Abstract
Hepatitis C virus (HCV) sets up persistent infection in the majority of those exposed. It is likely that, as with other persistent viral infections, the efficacy of T-lymphocyte responses influences long-term outcome. However, little is known about the functional capacity of HCV-specific T-lymphocyte responses induced after acute infection. We investigated this by using major histocompatibility complex class I-peptide tetrameric complexes (tetramers), which allow direct detection of specific CD8+ T lymphocytes ex vivo, independently of function. Here we show that, early after infection, virus-specific CD8+ T lymphocytes detected with a panel of four such tetramers are abnormal in terms of their synthesis of antiviral cytokines and lytic activity. Furthermore, this phenotype is commonly maintained long term, since large sustained populations of HCV-specific CD8+ T lymphocytes were identified, which consistently had very poor antiviral cytokine responses as measured in vitro. Overall, HCV-specific CD8+ T lymphocytes show reduced synthesis of tumor necrosis factor alpha (TNF-α) and gamma interferon (IFN-γ) after stimulation with either mitogens or peptides, compared to responses to Epstein-Barr virus and/or cytomegalovirus. This behavior of antiviral CD8+ T lymphocytes induced after HCV infection may contribute to viral persistence through failure to effectively suppress viral replication.
Hepatitis C virus (HCV) infects approximately 170 million people worldwide and is a significant cause of liver failure. The virus is able to set up chronic infection in about 80% of those infected, although a minority are able to clear the virus from blood after acute exposure. Although dynamic studies of infection are hampered by the lack of acute symptoms in most patients, there is nevertheless accumulating evidence that multiple immune responses are involved (8, 11, 19, 21, 24).
A role for CD4+ T-helper lymphocytes is supported by functional and genetic linkage studies (11). (7, 25, 28, 30). Recent studies of acute disease have also indicated that antiviral antibody responses directed against envelope proteins (E2) influence viral evolution and disease outcome (9). CD8+ T lymphocytes specific for HCV also arise early after infection (19–21) and, by analogy with hepatitis B virus infection, theoretically could suppress viral replication by secretion of antiviral cytokines (13). However, these populations are not sustained and generally become difficult to detect in blood, especially when techniques that rely on detection of gamma interferon (IFN-γ) are used (29), although they can be recovered upon stimulation in vitro (4, 17, 27, 32). There is evidence from murine models that failure of T-cell responses to control early-stage infection can lead to shifts in immune-selective forces and viral escape (6, 16). We therefore asked whether a specific dysfunction of antiviral CD8+ T lymphocytes may occur after infection with HCV and addressed this question by analyzing T-lymphocyte responses directly ex vivo with peptide-major histocompatibility complex (MHC) class I tetramers (1, 3, 12) combined with assays for lymphocyte function.
MATERIALS AND METHODS
Patients and blood samples.
Patients were recruited from clinics in Munich, Germany; Oxford, United Kingdom; Sydney, Australia; and Boston, Mass. Peripheral blood mononuclear cells (PBMCs) were prepared and frozen as previously described(19–21). For assays comparing different time points from the same individual, samples were thawed and stained simultaneously. Informed consent for venipuncture was obtained in all cases. For subjects A and B (both HLA-A*0201-positive female intravenous drug users who presented acutely and are studied here in the most detail), seroconversion to anti-HCV antibody positivity was determined at the time of presentation. Liver histology was not obtained for subjects A and B, because it was not indicated in acute disease. Their clinical details are shown in Table 1. Of the other subjects, not studied here during acute infection, subject C also acquired virus via intravenous drug use, subject D probably acquired the virus via sexual contact, and subject E acquired the virus via infected blood products (subjects 1 and 3 and 15 in reference 19). The time of acquisition of virus was determined from clinical history and from transfusion data. The full details of the cohorts from which these subjects were selected, including age, tissue type, sex, PCR and treatment status, and, where appropriate, genotype and liver histology, have been published previously (19–21).
TABLE 1.
Clinical details of subjects studied during or after acute infection
| Subject | Sex | Age (yr) | Time of analysis | HLA class I | Clinical outcome |
|---|---|---|---|---|---|
| A | Female | 31 | During acute hepatitis | A2, A26, B38, B50 | Relapse |
| B | Female | 37 | During acute hepatitis | A1, A2, B8, B40 | Sustained PCR negative in blood |
| C | Male | 42 | After acute hepatitis | A2, A26, B37 | Sustained PCR negative in blood |
| D | Female | 32 | After acute hepatitis | A2, A3, B41, B44 | Sustained PCR negative in blood |
Class I-peptide tetramers.
Class I-peptide tetramers were prepared and validated exactly as previously described. The following peptides were obtained from Research Genetics (Huntsville, Ala.): NS3 peptide 1073–1081 (abbreviated here as NS3 1073; CINGVWCTV), NS5B peptide 2594–2602 (NS5 2594; ALYDVVTKL), NS4B 1406–1415 (NS4 1406; KLVALGINAV), and NS4B 1807–1816 (NS4 1807; LLFNILGGWV). The tetramers for the control Epstein-Barr virus (EBV) and cytomegalovirus (CMV) peptides restricted by HLA-A2 (EBV BMLF-1 [GLCTLVAML] and CMV pp65 [NLVPMVATV]) were validated by using peripheral blood samples from EBV- and CMV-seropositive, healthy individuals as described previously (19, 21). In screening the HCV patients, where appropriate, tetramers for two HCV epitopes restricted by HLA-B8 (HSKKKLDEL and LIRLKPTL) and two restricted by HLA-B7 (GPRLGVRAT and DPRRRSRNL) were also used, as previously described (19, 20). However, since these did not yield large tetramer-positive populations amenable to functional analysis, they are not detailed further.
Tetramer staining and functional assays.
