Key Points
Question
What symptoms are differentially present in SARS-CoV-2–infected individuals 6 months or more after infection compared with uninfected individuals, and what symptom-based criteria can be used to identify postacute sequelae of SARS-CoV-2 infection (PASC) cases?
Findings
In this analysis of data from 9764 participants in the RECOVER adult cohort, a prospective longitudinal cohort study, 37 symptoms across multiple pathophysiological domains were identified as present more often in SARS-CoV-2–infected participants at 6 months or more after infection compared with uninfected participants. A preliminary rule for identifying PASC was derived based on a composite symptom score.
Meaning
A framework for identifying PASC cases based on symptoms is a first step to defining PASC as a new condition. These findings require iterative refinement that further incorporates clinical features to arrive at actionable definitions of PASC.
Abstract
Importance
SARS-CoV-2 infection is associated with persistent, relapsing, or new symptoms or other health effects occurring after acute infection, termed postacute sequelae of SARS-CoV-2 infection (PASC), also known as long COVID. Characterizing PASC requires analysis of prospectively and uniformly collected data from diverse uninfected and infected individuals.
Objective
To develop a definition of PASC using self-reported symptoms and describe PASC frequencies across cohorts, vaccination status, and number of infections.
Design, Setting, and Participants
Prospective observational cohort study of adults with and without SARS-CoV-2 infection at 85 enrolling sites (hospitals, health centers, community organizations) located in 33 states plus Washington, DC, and Puerto Rico. Participants who were enrolled in the RECOVER adult cohort before April 10, 2023, completed a symptom survey 6 months or more after acute symptom onset or test date. Selection included population-based, volunteer, and convenience sampling.
Exposure
SARS-CoV-2 infection.
Main Outcomes and Measures
PASC and 44 participant-reported symptoms (with severity thresholds).
Results
A total of 9764 participants (89% SARS-CoV-2 infected; 71% female; 16% Hispanic/Latino; 15% non-Hispanic Black; median age, 47 years [IQR, 35-60]) met selection criteria. Adjusted odds ratios were 1.5 or greater (infected vs uninfected participants) for 37 symptoms. Symptoms contributing to PASC score included postexertional malaise, fatigue, brain fog, dizziness, gastrointestinal symptoms, palpitations, changes in sexual desire or capacity, loss of or change in smell or taste, thirst, chronic cough, chest pain, and abnormal movements. Among 2231 participants first infected on or after December 1, 2021, and enrolled within 30 days of infection, 224 (10% [95% CI, 8.8%-11%]) were PASC positive at 6 months.
Conclusions and Relevance
A definition of PASC was developed based on symptoms in a prospective cohort study. As a first step to providing a framework for other investigations, iterative refinement that further incorporates other clinical features is needed to support actionable definitions of PASC.
This study aims to develop a definition of postacute sequelae of SARS-CoV-2 infection (PASC) based on self-reported symptoms and describe PASC frequencies across cohorts, vaccination status, and number of infections using a cohort of adults with and without SARS-CoV-2 infection.
Introduction
More than 658 million people worldwide have been infected with SARS-CoV-2.1 Postacute sequelae of SARS-CoV-2 infection (PASC), also known as long COVID and defined as ongoing, relapsing, or new symptoms or conditions present 30 or more days after infection, is a major clinical and public health concern.2,3,4,5,6 Short- and long-term effects of PASC have substantial impacts on health-related quality of life, earnings, and health care costs.7,8 Most existing PASC studies have focused on individual symptom frequency and have generated widely divergent estimates of prevalence due to their retrospective design and lack of an uninfected comparison group. Moreover, defining PASC precisely is difficult because it is heterogeneous, composed of conditions with variable and potentially overlapping etiologies (eg, organ injury, viral persistence, immune dysregulation, autoimmunity, and gut dysbiosis).9,10
It is of significant public health and scientific importance to better research the underlying mechanisms of PASC and potential preventive and therapeutic interventions. This effort requires data collection on SARS-CoV-2–infected and –uninfected individuals in a large prospective cohort study designed specifically to characterize PASC. Additionally, simultaneous consideration of multiple symptoms that persist over time and application of appropriate analytical techniques are essential. Further consideration of changes in PASC frequency and its manifestations over the course of the COVID-19 pandemic, due to variable SARS-CoV-2 strains, new treatment and prevention strategies, and repeat infections, is important.
This study is part of the National Institutes of Health’s Researching COVID to Enhance Recovery (RECOVER) Initiative, which seeks to understand, treat, and prevent PASC (https://recovercovid.org/). In this first analysis of data from the RECOVER adult cohort, criteria for identifying PASC based on self-reported symptoms are delineated and several distinctive PASC subphenotypes with varying impacts on well-being and physical health are described. This study was enriched with self-referred participants to promote inclusive participation. Estimates were expected to be more accurate in the subcohort of participants enrolled within 30 days of acute infection, for whom selection bias based on PASC would be minimal.
Unlike electronic health records and most existing cohort studies, data from this study captured PASC-specific self-reported symptoms based on standardized questionnaires developed with input from patient representatives. This report is an adequately powered, prospective study of PASC based on participant-reported symptoms that included both infected and uninfected individuals over the course of the pandemic. Notably, unlike prior reports, the paradigm presented here does not rely on predefined clinical symptoms; instead, a definition of PASC as a new condition specific to SARS-CoV-2 infection is proposed.
Methods
Institutional review boards at NYU Grossman School of Medicine, serving as a single institutional review board, and other participating institutions reviewed and approved the protocol. All participants provided written informed consent to participate in research.
Study Design
The RECOVER adult cohort study included SARS-CoV-2–infected and –uninfected participants (the trial protocol is in Supplement 1 and the statistical analysis plan in Supplement 2). All infected participants met World Health Organization suspected, probable, or confirmed criteria.11 Index for infected participants was defined as date of first positive SARS-CoV-2 test result or COVID-19 symptom onset. Uninfected participants had no known history of SARS-CoV-2 infection and index was defined as a past negative SARS-CoV-2 test result date. Participants belonged to either the acute cohort (enrolled ≤30 days since index) or the postacute cohort (enrolled >30 days to 3 years after index). Participants were recruited from 85 sites across the United States and completed office visits and remote surveys developed with early engagement of patients, support group stakeholders, and multidisciplinary clinical experts.12
Participants
Adult participants enrolled prior to April 10, 2023 (N = 13 754) were considered (Figure 1). Enrollment is ongoing, and not all enrolled participants have reached eligibility for inclusion. The analysis cohort included participants with a study visit completed 6 months or more after the index date (Table 1). Uninfected participants with a reported on-study infection and participants who had no symptom survey data were excluded. A subgroup of participants also belonged to the RECOVER pregnancy cohort. Race and ethnicity were captured via participant self-report using fixed categories to better understand racial and ethnic differences in sequelae due to SARS-CoV-2 infection (eMethods in Supplement 3).
