Abstract
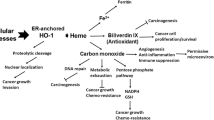
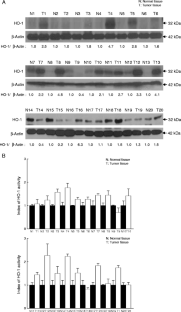
Heme oxygenase-1 (HO-1) catabolizes heme into carbon monoxide, biliverdin, and free iron which mediate its protective effect against oxidative stress. The aim of the present study was to determine the expression level and activity of HO-1 in Korean colon cancer tissues and cell lines. HO-1 protein expression was higher (>1.5-fold) in tumor tissues than in adjacent normal tissues in 14 of 20 colon cancer patients, and HO-1 protein expression was closely correlated with HO-1 enzyme activity in cancer tissues. Immunohistochemical data confirmed that HO-1 protein was expressed at a higher level in colon cancer tissues than in normal mucosa. Furthermore, HO-1 mRNA and protein expression and enzyme activity were higher in the colon cancer cell lines Caco-2, SNU-407, SNU-1033, HT-29, and SW-403 than in the normal fetal human colon cell line FHC. Treatment with the HO-1 inhibitor zinc protoporphyrin decreased the viability of colon cancer cell lines. These data indicate that HO-1 may serve as a clinically useful biomarker of colon cancer and as a target for anticolon cancer drugs.



Similar content being viewed by others
References
Ministry of Health and Welfare of Korea. Reports of cancer incidence 07-27. Available from: http://www.mohw.go.kr/in-dex.jsp.
Li M, Gu J. Changing patterns of colorectal cancer in China over a period of 20 years. World J Gastoenterol. 2005;11:4685–8.
Matos E, Brandani A. Review on meat consumption and cancer in South America. Mutat Res. 2002;506–507:243–9.
Nkondjock A, Ghadirian P. Associated nutritional risk of breast and colon cancers: a population-based case control study in Montreal, Canada. Cancer Lett. 2005;223:85–91.
Glei M, Latunde-Dada GO, Klinder A, Becker TW, Hermann U, Voigt K, et al. Iron-overload induces oxidative DNA damage in the human colon carcinoma cell line HT29 clone 19A. Mutat Res. 2002;519:151–61.
Sanders LM, Henderson CE, Hong MY, Barhoumi R, Burghardt RC, Carroll RJ, et al. Pro-oxidant environment of the colon compared to the small intestine may contribute to greater cancer susceptibility. Cancer Lett. 2004;208:155–61.
Ryter SW, Choi AM. Heme oxygenase-1: molecular mechanisms of gene expression in oxygen-related stress. Antioxid Redox Signal. 2002;4:625–32.
Applegate LA, Luscher P, Tyrrell RM. Induction of heme oxygenase: a general response to oxidant stress in cultured mammalian cells. Cancer Res. 1991;51:974–8.
Rizzardini M, Carelli M, Cabello Porras MR, Cantoni L. Mechanisms of endotoxin-induced haem oxygenase mRNA accumulation in mouse liver: synergism by glutathione depletion and protection by N-acetylcysteine. Biochem J. 1994;304:477–83.
Rossi A, Santoro MG. Induction by prostaglandin A1 of haem oxygenase in myoblastic cells: an effect independent of expression of the 70 kDa heat shock protein. Biochem J. 1995;308:455–63.
Choi AM, Alam J. Heme oxygenase-1: function, regulation, and implication of a novel stress-inducible protein in oxidant-induced lung injury. Am J Respir Cell Mol Biol. 1996;15:9–19.
Cisowski J, Loboda A, Józkowicz A, Chen S, Agarwal A, Dulak J. Role of heme oxygenase-1 in hydrogen peroxide-induced VEGF synthesis: effect of HO-1 knockout. Biochem Biophys Res Commun. 2005;326:670–6.
Was H, Dulak J, Jozkowicz A. Heme oxygenase-1 in tumor biology and therapy. Curr Drug Targets Rev. 2010;11:1551–70.
Lee J, Lee SK, Lee BU, Lee HJ, Cho NP, Yoon JH, et al. Upregulation of heme oxygenase-1 in oral epithelial dysplasias. Int J Oral Maxillofac Surg. 2008;37:287–92.
Nuhn P, Künzli BM, Hennig R, Mitkus T, Ramanauskas T, Nobiling R, et al. Heme oxygenase-1 and its metabolites affect pancreatic tumor growth in vivo. Mol Cancer. 2009;8:37.