The following conditions were used for staining. From 0.5 to 1 million PBMCs were coincubated with tetramer for 20 min at 37°C, followed by washing and phenotypic staining or stimulation. The following monoclonal antibodies (MAbs) were used: anti-CD8-PerCP, anti-CD27–fluorescein isothiocyanate (FITC) conjugate, anti-CD45RO–allophycocyanin (APC), anti-CD45RA–FITC (all Becton Dickinson Immunocytometry Systems), anti-CC chemokine receptor 5 (CCR-5)–FITC, anti-CD38–PC, anti-CD69–APC (all PharMingen), anti-human MHC class II–FITC (HLA-DR,-DP, and -DQ; Dako), anti-Ki 67–FITC (Coulter Immunotech, Marseille, France), anti-perforin–FITC (Pharmingen), and FITC-conjugated anti-T-cell receptor (TCR) (TCR Vβ1, -2, -3, -5.1, -5.2, -8, -12, -13.1, -14, -16, -17, -20, and -22; unlabeled anti-TCR Vβ5.3, -9, and -23 [all Coulter Immunotech]). Unlabeled anti-TCR Vβ antibodies were detected with FITC-conjugated antimouse immunoglobulin (Ig) [F(ab′)2; Biosource, Camarillo, Calif.]. Phorbol myristate acetate (PMA)-ionomycin stimulation was performed exactly as described in reference 21, but the tetramer staining was done first (20 min), and then a PMA-ionomycin mixture was added without washing. Determination of peptide stimulation and intracellular cytokine secretion was performed in the presence of 10 μM cognate peptide or control and 1 μg of costimulatory anti-CD28 (Pharmingen, San Diego, Calif.) and anti-CD49b antibodies (Becton Dickinson, San Jose, Calif.) per ml (6 h). Peptide stimulation and CD69 staining were performed in the absence of costimulation (4 h). Flow cytometric analysis was performed with a Becton Dickinson FACSCalibur fluorescence-activated cell sorter (FACS), and analysis was performed with CellQuest software.
CD4 proliferative assays.
CD4 proliferative assays were performed with recombinant HCV proteins and tritium incorporation exactly as previously described (11).
RESULTS
Analysis of CD8+ T-lymphocyte responses ex vivo after acute HCV infection.
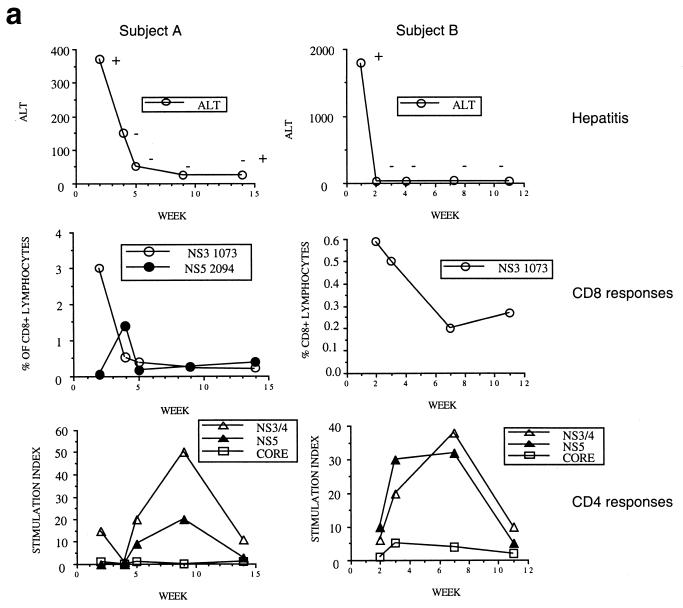
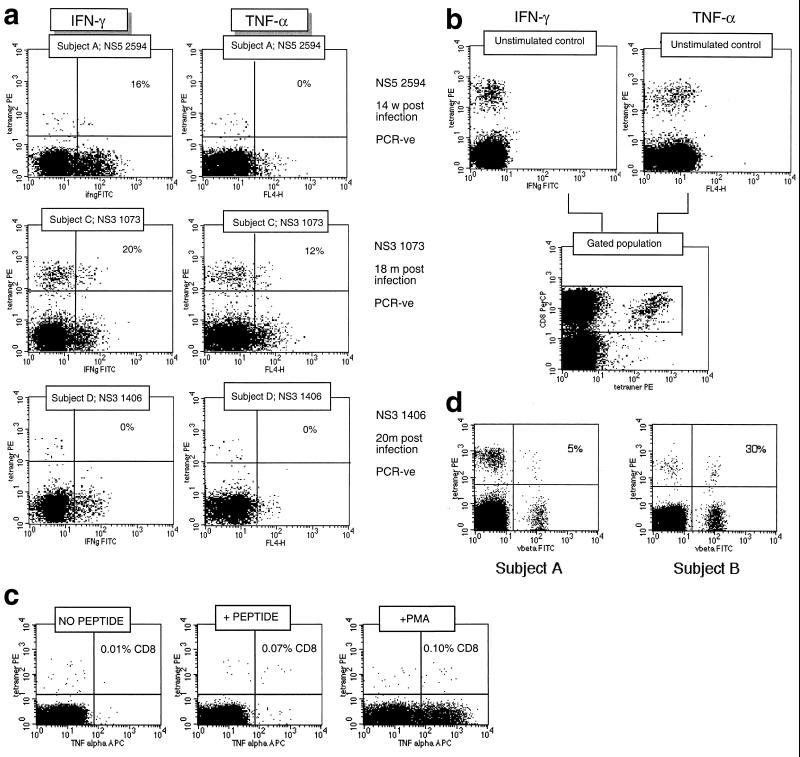
We first tracked T-lymphocyte responses in a set of four HLA-A*0201-positive patients for whom a clear date of onset of infection was available (Table 1). Two examples (subjects A and B) in which samples were obtained very close to the time of acquisition of virus are illustrated in detail in Fig. 1a and described in Table 1. We analyzed CD8+ T-lymphocyte responses against four HLA-A2-restricted HCV peptides by using peptide-MHC class II tetramers, as previously described (Fig. 1a, lower panel) (19, 21). In subject A, a significant response was observed against NS3 1073 (CINGVWCTV [shown in Fig. 1a, middle panel]) and NS5 2594 (ALYDVVTKL), but not NS4 1406 (KLVALGINAV) or NS4 1807 (LLFNILGGWV) (not shown). In subject B, only a response to NS3 1073 was observed, but in this instance, we also had the opportunity to compare this response with established memory responses to HLA-A2-restricted epitopes from either EBV (GLCTLVAML) or CMV (NLVPMVATV [0.74 and 0.25% of CD8+ lymphocytes, respectively]).
