Abstract
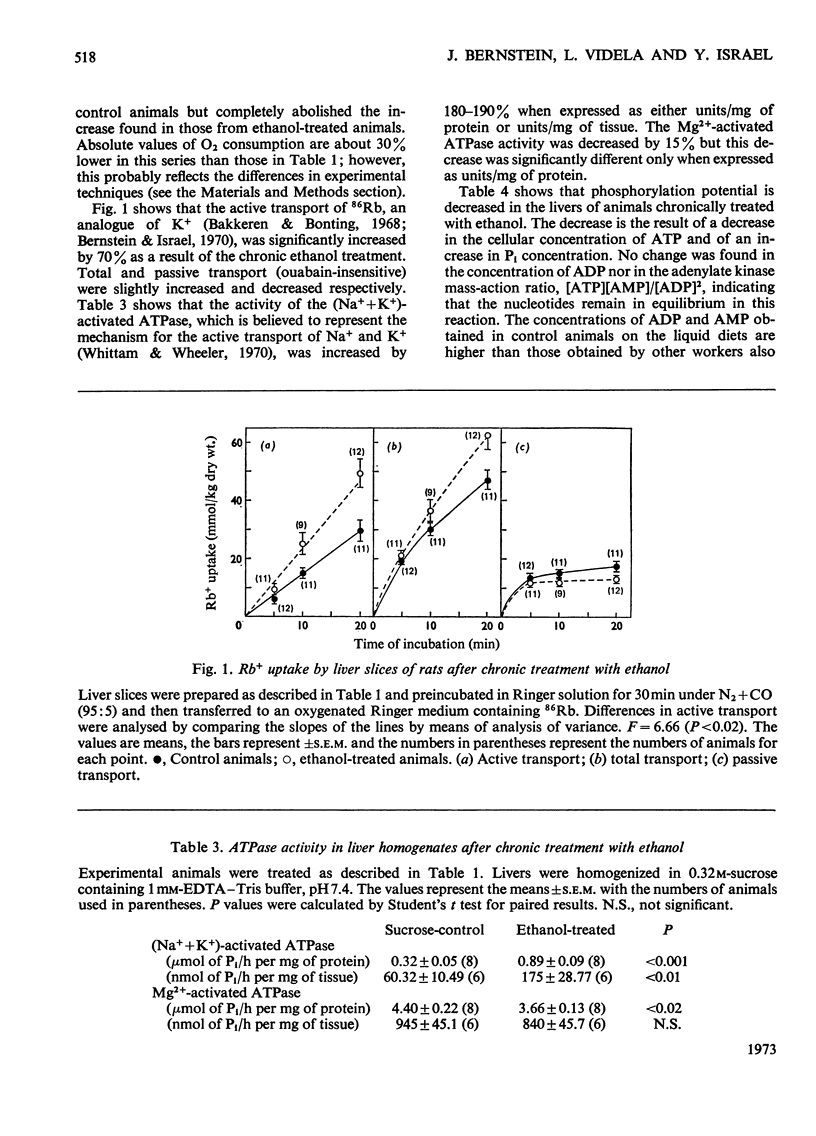
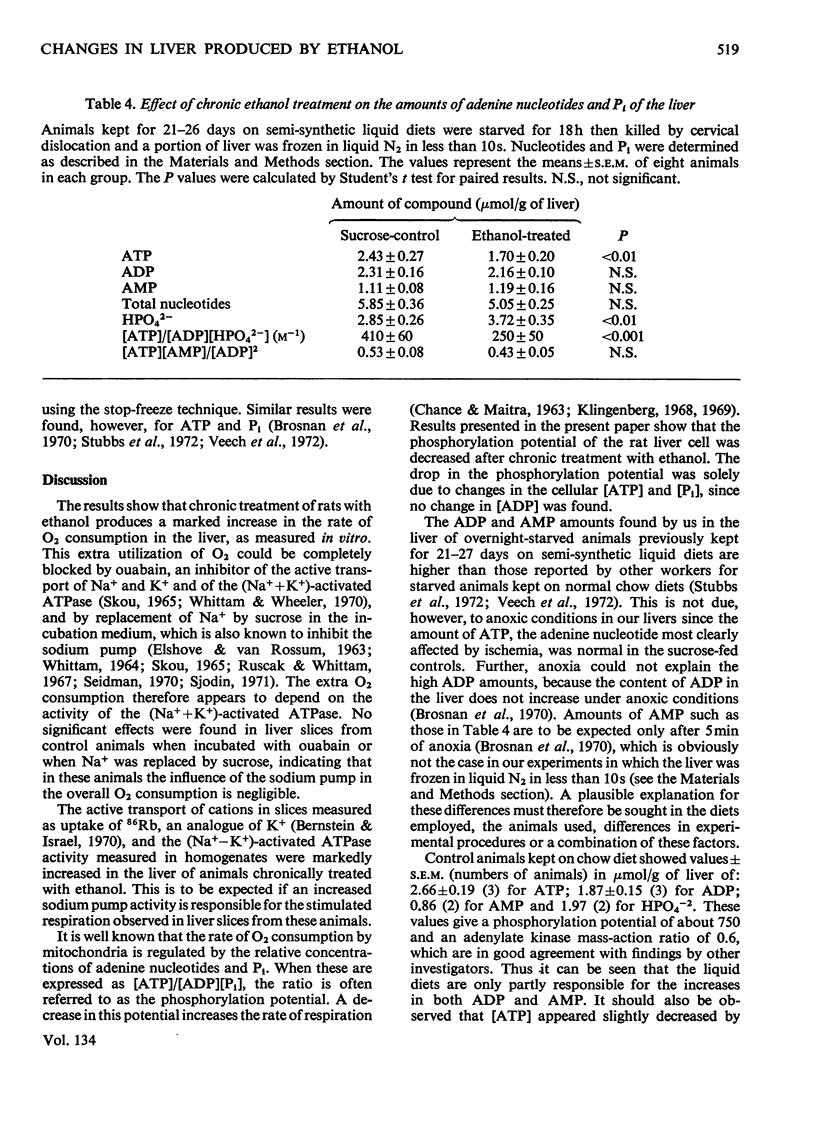
1. Chronic ethanol administration to rats for 21–27 days increases the rate of O2 consumption as measured in liver slices. The extra respiration can be abolished by inhibition of the active transport of Na+ and K+. Dinitrophenol activates the respiratory rate in the liver of the treated animals only in the presence of ouabain. 2. Active (ouabain-sensitive) transport of 86Rb and (Na++K+)-stimulated adenosine triphosphatase activity were increased in the livers of the ethanol-treated animals. 3. Chronic ethanol administration also led to a decrease in the phosphorylation potential ([ATP]/[ADP][Pi]) in the liver cell owing to a decrease in [ATP] and an increase in [Pi]. 4. It is suggested that an increased sodium pump activity is responsible for the increased oxidative capacity and for the insensitivity to dinitrophenol observed in the livers of ethanol-treated animals.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ammon H. P., Estler C. J. Influence of acute and chronic administration of alcohol on carbohydrate breakdown and energy metabolism in the liver. Nature. 1967 Oct 14;216(5111):158–159. doi: 10.1038/216158a0. [DOI] [PubMed] [Google Scholar]
- Bakkeren J. A., Bonting S. L. Studies on (Na+-K+)-activated ATPase. XX. Properties of (Na+-K+)-activated ATPase in rat liver. Biochim Biophys Acta. 1968 Apr 29;150(3):460–466. doi: 10.1016/0005-2736(68)90145-4. [DOI] [PubMed] [Google Scholar]
- Bernstein J. C., Israel Y. Active transport of Rb86 in human red cells and rat brain slices. J Pharmacol Exp Ther. 1970 Aug;174(2):323–329. [PubMed] [Google Scholar]
- Brosnan J. T., Krebs H. A., Williamson D. H. Effects of ischaemia on metabolite concentrations in rat liver. Biochem J. 1970 Mar;117(1):91–96. doi: 10.1042/bj1170091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- EGGLESTON L. V., HEMS R. Separation of adenosine phosphates by paper chromotography and the equilibrium constant of the myokinase system. Biochem J. 1952 Sep;52(1):156–160. doi: 10.1042/bj0520156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELSHOVE A., VAN ROSSUMG NET MOVEMENTS OF SODIUM AND POTASSIUM, AND THEIR RELATION TO RESPIRATION, IN SLICES OF RAT LIVER INCUBATED IN VITRO. J Physiol. 1963 Oct;168:531–553. doi: 10.1113/jphysiol.1963.sp007206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French S. W. Effect of acute and chronic ethanol ingestion on rat liver ATP. Proc Soc Exp Biol Med. 1966 Mar;121(3):681–685. doi: 10.3181/00379727-121-30860. [DOI] [PubMed] [Google Scholar]
