Abstract
The Francisella tularensis-containing phagosome (FCP) matures to a late-endosome-like phagosome prior to bacterial escape into the cytosols of macrophages, where bacterial proliferation occurs. Our data show that within the first 15 min after infection of primary human monocyte-derived macrophages (hMDMs), ∼90% of the FCPs acquire the proton vacuolar ATPase (vATPase) pump and the lysomotropic dye LysoTracker, which concentrates in acidic compartments, similar to phagosomes harboring the Listeria monocytogenes control. The acquired proton vATPase pump and lysomotropic dye are gradually lost by 30 to 60 min postinfection, which coincides with bacterial escape into the cytosols of hMDMs. Colocalization of phagosomes harboring the iglD mutant with the vATPase pump and the LysoTracker dye was also transient, and the loss of colocalization was faster than that observed for the wild-type strain, which is consistent with the faster escape of the iglD mutant into the macrophage cytosol. In contrast, colocalization of both makers with phagosomes harboring the iglC mutant was persistent, which is consistent with fusion to the lysosomes and failure of the iglC mutant to escape into the macrophage cytosol. We have utilized a fluorescence microscopy-based phagosome integrity assay for differential labeling of vacuolar versus cytosolic bacteria, using antibacterial antibodies loaded into the cytosols of live hMDMs. We show that specific inhibition of the proton vATPase pump by bafilomycin A1 (BFA) blocks rapid bacterial escape into the cytosols of hMDMs, but 30% to 50% of the bacteria escape into the cytosol by 6 to 12 h after BFA treatment. The effect of BFA on the blocking of bacterial escape into the cytosol is completely reversible, as the bacteria escape after removal of BFA. We also show that the limited fusion of the FCP to lysosomes is not due to failure to recruit the late-endosomal fusion regulator Rab7. Therefore, within few minutes of its biogenesis, the FCP transiently acquires the proton vATPase pump to acidify the phagosome, and this transient acidification is essential for subsequent bacterial escape into the macrophage cytosol.
Francisella tularensis is a facultative intracellular bacterium that causes tularemia in many mammalian species, including humans (7, 10, 29). Due to its high infectivity, morbidity, and mortality, F. tularensis has been classified as a category A bioterrorism agent. There are four closely related subspecies of F. tularensis (F. tularensis subsp. tularensis, F. tularensis subsp. holarctica, F. tularensis subsp. mediasiatica, and “F. tularensis subsp. novicida”), and F. tularensis subsp. tularensis is the most virulent to humans (7, 10-12, 26, 28, 29). F. tularensis subsp. novicida is attenuated in humans but replicates robustly within primary human and mouse macrophages, causes disease in mice, and is an attractive model for studying the pathogenesis of tularemia (16, 26, 29). Importantly, intracellular trafficking of F. tularensis subsp. novicida within primary human and mouse macrophages is indistinguishable from that of the two virulent subspecies F. tularensis subsp. tularensis and F. tularensis subsp. holarctica (6, 8, 13, 24, 27). Therefore, F. tularensis subsp. novicida is a useful model for studying intracellular trafficking of F. tularensis subspecies.
Ingested particles are normally processed by macrophages through the “default” endosomal lysosomal degradation pathway, which is one of the first lines of defense against microbial infection (9, 14, 15, 26). The nascent phagosome matures to an early endosome stage regulated by Rab5, followed by maturation into a late endosome regulated by Rab7. The late endosome becomes acidified upon acquisition of the proton vacuolar ATPase (vATPase) pump, which imports hydrogen protons into the phagosome. The acidified late endosome then fuses to lysosomes and becomes a hydrolase-rich phagolysosome, within which most ingested particles are degraded. This process is very rapid and is completed within 15 to 30 min of formation of the phagosome (see reference 26 for a recent review). Therefore, many intracellular pathogens have evolved with idiosyncratic strategies to avoid fatal fates within the phagolysosomes. For example, Listeria monocytogenes escapes from the acidified late-endosome-like phagosome into the cytosol of the host cell. Escape of L. monocytogenes into the cytosol requires the vATPase pump to acidify the phagosome, resulting in activation of the pore-forming hemolysin listeriolysin O (22). Inhibition of the vATPase pump by bafilomycin A1 (BFA) (5, 21) blocks escape of L. monocytogenes from the phagosome into the cytosol of the host cell (4).
