Abstract
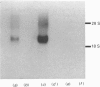
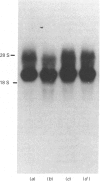
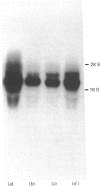
Starvation (48 h) decreased the concentration of mRNA of the insulin-responsive glucose transporter isoform (GLUT 4) in interscapular brown adipose tissue (IBAT) (56%) and tibialis anterior (10%). Despite dramatic [7-fold (tibialis anterior) and 40-fold (IBAT)] increases in glucose utilization after 2 and 4 h of chow re-feeding, no significant changes in GLUT 4 mRNA concentration were observed in these tissues over this re-feeding period. The results exclude changes in GLUT 4 mRNA concentration in mediating the responses of glucose transport in these tissues to acute re-feeding after prolonged starvation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell G. I., Kayano T., Buse J. B., Burant C. F., Takeda J., Lin D., Fukumoto H., Seino S. Molecular biology of mammalian glucose transporters. Diabetes Care. 1990 Mar;13(3):198–208. doi: 10.2337/diacare.13.3.198. [DOI] [PubMed] [Google Scholar]
- Birnbaum M. J. Identification of a novel gene encoding an insulin-responsive glucose transporter protein. Cell. 1989 Apr 21;57(2):305–315. doi: 10.1016/0092-8674(89)90968-9. [DOI] [PubMed] [Google Scholar]
- Charron M. J., Kahn B. B. Divergent molecular mechanisms for insulin-resistant glucose transport in muscle and adipose cells in vivo. J Biol Chem. 1990 May 15;265(14):7994–8000. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cooney G. J., Newsholme E. A. The maximum capacity of glycolysis in brown adipose tissue and its relationship to control of the blood glucose concentration. FEBS Lett. 1982 Nov 8;148(2):198–200. doi: 10.1016/0014-5793(82)80807-7. [DOI] [PubMed] [Google Scholar]
- Cushman S. W., Wardzala L. J. Potential mechanism of insulin action on glucose transport in the isolated rat adipose cell. Apparent translocation of intracellular transport systems to the plasma membrane. J Biol Chem. 1980 May 25;255(10):4758–4762. [PubMed] [Google Scholar]
- Cusin I., Terrettaz J., Rohner-Jeanrenaud F., Assimacopoulos-Jeannet F., Jeanrenaud B. Aspects of the regulation of glucose transport in insulin-sensitive tissues in normal conditions and in type-2 diabetes. Biochem Soc Trans. 1990 Dec;18(6):1127–1130. doi: 10.1042/bst0181127. [DOI] [PubMed] [Google Scholar]
- Felber J. P., Golay A., Felley C., Jéquier E. Regulation of glucose storage in obesity and diabetes: metabolic aspects. Diabetes Metab Rev. 1988 Nov;4(7):691–700. doi: 10.1002/dmr.5610040706. [DOI] [PubMed] [Google Scholar]
- Ferré P., Burnol A. F., Leturque A., Terretaz J., Penicaud L., Jeanrenaud B., Girard J. Glucose utilization in vivo and insulin-sensitivity of rat brown adipose tissue in various physiological and pathological conditions. Biochem J. 1986 Jan 1;233(1):249–252. doi: 10.1042/bj2330249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferré P., Leturque A., Burnol A. F., Penicaud L., Girard J. A method to quantify glucose utilization in vivo in skeletal muscle and white adipose tissue of the anaesthetized rat. Biochem J. 1985 May 15;228(1):103–110. doi: 10.1042/bj2280103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco-Perotto R., Assimacopoulos-Jeannet F., Jeanrenaud B. Insulin modifies the properties of glucose transporters in rat brown adipose tissue. Biochem J. 1987 Oct 1;247(1):63–68. doi: 10.1042/bj2470063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco-Perotto R., Zaninetti D., Assimacopoulos-Jeannet F., Bobbioni E., Jeanrenaud B. Stimulatory effect of cold adaptation on glucose utilization by brown adipose tissue. Relationship with changes in the glucose transporter system. J Biol Chem. 1987 Jun 5;262(16):7732–7736. [PubMed] [Google Scholar]