Figure 1. RECOVER Adult Analysis Cohort.
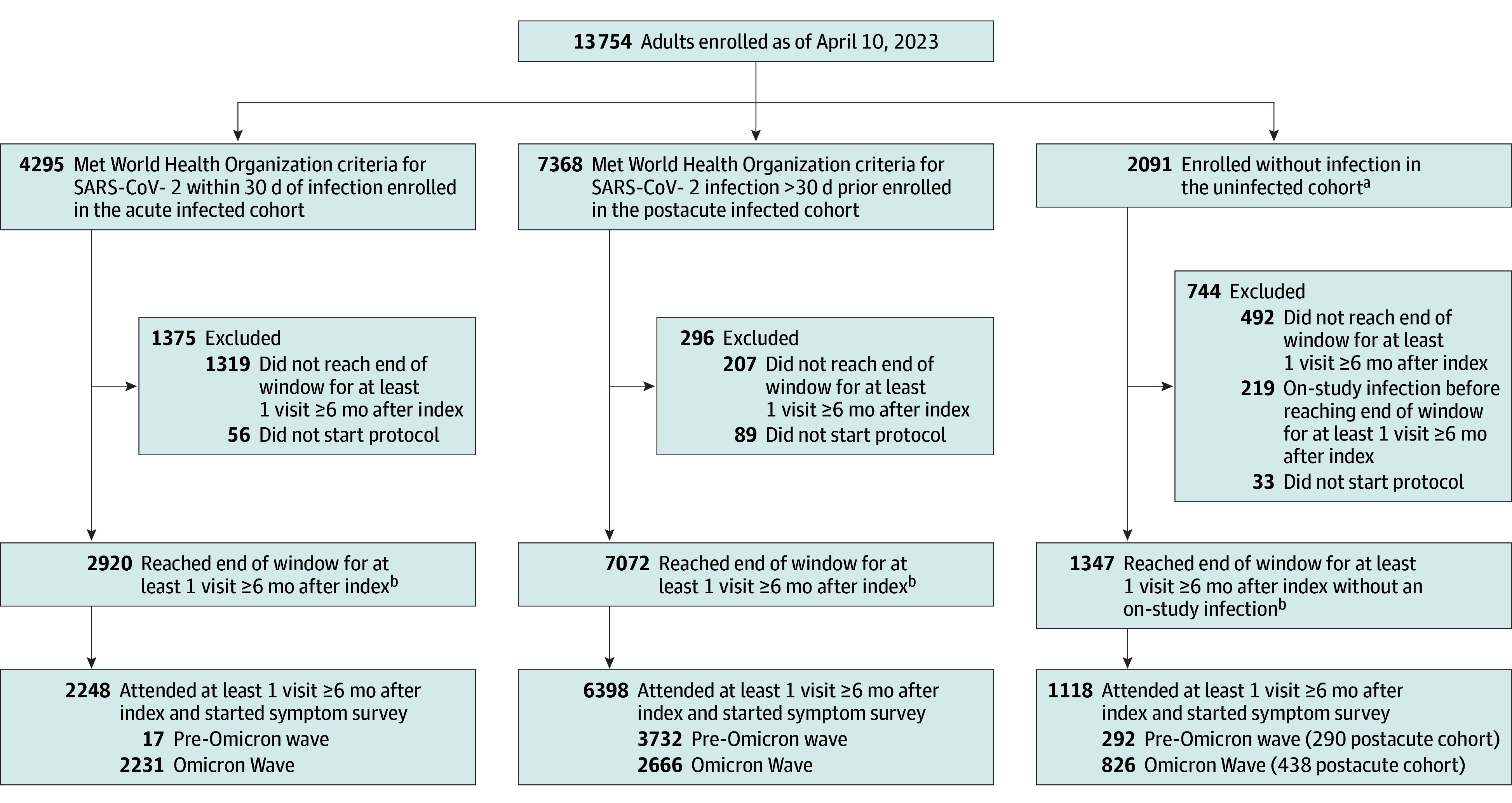
aUninfected included acute cohort (n = 1092) and postacute cohort (n = 999) participants. Uninfected participants had no known history of SARS-CoV-2 infection. Acute uninfected participants were enrolled within 30 days of a SARS-CoV-2 negative test result, while postacute uninfected participants were enrolled more than 30 days after a SARS-CoV-2 negative test result.
bParticipants who completed visit without reaching end of visit window were included in this count.
Table 1. RECOVER Adult Cohort Demographic Characteristics by Infection Status at Enrollment.
| Characteristica | No./total (%) | ||
|---|---|---|---|
| Infected (n = 8646) | Uninfected (n = 1118) | Uninfected with balancing weights (n = 1109), % | |
| Age at enrollment, y | |||
| Median (IQR) | 45 (34-59) | 55 (40-65) | 45 (35-60) |
| No. | 8637 | 1117 | 1109 |
| Age category at enrollment, y | |||
| 18-45 | 4389/8637 (51) | 377/1117 (34) | 51 |
| 46-65 | 3175/8637 (37) | 502/1117 (45) | 37 |
| >65 | 1073/8637 (12) | 238/1117 (21) | 12 |
| Race and ethnicityb | |||
| Asian, non-Hispanic | 428/8558 (5) | 73/1106 (7) | 5 |
| Black or African American, non-Hispanic | 1220/8558 (14) | 197/1106 (18) | 14 |
| Hispanic, Latino, or Spanish | 1473/8558 (17) | 119/1106 (11) | 17 |
| White, non-Hispanic | 5027/8558 (59) | 685/1106 (62) | 59 |
| Multiracial/multiethnic | 305/8558 (4) | 26/1106 (2) | 4 |
| Other | 105/8558 (1) | 6/1106 (1) | 1 |
| Sex assigned at birth | |||
| Female | 6221/8602 (72) | 711/1110 (64) | 72 |
| Male | 2377/8602 (28) | 399/1110 (36) | 28 |
| Intersex | 4/8602 (<1) | 0/1110 | 0 |
| Vaccination status at index datec | |||
| Unvaccinated | 3291/8538 (39) | 161/1095 (15) | 16 |
| Partially vaccinated | 154/8538 (2) | 21/1095 (2) | 2 |
| Fully vaccinated | 4725/8538 (55) | 860/1095 (79) | 77 |
| Date of last dose unknown | 368/8538 (4) | 53/1095 (5) | 5 |
| Cohort and prevalent SARS-CoV-2 strain at Indexd | |||
| Acute pre-Omicron | 17/8646 (<1) | 2/1118 (<1) | <1 |
| Acute Omicron | 2231/8646 (26) | 388/1118 (35) | 33 |
| Postacute pre-Omicron | 3732/8646 (43) | 290/1118 (26) | 28 |
| Postacute Omicron | 2666/8646 (31) | 438/1118 (39) | 39 |
| Medically underserved area | |||
| Yes | 2369/8646 (27) | 298/1118 (27) | 28 |
| No | 6277/8646 (73) | 820/1118 (73) | 72 |
| Rural participant | |||
| Yes | 465/8646 (5) | 45/1118 (4) | 4 |
| No | 8181/8646 (95) | 1073/1118 (96) | 96 |
| Education level | |||
| Did not complete high school/no diploma | 280/8564 (3) | 44/1107 (4) | 3 |
| High school/GED/some college/vocational/technical | 2790/8564 (33) | 306/1107 (28) | 26 |
| Bachelor’s/advanced degree | 5494/8564 (64) | 757/1107 (68) | 70 |
Additional characteristics were summarized in eTable 5 in Supplement 3. Summaries by subcohort were provided in eTable 6 in Supplement 3.