FIG. 1.
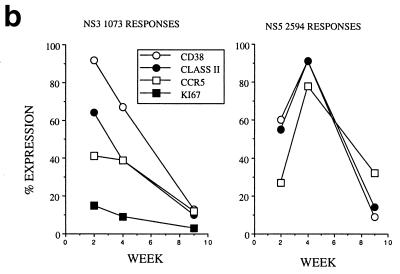
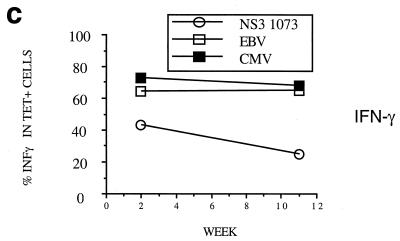
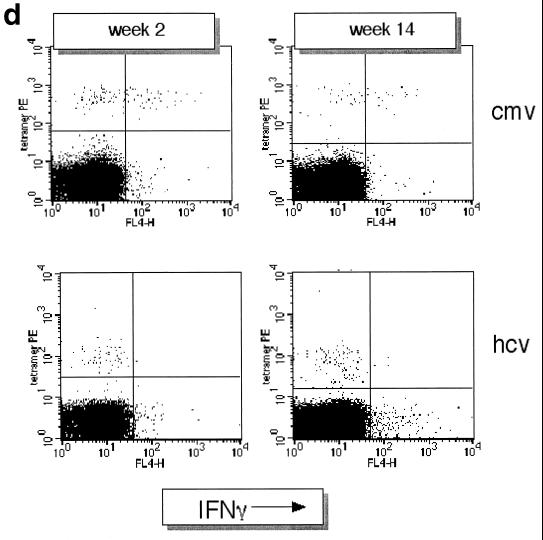
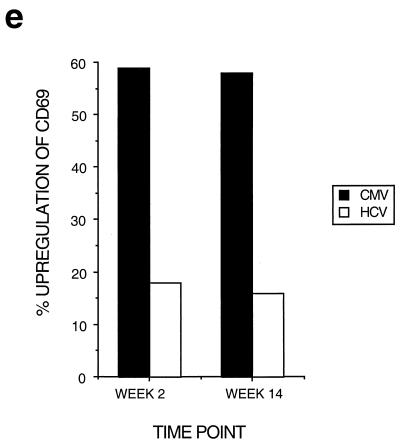
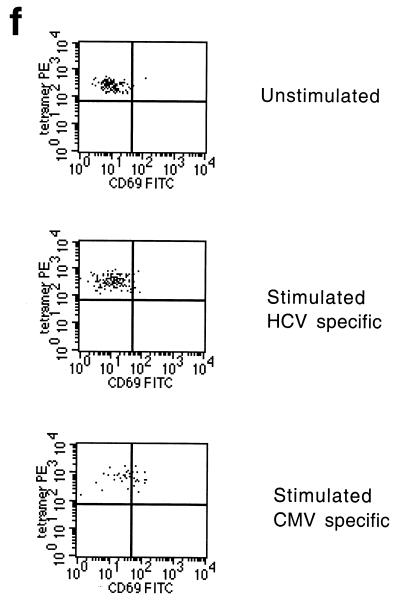
Dynamics of hepatitis and acute immune responses in two subjects. (a) Time course of disease and immune responses. (Upper panels) Time course of alanine aminotransferase (ALT [international units per milliliter]) in serum over time. Subject A was PCR positive (+) for HCV RNA at the first time point and subsequently became PCR negative (−) over time as indicated, with recrudescence at week 15. In subject B, virus did not recrudesce, even during longer follow-up periods of 1 year. (Middle panels) Dynamics of tetramer-positive responses. Frozen PBMCs were thawed and tested in parallel with tetramers for four HLA-A2-restricted peptides (NS3 1073, NS3 1406, NS4B 1807, and NS5B 2594). Thawed PBMCs were stained exactly as previously described (19, 21). Only positive stains are shown. The proportions were calculated after gating on live CD8+ lymphocytes. (Lower panels) Fresh PBMCs were tested in standard proliferation assays by incorporation of [3H]thymidine after stimulation with HCV antigens as previously described (11). (b) Phenotype of acute responses in subject A. Frozen PBMCs were thawed and stained in parallel with the tetramers for NS3 1073 and NS5 2094, shown to be positive, together with the antibodies for MHC class II CD38, CCR-5, or (after permeabilization) Ki-67 (see Materials and Methods). The proportions of tetramer-positive cells staining positive at each time point for each marker are shown. (c) PMA-ionomycin-stimulated cytokine synthesis over time in subject B. Frozen PBMCs were thawed, tetramer stained for 20 min, and then stimulated with PMA-ionomycin as previously described (21). Staining with PerCP-labeled anti-CD8 was followed by permeabilization as in panel b and intracellular staining with FITC–anti-IFN-γ (Becton Dickinson). After four-color flow cytometry, the proportion of tetramer-positive cells staining positive for intracellular IFN-γ was calculated. (d) Peptide-stimulated synthesis of IFN-γ in subject B: comparison of HCV and control response. Frozen PBMCs from subject B at weeks 2 and 14 were thawed, stained with tetramers for HCV NS3 1073 or CMV, stimulated with the appropriate peptide and costimulatory molecules, permeabilized, and stained for CD8 and intracellular IFN-γ (21). After flow cytometric analysis, the CD8+ population is displayed. The proportion of tetramer-positive cells staining positive for IFN-γ is shown. Staining of cells in the absence of peptide revealed stimulation of <2% in both cases. (e) Peptide-stimulated upregulation of CD69 in subject B: comparison of HCV and control responses. PBMCs from the same time points as in panel d above were stimulated in the same manner with peptide (21) and stained thereafter with PerCP–anti-CD8 and FITC–anti-CD69. The proportion of tetramer-positive cells expressing CD69 is illustrated. Expression in ex vivo samples or in the absence of peptide was <2%. (f) Example of CD69 upregulation in tetramer-positive populations by peptide stimulation. Examples from the first time point of CD69 surface staining in tetramer-positive cells. The tetramer-positive CD8+ population was gated upon and CD69 expression was analyzed after peptide stimulation. No upregulation of CD69 on tetramer-negative cells was observed (data not shown).