Sass G, Leukel P, Schmitz V, Raskopf E, Ocker M, Neureiter D, et al. Inhibition of heme oxygenase 1 expression by small interfering RNA decreases orthotopic tumor growth in livers of mice. Int J Cancer. 2008;123:1269–77.
Becker JC, Fukui H, Imai Y, Sekikawa A, Kimura T, Yamagishi H, et al. Colonic expression of heme oxygenase-1 is associated with a better long-term survival in patients with colorectal cancer. Scand J Gastroenterol. 2007;42:852–8.
Fang J, Akaike T, Maeda H. Antiapoptotic role of heme oxygenase (HO) and the potential of HO as a target in anticancer treatment. Apoptosis. 2004;9:27–35.
Tanaka S, Akaike T, Fang J, Beppu T, Ogawa M, Tamura F, et al. Antiapoptotic effect of haem oxygenase-1 induced by nitric oxide in experimental solid tumour. Br J Cancer. 2003;88:902–9.
Alaoui-Jamali MA, Bismar TA, Gupta A, Szarek WA, Su J, Song W, et al. A novel experimental heme oxygenase-1-targeted therapy for hormone-refractory prostate cancer. Cancer Res. 2009;69:8017–24.
Clark JE, Green CJ, Motterlini R. Involvement of the human oxygenase-carbon monoxide pathway in keratinocyte proliferation. Biochem Biophys Res Commun. 1997;241:215–20.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–8.
Carmichael J, DeGraff WG, Gazdar AF, Minna JD, Mitchell JB. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res. 1987;47:936–41.
Liu ZM, Chen GG, Ng EK, Leung WK, Sung JJ, Chung SC. Upregulation of heme oxygenase-1 and p21 confers resistance to apoptosis in human gastric cancer cells. Oncogene. 2004;23:503–13.
Sasaki T, Yoshida K, Kondo H, Ohmori H, Kuniyasu H. Heme oxygenase-1 accelerates protumoral effects of nitric oxide in cancer cells. Virchows Arch. 2005;446:525–31.
Jozkowicz A, Was H, Dulak J. Heme oxygenase-1 in tumor: is it a false friend? Antioxid Redox Signal. 2007;9:2099–118.
Fang J, Sawa T, Akaike T, Akuta T, Sahoo SK, Khaled G, et al. In vivo antitumor activity of pegylated zinc protoporphyrin: targeted inhibition of heme oxygenase in solid tumor. Cancer Res. 2003;63:3567–74.
Fang J, Sawa T, Akaike T, Greish K, Maeda H. Enhancement of chemotherapeutic response of tumor cells by a heme oxygenase inhibitor, pegylated zinc protoporphyrin. Int J Cancer. 2004;109:1–8.
Alcaraz MJ, Fernandez P, Guillen MI. Antiinflammatory actions of the heme oxygenase-1 pathway. Curr Pharm Des. 2003;9:2541–51.
Mayerhofer M, Florian S, Krauth MT, Aichberger KJ, Bilban M, Marculescu R, et al. Identification of heme oxygenase-1 as a novel BCR/ABL-dependent survival factor in chronic myeloid leukemia. Cancer Res. 2004;64:3148–54.
Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, et al. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86.
Hayes JD, McMahon M. NRF2 and KEAP1 mutations: permanent activation of an adaptive response in cancer. Trends Biochem Sci. 2009;34:176–88.
DeNicola GM, Karreth FA, Humpton TJ, Gopinathan A, Wei C, Frese K, et al. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature. 2011;475:106–9.
Kim KC, Kang KA, Zhang R, Piao MJ, Kim GY, Kang MY, et al. Up-regulation of Nrf2-mediated heme oxygenase-1 expression by eckol, a phlorotannin compound, through activation of Erk and PI3K/Akt. Int J Biochem Cell Biol. 2010;42:297–305.
Imai M, Kikuchi H, Denda T, Ohyama K, Hirobe C, Toyoda H. Cytotoxic effects of flavonoids against a human colon cancer derived cell line, COLO 201: a potential natural anti-cancer substance. Cancer Lett. 2009;276:74–80.
Acknowledgment
This study was supported by a grant from the National R&D Program for Cancer Control, Ministry of Health and Welfare, Republic of Korea (1120340).
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kang, K.A., Maeng, Y.H., Zhang, R. et al. Involvement of heme oxygenase-1 in Korean colon cancer. Tumor Biol. 33, 1031–1038 (2012). https://doi.org/10.1007/s13277-012-0336-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-012-0336-0