Race and ethnicity were captured via participant self-report using fixed categories to better understand racial and ethnic differences in sequelae due to SARS-CoV-2 infection (eMethods in Supplement 3).
Details on how vaccination status was defined were provided in eTable 4 in Supplement 3.
The pre-Omicron prevalent strain was defined as prior to December 1, 2021.
Exposures and Outcomes
The analysis used the first study visit at 6 months or more after the index date. The exposure was SARS-CoV-2 infection prior to study enrollment. Uninfected participants with antibody results at enrollment indicating prior infection were reclassified as infected and assigned an index date 90 days prior. The primary outcome was the presence of each of 44 symptoms (eTable 1 in Supplement 3). Using these symptoms, a PASC definition was developed. The primary analysis used symptom presence for inclusivity; sensitivity analysis considered new-onset symptoms. Results were reported for 3 additional age- and sex-dependent symptoms (eTable 1 in Supplement 3).
Statistical Analysis
Results were reported overall and within 3 subcohorts: acute Omicron (n = 2231 infected, n = 388 uninfected; index date on or after December 1, 2021); postacute pre-Omicron (n = 3732 infected, n = 290 uninfected; index date before December 1, 2021); and postacute Omicron (n = 2666 infected, n = 438 uninfected; index date on or after December 1, 2021). Acute cohort participants with a pre-Omicron index date (17 infected, 2 uninfected) were included in overall analyses.
Balancing weights were used to account for differences in the age, sex, and race and ethnicity distributions between infected and uninfected participants (eMethods in Supplement 3). Symptom frequency was defined as the proportion reporting a symptom and exceeding corresponding moderate to severe symptom severity threshold (eTables 1 and 2 in Supplement 3). Symptoms with frequency of 2.5% or greater were considered. Symptom frequencies by infection status were reported and adjusted odds ratios (aORs) were calculated using weighted logistic regression. In sensitivity analysis, new-onset symptom frequency was defined as the proportion of participants with the symptom at study visit among those without the symptom in the year prior to the index date. Symptom frequencies characterized the study cohort and were not unbiased estimators of population-level prevalence due to the cohort sampling strategies. Symptom frequency estimates within the acute Omicron subcohort were expected to be more aligned with the corresponding population frequencies.
A rule for identifying PASC was derived. Symptoms differentiating infected and uninfected participants were identified using least absolute shrinkage and selection operator (LASSO) with balancing weights.13 Each symptom was assigned a score based on the estimated coefficients and participants were assigned a total score by summing the symptom scores for each reported symptom. Using 10-fold cross-validation, an optimal score threshold for PASC was selected (eMethods in Supplement 3). Participants meeting the PASC score threshold were classified as PASC positive; others were classified as PASC unspecified. The proportions were reported.
Participants classified as PASC positive were clustered into subgroups using unsupervised learning (K-means consensus clustering14 followed by hierarchical clustering15) including symptoms identified with LASSO. Symptoms highly correlated with those identified by LASSO were reported. The distribution of PASC score and Patient-Reported Outcomes Measurement Information System (PROMIS) Global Health 10 general quality of life (Q2), general physical health (Q3), and ability to carry out everyday physical activities (Q6) (eTable 3 in Supplement 3) were reported.
Rates of PASC were assessed by infection status, sex, age, and vaccination status at the index date (eTable 4 in Supplement 3), reinfection (between index and analysis visit), and visit month. The proportion of participants meeting criteria for myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS, defined based on RECOVER survey; eMethods in Supplement 3) who were PASC positive at the same visit was reported. Sensitivity analyses removed the symptom severity thresholds and separately added well-being and physical health requirements for PASC (Q2 or Q3: fair or poor; Q6: moderate or worse). Inverse probability weighting was applied to account for loss to follow-up in the acute Omicron subcohort (eMethods in Supplement 3).
Results
Participants
A total of 9764 participants (8646 infected; 1118 uninfected) met study criteria (Figure 1, 71% female [6932/9712]; 16% Hispanic/Latino [1592/9664]; 15% non-Hispanic Black [1417/9664]; 58% fully vaccinated at the index date [5585/9633]; median age, 47 years [IQR, 35-60]). After application of balancing weights, the distributions of age, sex, and race and ethnicity were the same in infected and uninfected participants (Table 1). In the weighted cohort, uninfected participants were more likely to be fully vaccinated (77% vs 55%). Comorbidity frequencies were similar between infected and uninfected participants (eTable 5 in Supplement 3). Uninfected participants were more likely to be self-referrals or recruited via community outreach (eTable 5 in Supplement 3). A total of 1260 of 6932 female participants (18%) were in the pregnancy cohort.
Symptom Frequency
In the full cohort, 37 symptoms had frequency of 2.5% or greater and aORs were 1.5 or greater (infected vs uninfected participants) for all 37 (eFigure 1 in Supplement 3). Symptoms (using severity thresholds) with more than 15% absolute difference in frequencies (infected vs uninfected) included postexertional malaise (PEM) (28% vs 7%; aOR, 5.2 [95% CI, 3.9-6.8]), fatigue (38% vs 17%; aOR, 2.9 [95% CI, 2.4-3.4]), dizziness (23% vs 7%; aOR, 3.4 [95% CI, 2.6-4.4]), brain fog (20% vs 4%; aOR, 4.5 [95% CI, 3.2-6.2]), and gastrointestinal (GI) symptoms (25% vs 10%; aOR, 2.7 [95% CI, 2.2-3.4]).