Dynamics and phenotype and of antiviral CD8+ T-lymphocyte responses over time.
Interestingly, in subject A, a shift in dominance between the two detectable responses was noted over time. This occurred during a period in which the subject's blood was already negative for viral RNA by PCR, and a very similar pattern was observed under similar circumstances in a previously described case (21), as well as in cases later after infection (19, 21). The shift in numerical dominance was paralleled by a striking shift in expression of markers of activation between the two populations (Fig. 1b). The response, which peaked second (directed against NS5 2594), showed peak CD38 and class II expression 2 weeks later than the first wave of cells directed against NS3 1073. Also, both populations showed increased expression of chemokine receptor CCR5 at the time of their maximal activation, which might contribute to recruitment into the inflamed liver. The level of expression of the intracellular marker of proliferation Ki-67 was high for the first wave of cells observed, at a time when their numbers in blood were actually waning. This is consistent with either exhaustion of this response or redistribution into the liver.
Analysis of function of tetramer-positive cells ex vivo.
We next examined the function of these virus-specific CD8+ T-lymphocyte populations ex vivo by using an established assay for intracellular detection of IFN-γ synthesis after stimulation with PMA-ionomycin (21). These are illustrated in Fig. 1c to e in detail for subject B, for whom an internal comparison was available. In this individual, the level of IFN-γ synthesis after stimulation was low in the HCV-specific tetramer-positive population and was significantly lower at both time points tested than those in the control CMV- and EBV-specific populations (Fig. 1c). The release of IFN-γ in response to peptide stimulation (a more physiological stimulus, since triggering occurs through the TCR) showed even more profound defects (Fig. 1d) compared to the internal control of the CMV-specific response. This dysfunction was sustained over a period of 3 months. Consistent with this, a highly sensitive overnight ex vivo IFN-γ ELISpot assay (Mabtech, Upsalla, Sweden) performed with the same NS3 peptide was also completely negative throughout this period (data not shown) (18). Intermediate time points over this period showed a very similar profile of unresponsiveness. For example, at week 7 in subject B, at the peak of the CD4 responses (Fig. 1a, lower panels), the IFN-γ ELISpot remained negative for responses against NS3 1073, as was synthesis of tumor necrosis factor alpha (TNF-α) after PMA stimulation.
Analysis of CD69 upregulation in comparison to control responses.
To confirm that the defect in cytokine synthesis represented a failure of triggering (rather than a switch in secretion pattern to other cytokines), upregulation of CD69 after NS3 1073 peptide stimulation in vitro was also measured at these time points, as previously analyzed (21). Again, weak responses were observed, most obviously when compared with the internal CMV control (Fig. 1e and f).
Examination of tetramer-positive responses of different specificities and in different individuals.
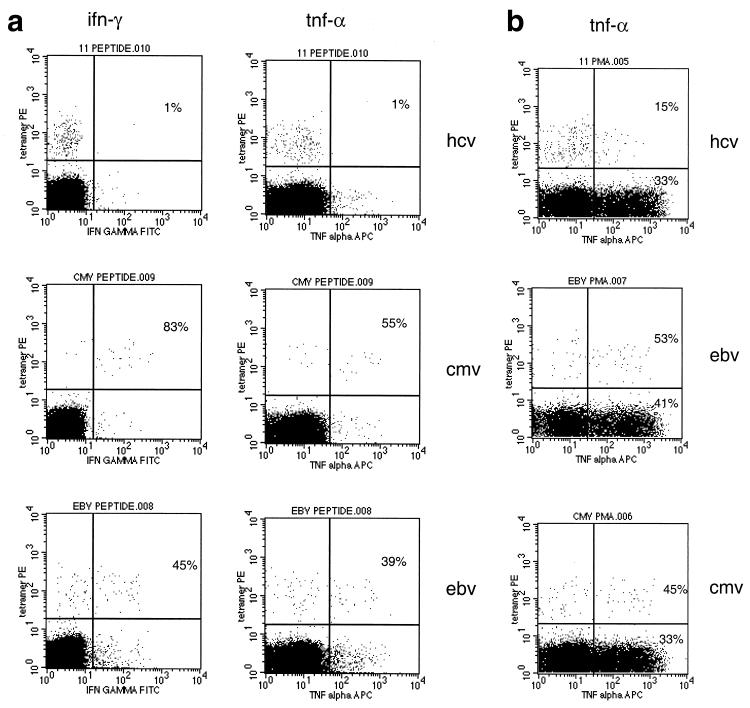
The specificities of tetramer-positive responses in different subjects were reproduced through analyses of other responses in other patients and other epitopes. Figure 2a illustrates cytokine staining patterns in three separate individuals in whom three distinct epitopes (NS3 1073, NS3 1406, and NS5 2594) from HCV were targeted. In these cases, little or no cytokine synthesis was measured after maximal stimulation with PMA-ionomycin. This was clear by measurement of IFN-γ and TNF-α. These individuals were all PCR negative at the time of study and had been so for many months. The plots illustrated demonstrate the extremes of the range of cytokine responses seen among HCV-specific CD8+ lymphocytes. In all cases, the proportion of cytokine-positive cells among the tetramer-positive population was lower than that in the tetramer-negative CD8+ population. Exactly the reverse result is seen in the case of CMV- and EBV-specific tetramer populations (described below; Fig. 3b). Again, no or very low levels of synthesis were also observed after peptide stimulation (data not shown).
FIG. 2.