- GIBBS R., RODDY P. M., TITUS E. PREPARATION, ASSAY, AND PROPERTIES OF AN NA+- AND K+-REQUIRING ADENOSINE TRIPHOSPHATASE FROM BEEF BRAIN. J Biol Chem. 1965 May;240:2181–2187. [PubMed] [Google Scholar]
- Goldstein D. B., Israel Y. Effects of ethanol on mouse brain (Na+K)-activated adenosine triphosphatase. Life Sci II. 1972 Oct 8;11(19):957–963. [PubMed] [Google Scholar]
- Israel Y. Cellular effects of alcohol. A review. Q J Stud Alcohol. 1970 Jun;31(2):293–316. [PubMed] [Google Scholar]
- Israel Y., Kalant H., LeBlanc E., Bernstein J. C., Salazar I. Changes in cation transport and (Na + K)-activated adenosine triphosphatase produced by chronic administration of ethanol. J Pharmacol Exp Ther. 1970 Aug;174(2):330–336. [PubMed] [Google Scholar]
- Israel Y., Videla L., Macdonald A., Bernstein J. Metabolic alterations produced in the liver by chronic ethanol administration. Comparison between the effects produced by ethanol and by thyroid hormones. Biochem J. 1973 Jun;134(2):523–529. doi: 10.1042/bj1340523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalant H., Mons W., Mahon M. A. Acute effects of ethanol on tissue electrolytes in the rat. Can J Physiol Pharmacol. 1966 Jan;44(1):1–12. doi: 10.1139/y66-001. [DOI] [PubMed] [Google Scholar]
- Knox W. H., Perrin R. G., Sen A. K. Effect of chronic administration of ethanol on (Na+K)-activated ATPase activity in sex areas of the cat brain. J Neurochem. 1972 Dec;19(12):2881–2884. doi: 10.1111/j.1471-4159.1972.tb03827.x. [DOI] [PubMed] [Google Scholar]
- Rubin E., Beattie D. S., Lieber C. S. Effects of ethanol on the biogenesis of mitochondrial membranes and associated mitochondrial functions. Lab Invest. 1970 Dec;23(6):620–627. [PubMed] [Google Scholar]