Symptom-Level Sensitivity Analyses
In infected participants, the frequencies of new-onset symptoms (with severity thresholds) were similar, including PEM (28%), fatigue (37%), dizziness (21%), brain fog (20%), and GI symptoms (20%) (eFigure 2 in Supplement 3). The corresponding observed symptom frequencies without severity thresholds were higher (eg, fatigue, 47%; brain fog, 40%) (eFigure 3 in Supplement 3).
Symptom-Level Analysis by Subcohort
The distributions of demographics and comorbidities were comparable across the acute Omicron, postacute pre-Omicron, and postacute Omicron subcohorts, though there was a higher proportion unvaccinated in the postacute pre-Omicron subcohort (eTable 6 in Supplement 3). Time from the index date to analysis visit ranged from 6 to 15 months in the acute Omicron and postacute Omicron subcohorts and 6 to 39 months in the postacute pre-Omicron subcohort (eFigure 4 in Supplement 3). Generally, symptom frequencies and the differences between infected and uninfected participants were lower in the acute Omicron subcohort, higher in the postacute Omicron subcohort, and highest in the postacute pre-Omicron subcohort (eFigures 5-7 in Supplement 3). Symptom frequencies in acute Omicron participants who were also fully vaccinated were the lowest (eFigure 8 in Supplement 3).
PASC Score
Using the full cohort, LASSO identified 12 symptoms with corresponding scores ranging from 1 to 8 (Table 2). The optimal PASC score threshold used was 12 or greater (Figure 2A). The symptoms (ordered by decreasing frequencies among participants with a qualifying PASC score) were PEM, fatigue, brain fog, dizziness, GI symptoms, palpitations, changes in sexual desire or capacity, loss of or change in smell or taste, thirst, chronic cough, chest pain, and abnormal movements. Symptoms correlated with the selected symptoms included dry mouth, weakness, headaches, tremor, muscle and abdominal pain, fever/sweats/chills, and sleep disturbance (eTable 7 in Supplement 3).
Table 2. Model-Selected Symptoms That Define PASC and Their Corresponding Scoresa.
| Symptom | Log odds ratio | Score |
|---|---|---|
| Smell/taste | 0.776 | 8 |
| Postexertional malaise | 0.674 | 7 |
| Chronic cough | 0.438 | 4 |
| Brain fogb | 0.325 | 3 |
| Thirst | 0.255 | 3 |
| Palpitations | 0.238 | 2 |
| Chest painb | 0.233 | 2 |
| Fatigueb | 0.148 | 1 |
| Sexual desire or capacity | 0.126 | 1 |
| Dizzines | 0.121 | 1 |
| Gastrointestinal | 0.085 | 1 |
| Abnormal movements | 0.072 | 1 |
| Hair loss | 0.049 | 0 |
Abbreviation: PASC, postacute sequelae of SARS-CoV-2 infection.
Least absolute shrinkage and selection operator was used to identify which symptoms defined PASC. A symptom score was assigned by dividing the estimated log odds ratio by 0.10 and rounding to the nearest integer. For each person, the total score was defined as the sum of the scores for each symptom a person reported.
Additional severity criteria required (eTables 1 and 2 in Supplement 3).
Figure 2. Defining the Postacute Sequelae of SARS-CoV-2 Infection (PASC) Score and a Decision Rule.
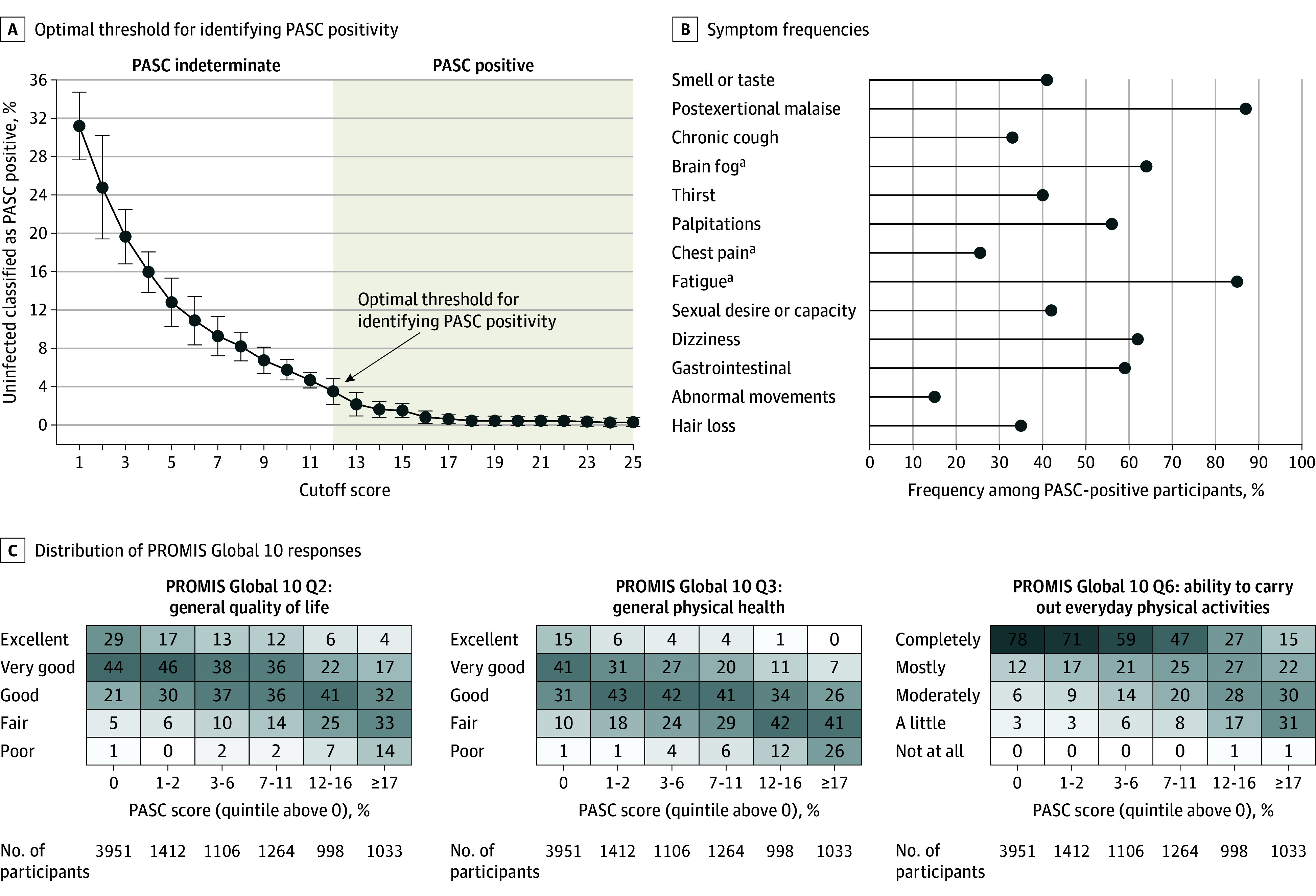
A, Optimal score cutoff for classifying a participant as PASC positive using cross-validation (eMethods in Supplement 3). The decision rule based on symptoms is intended to identify participants with PASC. PASC status for participants not meeting the score threshold requires consideration of additional data inputs.