Functional analysis of HCV-specific responses against three separate epitopes in three separate donors. (a) Cytokine release from CD8+ lymphocytes of different specificities. Frozen samples from three separate HCV antibody-positive individuals were thawed and tested for synthesis of antiviral cytokines after PMA-ionomycin stimulation exactly as in Fig. 1c. Both stains for TNF-α (right panels [marked FL4-H]) and IFN-γ (left panels [ifngFITC]) are illustrated. The numbers shown in each plot represent the percentage of tetramer-positive cells that stained positive for the particular cytokine. PCR-ve, PCR negative. (b) Control unstimulated cells and a reference for gating on the CD8 high population. Samples obtained from subject C (NS3 1073 specific) are illustrated. No synthesis of IFN-γ is seen. (c) Comparison of PMA-ionomycin and peptide stimulation. A CMV-specific response from a control HCV-negative patient is shown. The samples were tested in parallel for TNF-α synthesis according to the peptide stimulation and PMA stimulation protocols, and the CD8 high population is shown. Approximately similar proportions of CD8+ cytokine-positive cells were obtained, as indicated in the right upper quadrant of each FACS plot. (d) Analysis of Vβ usage of tetramer-positive cells in subjects A and B. PBMCs were anti-CD8 and tetramer stained as described above and costained with a panel of FITC-conjugated Vβ-specific MAbs (Immunotech, Marseille, France). Staining for Vβ3 only is shown, after gating on live CD8+ lymphocytes. A large population of Vβ3-positive, tetramer-positive lymphocytes is seen in subject B (30% of tetramer-positive cells), and a smaller population is seen in subject A (5%). No dominant Vβ usage was seen in subject A, C, or D. Tetramer-negative populations in these subjects did not reveal a major oligoclonal expansion when this restricted panel of antibodies was used (Vβ1, -2, -3, -5.1, -5.2, -5.3, -8, -9, -12, -13.1, -14, -16, -17, -20, -22, and -23).
FIG. 3.
Functional analysis of a long-term antiviral CD8+ T-lymphocyte response. (a) Peptide-stimulated synthesis of cytokines in subject E: comparison of HCV and control responses. Experiments were performed exactly as in Fig. 1c. The CD8+ population is shown, and the proportions within the tetramer-positive populations that stain positive for intracellular cytokine are indicated. The upper panels represent stimulation with HCV peptide NS3 1073 (also indicated as “peptide 11” in the FACS plot title line), and the middle and lower panels represent HLA-A2-restricted peptides from CMV and EBV, respectively. (b) PMA-ionomycin-stimulated TNF-α synthesis in patient E. Experiments were performed exactly as in Fig. 2a. The CD8+ population is shown, and the proportions within the tetramer-positive and tetramer-negative populations positive for intracellular cytokine are indicated.
A series of control experiments were performed to confirm that these results were not artifactual due to stimulation of T cells by tetramers or downregulation effects due to stimulation. The tetramer-positive controls left unstimulated for the duration of the assay are illustrated in Fig. 2b, which demonstrates that staining with the tetramer alone does not lead to synthesis of cytokines with this protocol. A typical control stain directly comparing PMA and peptide-stimulated responses is also shown (Fig. 2c), which demonstrates that the populations of CD8+ cells that make cytokine after peptide stimulation are comparable with both stimuli. This experiment also shows that the number of tetramer-stained cells remains high in phycoerythrin (PE) fluorescence under this assay system. Internalization of tetramer occurs normally within a few minutes of staining (31), and strong signals appear to be intact at the end of the assay in both cytokine-positive and cytokine-negative subsets with this protocol. This experiment also demonstrates the lack of cytokine secretion by tetramer alone in the presence of costimulatory antibodies.
Analysis of TCR Vβ usage in acutely expanded HCV-specific CD8+ T-lymphocyte populations.
We addressed whether the HCV-specific CD8+ phenotype was the result of expansion of a single aberrant clone by ex vivo analysis of Vβ usage with a panel of Vβ-specific MAbs in the large expansions in subjects A to D. Only in subject B was an expansion of a single Vβ-bearing subset seen (Fig. 2d, right panel), comprising 30% of the tetramer-positive population at the first time point. This was not seen in the other patients (for example, subject A [Fig. 2d, left panel]) and was not sustained in subject B, in whom the proportion dropped to about 10% by week 14 (data not shown). Thus, the observed phenotype of weak or absent cytokine secretion upon stimulation appears to extend across populations of CD8+ T lymphocytes targeting distinct epitopes and using distinct TCRs.
Analysis of lytic function and perforin staining ex vivo.
We also had the opportunity with a single patient (subject A) to address whether the cells identified at the peak of activation during acute hepatitis were directly cytotoxic ex vivo. In this situation, at the first time point, 3% of CD8+ T lymphocytes were NS3 1073 specific and were highly activated (92% CD38 high and 64% MHC class II high), as previously observed in other patients (19) (Fig. 1b). Nevertheless at this time point, no specific lysis of peptide-pulsed targets was observed in a 5-h or extended assay for chromium release, even though the same targets were readily lysed by a specific clone (data not shown). Consistent with this finding, the cells were low in perforin (1.5% positive) and high in CD27 (90% positive), a phenotype which has been associated with a low lytic capacity and what has been described as an “immature” or “early differentiation” phenotype (2). Of eight responses tested for perforin staining, all were ≤10% positive (mean, 3%; range, 0 to 10%) (data not shown).
Analysis of function of CD8+ T lymphocytes ex vivo in patients later after infection.
We and others have previously reported that levels of virus-specific CD8+ lymphocytes (assessed by tetramer) in patients identified outside the setting of acute disease are generally very low, which renders analyses of phenotype and function technically difficult without in vitro manipulation (14, 19, 21). To further address the question of whether the dysfunction of these cells is sustained long term, we screened blood from another 56 HLA-A2-positive subjects from time points outside the first 24 months of infection. This was performed with tetramers containing the four different HLA-A2-restricted peptides exactly as used in subjects A to D (19–21). Where appropriate, we also tested subjects by using HLA-B8 and -B7 tetramers (see Materials and Methods). We obtained samples from a further three such individuals (two PCR negative and one PCR positive) with expansions of HCV-specific HLA-A2 tetramer-positive cells representing >0.1% of CD8+ lymphocytes in whom intracellular cytokine staining could be assessed after stimulation.