- Ruscák M., Whittam R. The metabolic response of brain slices to agents affecting the sodium pump. J Physiol. 1967 Jun;190(3):595–610. doi: 10.1113/jphysiol.1967.sp008230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHWARTZ A., LASETER A. H. Observations on the mechanism of action of ouabain on an active transport enzyme system. Life Sci. 1963 Jun;6:363–367. doi: 10.1016/0024-3205(63)90118-8. [DOI] [PubMed] [Google Scholar]
- SEN A. K., POST R. L. STOICHIOMETRY AND LOCALIZATION OF ADENOSINE TRIPHOSPHATE-DEPENDENT SODIUM AND POTASSIUM TRANSPORT IN THE ERYTHROCYTE. J Biol Chem. 1964 Jan;239:345–352. [PubMed] [Google Scholar]
- SKOU J. C. ENZYMATIC BASIS FOR ACTIVE TRANSPORT OF NA+ AND K+ ACROSS CELL MEMBRANE. Physiol Rev. 1965 Jul;45:596–617. doi: 10.1152/physrev.1965.45.3.596. [DOI] [PubMed] [Google Scholar]
- Stubbs M., Veech R. L., Krebs H. A. Control of the redox state of the nicotinamide-adenine dinucleotide couple in rat liver cytoplasm. Biochem J. 1972 Jan;126(1):59–65. doi: 10.1042/bj1260059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veech R. L., Guynn R., Veloso D. The time-course of the effects of ethanol on the redox and phosphorylation states of rat liver. Biochem J. 1972 Apr;127(2):387–397. doi: 10.1042/bj1270387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veech R. L., Raijman L., Krebs H. A. Equilibrium relations between the cytoplasmic adenine nucleotide system and nicotinamide-adenine nucleotide system in rat liver. Biochem J. 1970 Apr;117(3):499–503. doi: 10.1042/bj1170499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videla L., Bernstein J., Israel Y. Metabolic alterations produced in the liver by chronic ethanol administration. Increased oxidative capacity. Biochem J. 1973 Jun;134(2):507–514. doi: 10.1042/bj1340507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videla L., Israel Y. Factors that modify the metabolism of ethanol in rat liver and adaptive changes produced by its chronic administration. Biochem J. 1970 Jun;118(2):275–281. doi: 10.1042/bj1180275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittam R., Ager M. E. The connexion between active cation transport and metabolism in erythrocytes. Biochem J. 1965 Oct;97(1):214–227. doi: 10.1042/bj0970214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittam R., Wheeler K. P. Transport across cell membranes. Annu Rev Physiol. 1970;32:21–60. doi: 10.1146/annurev.ph.32.030170.000321. [DOI] [PubMed] [Google Scholar]