The intracellular fates of three F. tularensis groups (F. tularensis subsp. tularensis, the F. tularensis subsp. holarctica-derived live vaccine strain, and F. tularensis subsp. novicida) are similar but unique compared to those of other intracellular pathogens (6, 8, 13, 24, 27). The Francisella-containing phagosome (FCP) matures into a late-endosomal-like stage in which it acquires the late-endosomal marker LAMP-2, followed by bacterial escape into the macrophage cytosol. It is not known whether the FCP acquires the proton vATPase pump and becomes acidified transiently prior to bacterial escape into the cytosol by 30 to 60 min postinfection. The Francisella pathogenicity island (20) gene iglC and its regulator MglA are essential for bacterial escape into the cytosols of human monocyte-derived macrophages (hMDMs) (24, 27). In contrast, recent studies have shown that an F. tularensis mutant defective in the Francisella pathogenicity island gene iglD, which is downstream of iglC, escapes into the cytosols of hMDMs but fails to replicate within the cytosol (25). Therefore, F. tularensis transduces signals into the macrophage cytosol to render it hospitable for proliferation.
Based on earlier ultrastructural studies, disruption of the FCP was thought to occur by 4 to 6 h postinfection (8, 13, 24, 27). Based on these observations, it was determined that the lysomotropic agent LysoTracker, which concentrates in acidic compartments, does not concentrate around F. tularensis within macrophages at 4 h after infection, but earlier stages of the infection have not been examined (8). However, we and others have recently utilized different fluorescence-based assays for differential labeling of vacuolar versus cytosolic bacteria to determine more accurately the kinetics of disruption of the FCP in macrophages (17, 25). The data from two independent groups have shown that the FCP becomes disrupted by 30 to 60 min postinfection (6, 25). Therefore, the earlier study (8) that determined the status of acidification of the FCP presumed the FCP to be intact at 4 h postinfection, while the recent, more accurate studies have shown that the disrupted phagosome equilibrates with the cytosol within 30 to 60 min postinfection. Importantly, phagosome acidification normally occurs within 15 to 30 min after phagosome biogenesis from the plasma membrane.
Therefore, the objectives of the present study were to determine whether the FCPs acquire the vATPase proton pump and become acidified transiently prior to bacterial escape into the cytosol by 15 to 60 min postinfection. Our data show that the FCP transiently acquires the proton vATPase pump and becomes acidified within 15 to 30 min postinfection and that this acidification is essential for escape of F. tularensis into the cytosols of human macrophages, similar to what was found for L. monocytogenes.
MATERIALS AND METHODS
Bacteria and macrophages.
The wild-type F. tularensis subsp. novicida strain U112 and it isogenic iglC and iglD mutants have been described previously (16) and were grown on buffered-charcoal yeast extract agar plates. The tetracycline-resistant plasmid pKK214, encoding green fluorescent protein (GFP), was introduced to the F. tularensis strains (1). Legionella pneumophila strain AA100, expressing GFP, was grown as described previously (18). The hemolytic EGD strain (serovar 1/2a) of L. monocytogenes was grown on blood agar at 37°C for 24 h (2).
To prepare hMDMs, peripheral blood monocytes were isolated from healthy volunteers with no history of tularemia or Legionnaires’ disease and hMDMs were prepared as we described previously (24). Obtaining of blood was approved by the institutional internal review board with a consent form according to standard federal laws.
Confocal laser scanning microscopy.
For confocal microscopy, the hMDMs were allowed to attach to glass coverslips in 24-well culture plates. At different time points after infection, the cells were fixed, permeabilized, and blocked as we described previously (24). Colocalization of the FCPs with the vATPase proton pump was determined using rabbit anti-vATPase antiserum (Chemicon International), as we described previously (3). Anti-Rab7 rabbit antiserum was obtained from Sigma and used at a 1:100 dilution. Anti-rabbit and anti-mouse secondary antibodies conjugated to Alexa Fluor-594 were obtained from Molecular Probes (Eugene, OR).