B, Symptom frequencies among PASC-positive participants for symptoms that contribute to the PASC score. Many other symptoms have high frequency in PASC-positive participants (eTable 8 in Supplement 3).
C, Distribution of Patient-Reported Outcomes Measurement Information System (PROMIS) Global 10 responses among participants with a zero PASC score and among participants within nonzero PASC score quintiles. The PROMIS Global 10 provides an assessment of quality of life along 10 dimensions, each rated on a 5-point scale. The shading corresponds to frequency within each column on a scale from 0% to 100%.
aAdditional severity criteria required (eTables 1 and 2 in Supplement 3).
The proportion with a qualifying PASC score in the full cohort (subject to selection bias) was 1990 of 8646 infected participants (23%) and 41 of 1118 of uninfected participants (3.7%) (overall: 2031/9764 [21%]). Among participants with PASC, the most common symptoms were PEM (87%), fatigue (85%), brain fog (64%), dizziness (62%), GI (59%), and palpitations (57%) (Figure 2B; eTable 8 in Supplement 3). Higher PASC scores were associated with worse PROMIS Global 10 scores (Figure 2C).
PASC in Subcohorts
The proportion of infected participants with PASC in the acute Omicron subcohort was 10% (95% CI, 8.8%-11%; 224/2231). After adjustment for missing data, the estimated rate was 9.8% (95% CI, 8.6%-11%). It was greater in the postacute pre-Omicron (1320/3732 [35%; 95% CI, 34%-37%]) and postacute Omicron (442/2666 [17%; 95% CI, 15%-18%]) subcohorts (Table 3). Symptom frequencies among PASC-infected participants were similar across subcohorts, with a few notable exceptions, including brain fog, GI symptoms, and palpitations (eTable 8 in Supplement 3). The proportion of PASC positivity was lower among fully vaccinated than unvaccinated participants (acute Omicron: 9.7% vs 17%; postacute pre-Omicron: 31% vs 37%; postacute Omicron: 16% vs 22%) (Table 3). In the Omicron cohorts, the estimated proportion of PASC positivity was greater among reinfected participants compared with participants with 1 reported infection (acute Omicron: 20% vs 9.7%; postacute Omicron: 21% vs 16%) (Table 3).
Table 3. PASC Frequencies, Overall and Stratified by Subcohort, Infection, Vaccination, and Reinfection.
| Total No. | PASC positive, No. (%) | |
|---|---|---|
| PASC frequencies overall and stratified by subcohort and infection a | ||
| All participants (full cohort)a | 9764 | 2031 (21) |
| Infected | 8646 | 1990 (23) |
| Uninfected | 1118 | 41 (3.7) |
| Acute Omicron | ||
| Infected | 2231 | 224 (10) |
| Uninfected | 388 | 18 (4.6) |
| Postacute pre-Omicron | ||
| Infected | 3732 | 1320 (35) |
| Uninfected | 290 | 11 (3.8) |
| Postacute Omicron | ||
| Infected | 2666 | 442 (17) |
| Uninfected | 438 | 12 (2.7) |
| PASC frequencies stratified by study cohort, prevalent SARS-CoV-2 strain, and vaccination (infected participants only) b | ||
| Acute Omicron | ||
| Vaccinated | 2016 | 195 (9.7) |
| Not vaccinated | 86 | 15 (17) |
| Postacute pre-Omicron | ||
| Vaccinated | 491 | 154 (31) |
| Not vaccinated | 2967 | 1090 (37) |
| Postacute Omicron | ||
| Vaccinated | 2208 | 356 (16) |
| Not vaccinated | 232 | 50 (22) |
| PASC frequencies stratified by study cohort, prevalent SARS-CoV-2 strain, and reinfection (infected participants only) | ||
| Acute Omicron | ||
| 1 infection | 2150 | 208 (9.7) |
| >1 infections | 81 | 16 (20) |
| Postacute pre-Omicron | ||
| 1 infection | 2484 | 907 (37) |
| >1 infections | 1248 | 413 (33) |
| Postacute Omicron | ||
| 1 infection | 2200 | 345 (16) |
| >1 infections | 466 | 97 (21) |
Abbreviation: PASC, postacute sequelae of SARS-CoV-2 infection.
Acute cohort participants with a pre-Omicron index date were included in the full cohort analysis.
Participants who were partially vaccinated, missing vaccination status, or missing date of last dose (eTable 4 in Supplement 3) were excluded.
PASC-Stratified Analysis in the Full Cohort
Among infected participants in the full cohort, the proportions of PASC positivity were 39% (299/757) among hospitalized participants and 22% (1636/7387) among not hospitalized participants during acute infection; 19% (442/2377) among males and 25% (1540/6221) among females; and 20% (885/4389) among those aged 18 to 45 years and 28% (904/3175) (28%) among those aged 46 to 65 years. In cross-sectional analysis, the proportion of PASC-positive participants was consistent over the visit month used in analysis (eTable 9 in Supplement 3). In subgroups with repeated visits at 6 and 9 months after the index date, PASC positivity varied over study visit, though 68% of PASC-positive participants remained positive at the subsequent visit (eTable 10 in Supplement 3). Among infected participants meeting criteria for ME/CFS, 98% met the criteria for PASC.
PASC Sensitivity Analyses in the Full Cohort
The proportions of PASC increased to 27% (2300/8646) among infected participants and 4.7% (52/1118) among uninfected participants when severity scores were not included in the PASC score determination. After applying additional PROMIS Global 10 criteria to qualify as PASC positive, 17% (1434/8646) were PASC positive among infected participants and 3.0% (34/1118) among uninfected participants.