Examples of such an analysis (subject E) are shown in Fig. 3a and b. These show, respectively, a profound defect in synthesis of both TNF-α and IFN-γ after peptide stimulation and a relative defect in staining for TNF-α after maximal (PMA-ionomycin) stimulation, compared to CMV and EBV responses. Subject E had been PCR negative after IFN-α treatment 5 years previously, and thus the defect cannot be attributed to ongoing viremia. It was sustained over a period of 1 year, with the relatively large HCV-specific tetramer-positive population (0.3% of CD8+ lymphocytes) maintained in a quiescent state (low in CD38 and HLA class II; similar to the EBV- and CMV-specific populations; data not shown).
Internal and group comparisons between HCV-specific and control responses.
The relative lack of cytokine secretory capacity in HCV-specific CD8+ T lymphocytes was further emphasized by comparisons with EBV- and CMV-specific responses within and between individuals. In Fig. 4 (upper panel), the synthesis of IFN-γ after PMA stimulation from HCV-specific CD8+ lymphocytes is compared with that from EBV- and CMV-specific populations in all of those individuals in whom both responses were present. This shows a marked skewing of the responses in favor of cytokine release from the non-HCV-specific CD8+ populations (P < 0.002). In some individuals (as in subject A), more than one HCV-specific response was present, and both showed a similar phenotype by comparison with CMV or EBV.
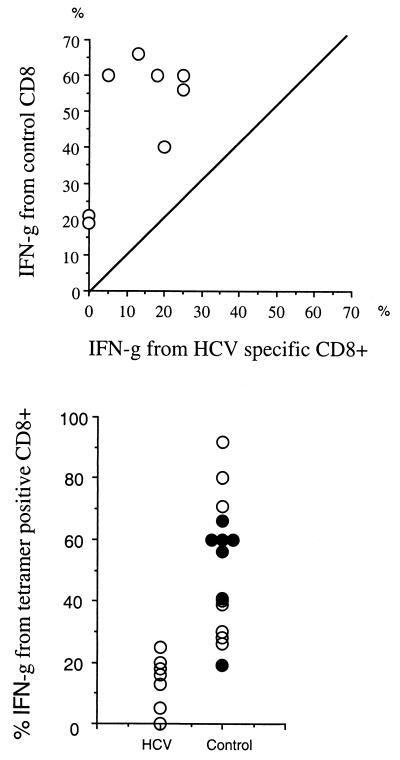
FIG. 4.
Overall comparisons of HCV function and control responses. (Upper panel) Internal comparison. Five HCV antibody-positive subjects (all HCV PCR negative) for whom HCV and control responses were available were tested simultaneously for IFN-γ staining after PMA-ionomycin stimulation, and the proportion of tetramer-positive cells was calculated as in Fig. 2a, upper panels. Each HCV response was compared with the EBV and/or CMV response in the same individual (a total of eight comparisons). (Lower panel) Group comparison. Cytokine synthesis from HCV tetramer-positive populations in seven HCV antibody-positive subjects (five PCR negative) were tested exactly as described above (left-hand group, marked HCV) and compared with EBV and/or CMV responses within themselves and in seven normal controls (right-hand group). CMV and EBV responses from HCV antibody-positive subjects are shown by solid circles, and those from control subjects are shown by open circles.
In Fig. 4 (lower panel), the IFN-γ responses after PMA stimulation are compared between HCV tetramer-positive populations and non-HCV (EBV or CMV) populations in seven HCV antibody-positive (dark dots) and seven control subjects (clear dots). The overall level of responsiveness is again lower at the population level (mean, 13.5% versus 60%; P < 0.0001). There was no significant difference between non-HCV (EBV and/or CMV) responses in HCV antibody-positive and antibody-negative subjects.
DISCUSSION
These data indicate that HCV-specific CD8+ T lymphocytes possess a distinct phenotype, which may be maintained well after virus is cleared from blood. Large populations of HCV-specific CD8+ lymphocytes are induced early and are highly activated, as judged by surface expression of appropriate marker molecules (21) (Fig. 1b), but have various degrees of impairment of antiviral functions, as assessed in vitro. These include an impaired ability to respond to peptide and to mitogen and are most obvious at the level of secretion of antiviral cytokines.
The essential differences in response dynamics or function dictating clearance versus persistence of HCV were not revealed in this study. Relative defects in cytokine secretion were seen in those who had cleared virus in the long term, and levels of intracellular perforin were universally low among HCV-specific CD8+ lymphocytes. It is possible that the essential mechanisms that control HCV in the long term lie outside of these conventional functions or indeed are displayed by some other subset of immune mediators, as has been clearly demonstrated in murine models (26). An alternative explanation is that the circulating CD8+ lymphocytes represent an unusual subset that differs from the truly functional lymphocytes. While this seems likely during chronic hepatitis infection, where compartmentalization in the liver has been observed (23), it seems unlikely in situations in which virus has been cleared and no hepatitis is detectable.
These populations differ from previously identified dysfunctional CD8+ lymphocytes in that the phenotype is sustained (21) and may occur in the presence of adequate CD4+ help (33), and the surface phenotype of the lymphocytes is otherwise normal (e.g., CD45RO high; data not shown) (22). The stability of these populations, compared with the dysfunction seen in lymphocytes undergoing exhaustion (10), indicates a long-lasting influence on function induced by the virus in an antigen-specific manner. It is possible that the inducing environment or potentially some influence on antigen-presenting cell function (15) is responsible. For example, in murine models, a similar sustained phenotype has been seen after vaccination in the presence of interleukin-10 (5). In this context, a description of “stunted” as opposed to “stunned” may be more appropriate. How this may then have an impact on the persistence of HCV remains to be determined, especially in the context of the balance with other mediators of immunity (6, 9). Methods to reverse this defect or to induce new populations of functional T lymphocytes should be explored as part of immunotherapeutic strategies and vaccine design.
ACKNOWLEDGMENTS
Norbert Gruener and Franziska Lechner contributed equally to this paper.