To load the cytosols of live hMDMs with antibacterial antibodies, we used the glass bead “loading” technique (17, 23) that we have recently adapted for the infection of hMDMs by L. pneumophila (19) and F. tularensis (25). Briefly, the cytoplasms of live hMDMs were loaded with antibacterial antibodies by using glass beads to permeabilize the plasma membrane. Infections were carried out using a multiplicity of infection (MOI) of 10, and the infection was synchronized by centrifugation at 500 × g for 5 min. After 1 h of infection, cells were washed three times with phosphate-buffered saline (PBS) and 400 μl of antibacterial monoclonal antibodies was added on the top of the coverslips, along with an aliquot of 0.5 g of acid-washed sterile glass beads (425 to 600 micrometers; Sigma). The beads were rolled over the cells 12 times, which had no detectable effect on the viability of the cells, as confirmed by trypan blue exclusion. The glass beads were washed off immediately with PBS, and the cells were incubated at 37°C for 1 h to allow a sufficient time for the antibodies to bind. The cells were then fixed and processed with conjugated secondary antibodies. Controls were either cells subjected to the same treatment without the glass beads or cells fixed and permeabilized with 0.05% Triton X-100 for 15 min on ice.
Acidification of the F. tularensis-containing phagosomes was determined using the lysomotropic agent LysoTracker red DND-99 (Molecular Probes). Briefly, hMDMs were grown on glass coverslips in 24-well plates and then used for subsequent invasion assays with live or heat-killed bacteria as described above. Thirty minutes prior to the time point, the cells were washed and incubated with 1 μM LysoTracker/ml, washed three times with PBS, fixed with 4% paraformaldehyde, and then mounted on glass slides for confocal microscopy analysis. Inhibition of the vATPase pump was accomplished by pretreating the cells for 1 h with 25 nM BFA, which was maintained during infection. All confocal microscopy analyses were performed on 100 infected cells from three different coverslips for each time point in each experiment, and all experiments were performed three times. The images shown in the figures are stacks of 12 to 15 1-μm-thick z-series sections.
RESULTS AND DISCUSSION
Early acquisition of the proton vATPase pump by the FCP.
Previous studies have demonstrated that upon entry of F. tularensis into macrophages, the FCP matures into a late-endosome-like stage in which it colocalizes with the LAMP late-endosomal markers, followed by phagosomal disruption during the first few hours of phagosome biogenesis (8). However, since recent and more-accurate studies have shown that the FCPs are disrupted by 30 to 60 min postinfection (6, 25), examination of intact vacuoles for any functional or cellular assay should be done prior to its disruption. It is not known whether the FCP acquires the proton vATPase pump or whether this acquisition is required for disruption of the phagosome and bacterial escape into the cytosol. Since phagosome acidification is exhibited within 15 to 30 min of phagosome biogenesis, we determined whether the FCP acquires the proton vATPase pump within the first 15 to 60 min postinfection. Since L. pneumophila excludes the vATPase proton pump from its phagosome, L. pneumophila was used as a negative control (3). Since phagosomes harboring Listeria monocytogenes acquire the proton vATPase to acidify the phagosome (22) prior to bacterial escape into the host cell cytosol, L. monocytogenes was used as a positive control.