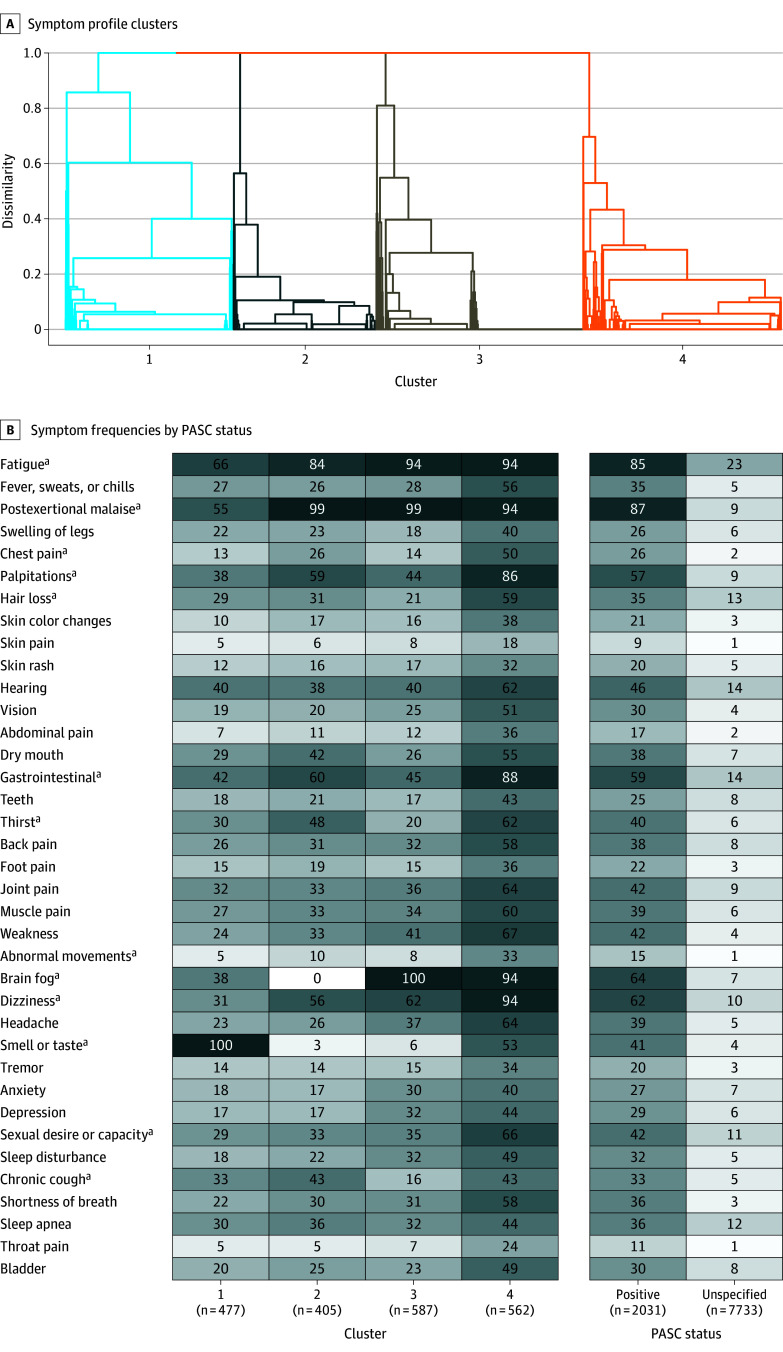
PASC Subgroups
Four PASC subgroups were identified (Figure 3A). Features of PASC subgroups included loss of or change in smell or taste (100%) in cluster 1 (n = 477); PEM (99%) and fatigue (84%) in cluster 2 (n = 405); brain fog (100%), PEM (99%), and fatigue (94%) in cluster 3 (n = 587); and fatigue (94%), PEM (94%), dizziness (94%), brain fog (94%), GI (88%), and palpitations (86%) in cluster 4 (n = 562) (Figure 3B). Twenty-six percent of the PASC-unspecified group also met PROMIS Global 10 criteria compared with 53% of participants in cluster 1, 69% in cluster 2, 77% in cluster 3, and 86% in cluster 4. Among infected participants, 456 of 1540 females (30%) and 102 of 442 males (23%) with PASC were in cluster 4. A total of 277 of 885 participants aged 18 to 45 years (31%), 254 of 904 participants aged 46 to 65 years (28%), and 29 of 198 participants aged older than 65 years (15%) with PASC were in cluster 4.
Figure 3. Identification of Postacute Sequelae of SARS-CoV-2 Infection (PASC) Subgroups and Their Characteristics.
A, Dendrogram illustrating how PASC-positive participants with similar symptom profiles cluster. Each branch in the dendrogram represents a participant, and each cluster represents a subgroup of participants.
B. Heatmap of symptom frequencies within PASC unspecified and within each PASC-positive subgroup. The shading corresponds to frequency within each column on a scale from 0% to 100%.
aAlthough unsupervised learning uses 12 symptoms selected by least absolute shrinkage and selection operator (LASSO) (Figure 2), many other symptoms occur in combination with these 12.
PASC Subgroups by Subcohort
The proportion of PASC-positive infected participants in cluster 4 was higher within the postacute pre-Omicron (31%) than postacute Omicron (23%) and acute Omicron (23%) subcohorts (eTable 11A in Supplement 3). Overall, among PASC-positive infected participants, the proportion in cluster 4 among fully vaccinated compared with unvaccinated participants was 23% vs 32% (eTable 11B in Supplement 3). The distribution of clusters was similar for participants with a single reported infection compared with those with more than 1 infection, though the results varied by prevalent SARS-CoV-2 strain (eTable 11C in Supplement 3).
Discussion
This study reported early results from a prospective, survey-based cohort of adult SARS-CoV-2–infected and –uninfected individuals with ascertainment of patient-reported symptoms. A data-driven scoring framework was developed to classify PASC as a condition specific to SARS-CoV-2 infection. Based on this PASC score, 10% of participants first infected on or after December 1, 2021, and enrolled within 30 days of infection were classified as PASC positive at 6 months after infection. Increasing levels of the PASC score were associated with progressively worse measures of well-being and functioning. Although only 12 symptoms contributed to the PASC score, other symptoms correlated with this subgroup are individually important, considering their potential adverse impact on health-related quality of life.