Funding was obtained from the Wellcome Trust, the Wilhelm-Sander-Stiftung and the European Union (5th Framework, HCVacc, grant no. QLK2-CT-1999-00356), the Deutsche Forschungsgemein, the Australian Red Cross, the National Institutes of Health, and the Doris Duke Charitable Foundation.
We are grateful to Jane Collier and the staff of the hepatitis and immunology clinics at the John Radcliffe Hospital for providing patient samples to screen; Mike Bunce for tissue typing; Philip Goulder, Rod Dunbar, Richard Cornall, Victor Appay, and Andrew McMichael for helpful discussions; and A. Morse for preparation of the manuscript. We also thank Carmen Amsel, Barbara Becker, Jutta Döhrmann, and Marion Satzger for excellent technical assistance and personal encouragement.
REFERENCES
- 1.Altman J, Moss P A H, Goulder P, Barouch D, McHeyzer-Williams M, Bell J I, McMichael A J, Davis M M. Direct visualization and phenotypic analysis of virus-specific T lymphocytes in HIV-infected individuals. Science. 1996;274:94–96. [Google Scholar]
- 2.Appay V, Nixon D F, Donahoe S M, Gillespie G M, Dong T, King A, Ogg G S, Spiegel H M, Conlon C, Spina C A, Havlir D V, Richman D D, Waters A, Easterbrook P, McMichael A J, Rowland-Jones S L. HIV-specific CD8(+) T cells produce antiviral cytokines but are impaired in lytic function. J Exp Med. 2000;192:63–75. doi: 10.1084/jem.192.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Callan M, Tan L, Annels N, Ogg G, Wilson J, O'Callaghan C, Steven N, McMichael A, Rickinson A. Direct visualisation of antigen specific CD8+ T cells during the primary immune response to EBV in vivo. J Exp Med. 1998;187:1395–1402. doi: 10.1084/jem.187.9.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cerny A, McHutchinson J, Pasquellini C, Brown M, Brothers M, Grabschied B, Fowler P, Houghton M, Chisari F. CTL response to HCV-derived peptides containing the HLA-A2.1 binding motif. J Clin Investig. 1995;95:521–530. doi: 10.1172/JCI117694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chun S, Daheshia M, Lee S, Eo S K, Rouse B T. Distribution fate and mechanism of immune modulation following mucosal immunisation. J Immunol. 1999;163:2393–2402. [PubMed] [Google Scholar]
- 6.Ciurea A, Klenerman P, Hunziker L, Horvath E, Senn B M, Ochsenbein A F, Hengartner H, Zinkernagel R M. Viral persistence in vivo through selection of neutralizing antibody-escape variants. Proc Natl Acad Sci USA. 2000;97:2749–2754. doi: 10.1073/pnas.040558797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cramp M E, Rossol S, Chokshi S, Carucci P, Williams R, Naoumov N V. Hepatitis C virus-specific T-cell reactivity during interferon and ribavirin treatment in chronic hepatitis C. Gastroenterology. 2000;118:346–355. doi: 10.1016/s0016-5085(00)70217-4. [DOI] [PubMed] [Google Scholar]
- 8.Diepolder H M, Zachoval R, Hoffmann R M, Wierenga E A, Santantonio T, Jung M C, Eichenlaub D, Pape G R. Possible mechanism involving T-lymphocyte response to non-structural protein 3 in viral clearance in acute hepatitis C virus infection. Lancet. 1995;346:1006–1007. doi: 10.1016/s0140-6736(95)91691-1. [DOI] [PubMed] [Google Scholar]
- 9.Farci P, Shimoda A, Coiana A, Diaz G, Peddis G, Melpolder J C, Strazzera A, Chien D Y, Munoz S J, Balestrieri A, Purcell R H, Alter H J. The outcome of acute hepatitis C predicted by the evolution of the viral quasispecies. Science. 2000;288:339–344. doi: 10.1126/science.288.5464.339. [DOI] [PubMed] [Google Scholar]
- 10.Gallimore A, Glitheroe A, Godkin A, Tissot A, Hengartner H, Elliott T, Zinkernagel R. Induction and exhaustion of LCMV-specific CTL visualised using soluble tetrameric MHC-peptide complexes. J Exp Med. 1998;187:1383–1393. doi: 10.1084/jem.187.9.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerlach J, Diepolder H, Jung M-C, Gruener N, Schraut W, Zachoval R, Hoffman R, Schirren C, Santantonio T, Pape G. Recurrence of HCV after loss of virus specific CD4+ T cell response in acute hepatitis C. Gastroenterology. 1999;117:933–941. doi: 10.1016/s0016-5085(99)70353-7. [DOI] [PubMed] [Google Scholar]
- 12.Gillespie G M A, Wills M R, Appay V, O'Callaghan C, Murphy M, Smith N, Sissons P, Rowland-Jones S, Bell J I, Moss P A H. Functional heterogeneity and high frequencies of cytomegalovirus-specific CD8+ T lymphocytes in healthy seropositive donors. J Virol. 2000;74:8140–8150. doi: 10.1128/jvi.74.17.8140-8150.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guidotti L, Rochford R, Chung J, Shapiro M, Purcell R, Chisari F. Viral clearance without destruction of infected cells during acute HBV infection. Science. 1999;284:825–829. doi: 10.1126/science.284.5415.825. [DOI] [PubMed] [Google Scholar]
- 14.He X-S, Rehermann B, Lopez-Labrador F, Boisvert J, Cheung R, Mumm J, Wedermeyer H, Berenguer M, Wright T, Davis M. Quantitative analysis of HCV-specific CD8+ T cells in peripheral blood and liver using peptide-MHC tetramers. Proc Natl Acad Sci USA. 1999;96:5692–5697. doi: 10.1073/pnas.96.10.5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hiasa Y, Horiike N, Akbar S M, Saito I, Miyamura T, Matsuura Y, Onji M. Low stimulatory capacity of lymphoid dendritic cells expressing hepatitis C proteins. Biochem Biophys Res Commun. 1998;249:90–95. doi: 10.1006/bbrc.1998.9089. [DOI] [PubMed] [Google Scholar]