- Holness M. J., Liu Y. L., Beech J. S., Sugden M. C. Glucose utilization by interscapular brown adipose tissue in vivo during nutritional transitions in the rat. Biochem J. 1991 Jan 1;273(Pt 1):233–235. doi: 10.1042/bj2730233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holness M. J., MacLennan P. A., Palmer T. N., Sugden M. C. The disposition of carbohydrate between glycogenesis, lipogenesis and oxidation in liver during the starved-to-fed transition. Biochem J. 1988 Jun 1;252(2):325–330. doi: 10.1042/bj2520325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holness M. J., Sugden M. C. Glucose disposal by skeletal muscle in response to re-feeding after progressive starvation. Biochem J. 1991 Jul 15;277(Pt 2):429–433. doi: 10.1042/bj2770429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holness M. J., Sugden M. C. Glucose utilization in heart, diaphragm and skeletal muscle during the fed-to-starved transition. Biochem J. 1990 Aug 15;270(1):245–249. doi: 10.1042/bj2700245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issad T., Pénicaud L., Ferré P., Kandé J., Baudon M. A., Girard J. Effects of fasting on tissue glucose utilization in conscious resting rats. Major glucose-sparing effect in working muscles. Biochem J. 1987 Aug 15;246(1):241–244. doi: 10.1042/bj2460241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James D. E., Kraegen E. W., Chisholm D. J. Muscle glucose metabolism in exercising rats: comparison with insulin stimulation. Am J Physiol. 1985 May;248(5 Pt 1):E575–E580. doi: 10.1152/ajpendo.1985.248.5.E575. [DOI] [PubMed] [Google Scholar]
- Kahn B. B., Cushman S. W., Flier J. S. Regulation of glucose transporter-specific mRNA levels in rat adipose cells with fasting and refeeding. Implications for in vivo control of glucose transporter number. J Clin Invest. 1989 Jan;83(1):199–204. doi: 10.1172/JCI113859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn B. B., Flier J. S. Regulation of glucose-transporter gene expression in vitro and in vivo. Diabetes Care. 1990 Jun;13(6):548–564. doi: 10.2337/diacare.13.6.548. [DOI] [PubMed] [Google Scholar]
- Kahn B. B., Rossetti L., Lodish H. F., Charron M. J. Decreased in vivo glucose uptake but normal expression of GLUT1 and GLUT4 in skeletal muscle of diabetic rats. J Clin Invest. 1991 Jun;87(6):2197–2206. doi: 10.1172/JCI115254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koranyi L., James D., Mueckler M., Permutt M. A. Glucose transporter levels in spontaneously obese (db/db) insulin-resistant mice. J Clin Invest. 1990 Mar;85(3):962–967. doi: 10.1172/JCI114526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roychoudhury R., Wu R. Terminal transferase-catalyzed addition of nucleotides to the 3' termini of DNA. Methods Enzymol. 1980;65(1):43–62. doi: 10.1016/s0076-6879(80)65009-5. [DOI] [PubMed] [Google Scholar]
- Sivitz W. I., DeSautel S. L., Kayano T., Bell G. I., Pessin J. E. Regulation of glucose transporter messenger RNA in insulin-deficient states. Nature. 1989 Jul 6;340(6228):72–74. doi: 10.1038/340072a0. [DOI] [PubMed] [Google Scholar]
- Sokoloff L., Reivich M., Kennedy C., Des Rosiers M. H., Patlak C. S., Pettigrew K. D., Sakurada O., Shinohara M. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem. 1977 May;28(5):897–916. doi: 10.1111/j.1471-4159.1977.tb10649.x. [DOI] [PubMed] [Google Scholar]
- Sugden M. C., Holness M. J. Substrate interactions in the development of insulin resistance in type II diabetes and obesity. J Endocrinol. 1990 Nov;127(2):187–190. doi: 10.1677/joe.0.1270187. [DOI] [PubMed] [Google Scholar]
- Sugden M. C., Liu Y. L., Holness M. J. Glucose utilization and disposal in cardiothoracic and skeletal muscles during the starved-to-fed transition in the rat. Biochem J. 1990 Nov 15;272(1):133–137. doi: 10.1042/bj2720133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden P. H., Fuller S. J. Regulation of protein turnover in skeletal and cardiac muscle. Biochem J. 1991 Jan 1;273(Pt 1):21–37. doi: 10.1042/bj2730021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiébaud D., DeFronzo R. A., Jacot E., Golay A., Acheson K., Maeder E., Jéquier E., Felber J. P. Effect of long chain triglyceride infusion on glucose metabolism in man. Metabolism. 1982 Nov;31(11):1128–1136. doi: 10.1016/0026-0495(82)90163-9. [DOI] [PubMed] [Google Scholar]
- Wardzala L. J., Jeanrenaud B. Potential mechanism of insulin action on glucose transport in the isolated rat diaphragm. Apparent translocation of intracellular transport units to the plasma membrane. J Biol Chem. 1981 Jul 25;256(14):7090–7093. [PubMed] [Google Scholar]
- Zaninetti D., Greco-Perotto R., Assimacopoulos-Jeannet F., Jeanrenaud B. Effects of insulin on glucose transport and glucose transporters in rat heart. Biochem J. 1988 Feb 15;250(1):277–283. doi: 10.1042/bj2500277. [DOI] [PMC free article] [PubMed] [Google Scholar]