1007/s40262-017-0610-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-017-0610-9