- 16.Klenerman P, Lechner F, Kantzanou M, Ciurea A, Hengartner H, Zinkernagel R. Viral escape and the failure of cellular immune responses. Science. 2000;289:2003a. doi: 10.1126/science.289.5487.2003a. [DOI] [PubMed] [Google Scholar]
- 17.Koziel M J, Dudley D, Afdhal N, Grakoui A, Rice C M, Choo Q L, Houghton M, Walker B D. HLA class I-restricted cytotoxic T lymphocytes specific for hepatitis C virus. Identification of multiple epitopes and characterization of patterns of cytokine release. J Clin Investig. 1995;96:2311–2321. doi: 10.1172/JCI118287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lalvani A, Brookes R, Hambleton S, Britton W J, Hill A V, McMichael A J. Rapid effector function in CD8+ memory T cells. J Exp Med. 1997;186:859–865. doi: 10.1084/jem.186.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lechner F, Gruener N H, Urbani S, Uggeri J, Santantonio T, Kammer A R, Cerny A, Phillips R, Ferrari C, Pape G R, Klenerman P. CD8+ T lymphocyte responses are induced during acute hepatitis C virus infection but are not sustained. Eur J Immunol. 2000;30:2479–2487. doi: 10.1002/1521-4141(200009)30:9<2479::AID-IMMU2479>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 20.Lechner F, Sullivan J, Spiegel H, Nixon D, Ferrari B, Davis A, Borkowski B, Pollack H, Barnes E, Dusheiko G, Klenerman P. Why do cytotoxic T lymphocytes fail to eliminate HCV? Lessons from studies using MHC class I tetramers. Phil Trans R Soc Lond B. 2000;355:1085–1092. doi: 10.1098/rstb.2000.0646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lechner F, Wong D K, Dunbar P R, Chapman R, Chung R T, Dohrenwend P, Robbins G, Phillips R, Klenerman P, Walker B D. Analysis of successful immune responses in persons infected with hepatitis C virus. J Exp Med. 2000;191:1499–1512. doi: 10.1084/jem.191.9.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee P P, Yee C, Savage P A, Fong L, Brockstedt D, Weber J S, Johnson D, Swetter S, Thompson J, Greenberg P D, Roederer M, Davis M M. Characterization of circulating T cells specific for tumor-associated antigens. Nat Med. 1999;5:677–685. doi: 10.1038/9525. [DOI] [PubMed] [Google Scholar]
- 23.Minutello M, Pileri P, Unutmaz D, Censini S, Kuo G, Houghton M, Brunetto M, Bonino F, Abrignani S. Compartmentalization of T lymphocytes to the site of disease: intrahepatic T cells specific for the NS4 protein of HCV in patients with chronic hepatitis C. J Exp Med. 1993;178:17–25. doi: 10.1084/jem.178.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Missale G, Bertoni R, Lamonaca V, Valli A, Massari M, Mori C, Rumi M, Houghton M, Fiaccadori F, Ferrari C. Different clinical behaviours of acute HCV infection are associated with different vigor of the anti-viral T cell response. J Clin Investig. 1996;98:706–714. doi: 10.1172/JCI118842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naoumov N V. Hepatitis C virus-specific CD4(+) T cells: do they help or damage? Gastroenterology. 1999;117:1012–1014. doi: 10.1016/s0016-5085(99)70361-6. [DOI] [PubMed] [Google Scholar]
- 26.Planz O, Ehl S, Furrer E, Horvath E, Brundler M, Hengartner H, Zinkernagel R. A critical role for neutralising antibody-producing B cells, CD4+ cells and interferons in persistent infections of mice with LCMV: implications for adoptive immunotherapy of virus carriers. Proc Natl Acad Sci USA. 1997;94:6874–6879. doi: 10.1073/pnas.94.13.6874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rehermann B, Chang K, McHutchinson J, Kokka R, Houghton M, Chisari F. Quantitative analysis of the peripheral blood CTL response in patients with chronic HCV infection. J Clin Investig. 1996;98:1432–1440. doi: 10.1172/JCI118931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosen H R, Hinrichs D J, Gretch D R, Koziel M J, Chou S, Houghton M, Rabkin J, Corless C L, Bouwer H G. Association of multispecific CD4(+) response to hepatitis C and severity of recurrence after liver transplantation. Gastroenterology. 1999;117:926–932. doi: 10.1016/s0016-5085(99)70352-5. [DOI] [PubMed] [Google Scholar]
- 29.Takaki A, Wiese M, Maertens G, Depla E, Seifert U, Liebetrau A, Miller J L, Manns M P, Rehermann B. Cellular immune responses persist and humoral responses decrease two decades after HCV infection. Nat Med. 2000;6:578–582. doi: 10.1038/75063. [DOI] [PubMed] [Google Scholar]
- 30.Thursz M, Yallop R, Goldin R, Trepo C, Thomas H C. Influence of MHC class II genotype on outcome of infection with hepatitis C virus. The HENCORE group. Hepatitis C European Network for Cooperative Research. Lancet. 1999;354:2119–2124. doi: 10.1016/s0140-6736(99)91443-5. [DOI] [PubMed] [Google Scholar]
- 31.Whelan J A, Dunbar P R, Price D A, Purbhoo M A, Lechner F, Ogg G S, Griffiths G, Phillips R E, Cerundolo V, Sewell A K. Specificity of CTL interactions with peptide-MHC class I tetrameric complexes is temperature dependent. J Immunol. 1999;163:4342–4348. [PubMed] [Google Scholar]
- 32.Wong D, Dudley D, Afdhal N, Dienstag J, Rice C, Wang L, Houghton M, Walker B, Koziel M. Liver derived CTL in HCV infection: breadth and specificity of responses in a cohort of patients with chronic infection. J Immunol. 1998;160:1479–1488. [PubMed] [Google Scholar]
- 33.Zajac A, Blattman J, Murali-Krishna K, Sourdive D, Suresh M, Altman J, Ahmed R. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 1998;188:2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]