PASC positivity was more common and associated with more severe manifestation in participants infected in the pre-Omicron era. Though participants with earlier infection may have been more likely to enroll in the RECOVER adult cohort because of known PASC, multiple studies reported an association between PASC and early pandemic variants.16 Among participants with a first infection during the Omicron era, PASC frequency was higher among those with recurrent infections, corroborating electronic health record–based studies.4,17,18,19,20,21,22,23 Though studies on the effect of vaccination are conflicting, these findings of modest reduction in PASC frequency among fully vaccinated participants align with recent systematic reviews.24,25
This study found that long-term symptoms associated with SARS-CoV-2 infection spanned multiple organ systems. The diversity of symptoms may be related to persistent viral reservoirs, autoimmunity, or direct differential organ injury. The symptoms identified are consistent with those reported in studies that assessed PASC manifestations (eMethods in Supplement 3). However, by simultaneously considering the contributions of multiple self-reported symptoms, a PASC-scoring algorithm that provides a framework for diagnosing PASC was developed.
Given the heterogeneity of PASC symptoms, determining whether PASC represents one unified condition or reflects a group of unique phenotypes is important. Recent evidence supports the presence of PASC phenotypes, although characterization of these phenotypes is inconsistent and largely dependent on available data.2,6,22,23 Accurate phenotypic stratification has important implications for investigations into the pathophysiological processes underlying PASC and clinical trial design. PASC subgroups that demonstrate overlap with conditions previously described in clinical practice are detailed here, including olfactory dysfunction, cardiopulmonary sequelae, neurocognitive impairment, ME/CFS, and dysautonomia26,27,28,29,30 and overlap with those reported by the National COVID Cohort Collaborative.6 Biological samples from these participants may enable the development of biomarkers of PASC and reveal insights into the mechanistic underpinnings of PASC that inform choice of therapeutic interventions and case selection in upcoming clinical trials for PASC.
Limitations
First, the proposed paradigm and accompanying decision rule require iterative refinement as additional data become available. The PASC score provides an operational definition of PASC and requires further refinement and validation. RECOVER recruitment is ongoing, and not all participants have reached the analysis stage. Evolution and refinement of the phenotypes are anticipated as additional data become available.
Second, selection bias was likely among postacute cohort participants that may have affected frequency estimates including distribution of subphenotypes because PASC severity may impact study participation. Differential attrition of symptomatic and asymptomatic participants at follow-up visits could also have biased frequency estimates though use of inverse probability weighting in the acute cohorts mitigated this bias.
Third, uninfected participants may have had prior asymptomatic SARS-CoV-2 infections not detected due to variations in antibody production and persistence, weakening the discriminant characteristics of this PASC score threshold.
Fourth, symptoms were self-reported and only some symptoms integrate severity scales. Participants could report other symptoms as free text; these were not included in this analysis.
Fifth, confounding may have impacted effect sizes, eg, vaccination status may have been higher in participants at higher risk of PASC, attenuating a vaccination effect. Additionally, PASC status can change over time, perhaps due to underlying mechanistic changes.
Sixth, more than 200 symptoms of PASC have been reported, each with the potential of being life-altering and debilitating, and the symptoms highlighted herein may not reflect the severity or impact of other symptoms.
Conclusions
This symptom-based PASC definition represents a first step for identifying PASC cases and serves as a launching point for further investigations. Definition of a classification rule for PASC requires an updated algorithm that incorporates symptoms as well as biological features. Future analyses must consider the relationships among age, sex, race and ethnicity, social determinants of health, vaccination status after index date, comorbidities, and pregnancy status during infection on the risk of PASC and the distribution of PASC subgroups.
Trial Protocol
Statistical Analysis Plan
eMethods
eReferences
eTable 1. Symptoms Considered in Analysis
eTable 2. Additional Severity Criteria Applied to Symptoms for Analysis
eTable 3. PROMIS Global-10 Questions Used
eTable 4. Vaccination Category Definitions
eTable 5. RECOVER-Adult Additional Demographic and Clinical Characteristics by Infection Status
eTable 6. RECOVER-Adult Demographic Characteristics by Sub-cohort
eTable 7. Symptoms That Correlate With Symptoms Contributing to PASC Score
eTable 8. Symptom Frequencies Among PASC-Positive Participants
eTable 9. PASC Frequencies by Time Since Index Date, Infected Participants
eTable 10. PASC Onset and Resolution Over Time
eTable 11. PASC Subgroup Distributions
eFigure 1. Symptom Frequency, Full Analysis Cohort
eFigure 2. New Onset Symptom Frequency, Full Analysis Cohort
eFigure 3. Symptom Frequency, Full Cohort, Without Severity Scores
eFigure 4. Distribution of Time From Index to Analysis Visit Date
eFigure 5. Symptom Frequency, Acute, Omicron
eFigure 6. Symptom Frequency, Post-Acute, Pre-Omicron
eFigure 7. Symptom Frequency, Post-Acute, Omicron
eFigure 8. Symptom Frequency, Acute, Fully Vaccinated, Omicron
Nonauthor Collaborators. RECOVER Consortium
Data Sharing Statement
References
- 1.World Health Organization . WHO coronavirus (COVID-19) Dashboard. Accessed February 28, 2023. https://covid19.who.int/
- 2.Danesh V, Arroliga AC, Bourgeois JA, et al. Symptom clusters seen in adult COVID-19 recovery clinic care seekers. J Gen Intern Med. 2023;38(2):442-449. doi:10.1007/s11606-022-07908-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davis HE, Assaf GS, McCorkell L, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38:101019. doi:10.1016/j.eclinm.2021.101019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.FAIR Health . A detailed study of patients with long-haul COVID: an analysis of private healthcare claims. June 15, 2021. Accessed February 28, 2023. https://collections.nlm.nih.gov/catalog/nlm:nlmuid-9918334383006676-pdf
- 5.Huang C, Huang L, Wang Y, et al. 6-Month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220-232. doi:10.1016/S0140-6736(20)32656-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reese JT, Blau H, Casiraghi E, et al. ; N3C Consortium; RECOVER Consortium . Generalisable long COVID subtypes: findings from the NIH N3C and RECOVER programmes. EBioMedicine. 2023;87:104413. doi:10.1016/j.ebiom.2022.104413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cutler DM. The costs of long COVID. JAMA Health Forum. 2022;3(5):e221809. doi:10.1001/jamahealthforum.2022.1809 [DOI] [PubMed] [Google Scholar]
- 8.Perlis RH, Trujillo KL, Safarpour A, et al. Research letter: association between long COVID symptoms and employment status. medRxiv. November 18, 2022. doi:10.1101/2022.11.17.22282452 [DOI]
- 9.Davis HE, McCorkell L, Vogel JM, Topol EJ. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023;21(3):133-146. doi:10.1038/s41579-022-00846-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Merad M, Blish CA, Sallusto F, Iwasaki A. The immunology and immunopathology of COVID-19. Science. 2022;375(6585):1122-1127. doi:10.1126/science.abm8108 [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization . Public health surveillance for COVID-19: interim guidance. July 22, 2022. Accessed February 28, 2023. https://www.who.int/publications/i/item/WHO-2019-nCoV-SurveillanceGuidance-2022.2
- 12.RECOVER . Research Protocols. Accessed February 28, 2023. https://recovercovid.org/protocols
- 13.Hastie T, Tibshirani R, Friedman JH, Friedman JH. The Elements of Statistical Learning: Data Mining, Inference, and Prediction. Vol 2. Springer; 2009. doi:10.1007/978-0-387-84858-7 [DOI] [Google Scholar]
- 14.Monti S, Tamayo P, Mesirov J, Golub T. Consensus clustering: a resampling-based method for class discovery and visualization of gene expression microarray data. Mach Learn. 2003;52:91-118. doi:10.1023/A:1023949509487 [DOI] [Google Scholar]
- 15.Murtagh F, Contreras P. Algorithms for hierarchical clustering: an overview. Wiley Interdiscip Rev Data Min Knowl Discov. 2012;2(1):86-97. doi:10.1002/widm.53 [DOI] [Google Scholar]
- 16.Fernández-de-Las-Peñas C, Notarte KI, Peligro PJ, et al. Long-COVID symptoms in individuals infected with different SARS-CoV-2 variants of concern: a systematic review of the literature. Viruses. 2022;14(12):2629. doi:10.3390/v14122629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594(7862):259-264. doi:10.1038/s41586-021-03553-9 [DOI] [PubMed] [Google Scholar]
- 18.Estiri H, Strasser ZH, Brat GA, Semenov YR, Patel CJ, Murphy SN; Consortium for Characterization of COVID-19 by EHR (4CE) . Evolving phenotypes of non-hospitalized patients that indicate long COVID. BMC Med. 2021;19(1):249. doi:10.1186/s12916-021-02115-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horberg MA, Watson E, Bhatia M, et al. Post-acute sequelae of SARS-CoV-2 with clinical condition definitions and comparison in a matched cohort. Nat Commun. 2022;13(1):5822. doi:10.1038/s41467-022-33573-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mizrahi B, Sudry T, Flaks-Manov N, et al. Long COVID outcomes at one year after mild SARS-CoV-2 infection: nationwide cohort study. BMJ. 2023;380:e072529. doi:10.1136/bmj-2022-072529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roessler M, Tesch F, Batram M, et al. Post-COVID-19-associated morbidity in children, adolescents, and adults: a matched cohort study including more than 157,000 individuals with COVID-19 in Germany. PLoS Med. 2022;19(11):e1004122. doi:10.1371/journal.pmed.1004122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wulf Hanson S, Abbafati C, Aerts JG, et al. ; Global Burden of Disease Long COVID Collaborators . Estimated global proportions of individuals with persistent fatigue, cognitive, and respiratory symptom clusters following symptomatic COVID-19 in 2020 and 2021. JAMA. 2022;328(16):1604-1615. doi:10.1001/jama.2022.18931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang Y, Pinto MD, Borelli JL, et al. COVID symptoms, symptom clusters, and predictors for becoming a long-hauler looking for clarity in the haze of the pandemic. Clin Nurs Res. 2022;31(8):1390-1398. doi:10.1177/10547738221125632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brannock MD, Chew RF, Preiss AJ, et al. Long COVID risk and pre-COVID vaccination: an EHR-based cohort study from the RECOVER program. medRxiv. October 7, 2022. doi:10.1101/2022.10.06.22280795 [DOI] [PMC free article] [PubMed]
- 25.Kuodi P, Gorelik Y, Zayyad H, et al. Association between BNT162b2 vaccination and reported incidence of post-COVID-19 symptoms: cross-sectional study 2020-21, Israel. NPJ Vaccines. 2022;7(1):101. doi:10.1038/s41541-022-00526-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haffke M, Freitag H, Rudolf G, et al. Endothelial dysfunction and altered endothelial biomarkers in patients with post-COVID-19 syndrome and chronic fatigue syndrome (ME/CFS). J Transl Med. 2022;20(1):138. doi:10.1186/s12967-022-03346-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kedor C, Freitag H, Meyer-Arndt L, et al. A prospective observational study of post-COVID-19 chronic fatigue syndrome following the first pandemic wave in Germany and biomarkers associated with symptom severity. Nat Commun. 2022;13(1):5104. doi:10.1038/s41467-022-32507-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Larsen NW, Stiles LE, Shaik R, et al. Characterization of autonomic symptom burden in long COVID: a global survey of 2,314 adults. Front Neurol. 2022;13:1012668. doi:10.3389/fneur.2022.1012668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mancini DM, Brunjes DL, Lala A, Trivieri MG, Contreras JP, Natelson BH. Use of cardiopulmonary stress testing for patients with unexplained dyspnea post-coronavirus disease. JACC Heart Fail. 2021;9(12):927-937. doi:10.1016/j.jchf.2021.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Twomey R, DeMars J, Franklin K, Culos-Reed SN, Weatherald J, Wrightson JG. Chronic fatigue and postexertional malaise in people living with long COVID: an observational study. Phys Ther. 2022;102(4):pzac005. doi:10.1093/ptj/pzac005 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Statistical Analysis Plan
eMethods
eReferences
eTable 1. Symptoms Considered in Analysis
eTable 2. Additional Severity Criteria Applied to Symptoms for Analysis
eTable 3. PROMIS Global-10 Questions Used
eTable 4. Vaccination Category Definitions
eTable 5. RECOVER-Adult Additional Demographic and Clinical Characteristics by Infection Status
eTable 6. RECOVER-Adult Demographic Characteristics by Sub-cohort
eTable 7. Symptoms That Correlate With Symptoms Contributing to PASC Score
eTable 8. Symptom Frequencies Among PASC-Positive Participants
eTable 9. PASC Frequencies by Time Since Index Date, Infected Participants
eTable 10. PASC Onset and Resolution Over Time
eTable 11. PASC Subgroup Distributions
eFigure 1. Symptom Frequency, Full Analysis Cohort
eFigure 2. New Onset Symptom Frequency, Full Analysis Cohort
eFigure 3. Symptom Frequency, Full Cohort, Without Severity Scores
eFigure 4. Distribution of Time From Index to Analysis Visit Date
eFigure 5. Symptom Frequency, Acute, Omicron
eFigure 6. Symptom Frequency, Post-Acute, Pre-Omicron
eFigure 7. Symptom Frequency, Post-Acute, Omicron
eFigure 8. Symptom Frequency, Acute, Fully Vaccinated, Omicron
Nonauthor Collaborators. RECOVER Consortium
Data Sharing Statement