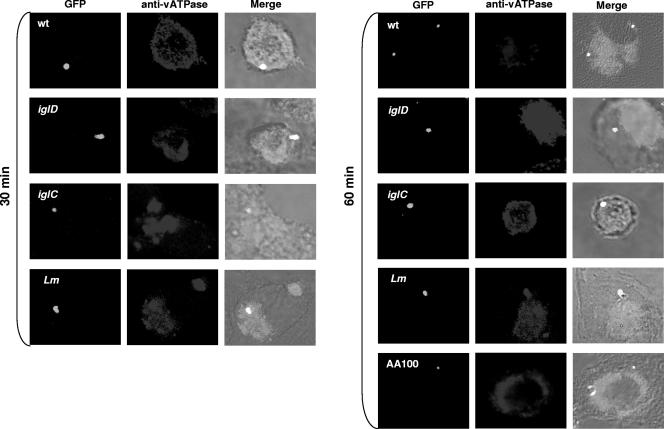
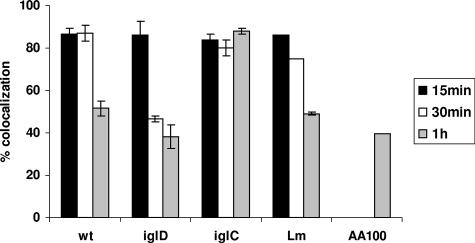
Our data showed that the Legionella-containing phagosomes excluded the proton vATPase pump (Fig. 1) while the Listeria-containing phagosomes acquired the proton vATPase pump (Fig. 1 and 2). Approximately 90% of the wild-type-strain FCPs acquired the proton vATPase pump by 15 min postinfection. Colocalization of the FCP with the vATPase pump decreased to ∼55% by 60 min postinfection (Student's t test; P < 0.002), which coincided with disruption of the FCP and bacterial escape into the cytosol by this time point (Fig. 1 and 2). We conclude that the FCPs acquire the proton vATPase pump transiently within the first 15 min postinfection and that this acquisition is gradually and rapidly lost, which coincides with bacterial escape into the macrophage cytosol.
FIG. 1.
Transient acquisition of the vATPase proton pump by the FCP during early stages of infection. Representative confocal microscopy images of acquisition of the vATPase proton pump by the FCP within hMDMs are shown. Colocalization of the FCP with the vATPase proton pump by the GFP-expressing wild-type (wt) strain and its isogenic iglC or iglD mutant was examined at 15 (not shown), 30, and 60 min postinfection. The wild-type strain AA100 of L. pneumophila, expressing GFP, was used as a negative control, and L. monocytogenes (Lm) was used as a positive control. The images are representatives of 100 infected cells examined from three different coverslips. The results shown are representative of three independent experiments.
FIG. 2.
Quantitative analyses of transient acquisition of the vATPase proton pump by the FCP. Quantification of colocalization of the FCPs of the wild-type (wt) strain and its isogenic iglC or iglD mutant with the vATPase pump at 15, 30, and 60 min postinfection is shown compared to that for the L. pneumophila strain AA100 and L. monocytogenes (Lm) controls. Analyses were based on examination of 100 infected cells from three different coverslips. The results shown are representative of three independent experiments, and the error bars represent standard deviations of triplicate samples.
Persistent acquisition of the vATPase pump by phagosomes harboring the iglC mutant and rapid loss by phagosomes harboring the iglD mutant.
The F. tularensis bicistronic intracellular growth locus iglCD is located within the F. tularensis pathogenicity island (27), and both genes are essential for intracellular proliferation. While the iglC mutant fails to escape from the phagosomes, which fuse to lysosomes (27), the iglD mutant bacteria escape into the macrophage cytosol faster than those of the parental strain but fail to replicate in the cytosols of hMDMs (25). When the iglD mutant-containing phagosomes were examined for acquisition of the vATPase pump, our data showed that ∼80% of them acquired the proton vATPase pump by 15 min postinfection (Fig. 1 and 2), followed by a rapid decrease to ∼40% at 30 min after infection (Fig. 1 and 2), which was significantly faster than the process for the wild-type strain (Student's t test; P < 0.01). This is consistent with the rapid escape of this mutant into the cytosol compared to the result for the wild-type strain (25). In contrast, 85% of the iglC mutant-containing phagosomes acquired and retained the proton vATPase pump at 15 to 60 min (Fig. 1 and 2). We conclude that the FCPs of the iglD mutant acquire the proton vATPase pump, which is lost, faster than those harboring the parental strain, consistent with the faster escape of the iglD mutant into the macrophage cytosol (25). However, the FCPs of the iglC mutant acquire and retain the vATPase pump, consistent with fusion of its phagosomes to lysosomes and the failure of the iglC mutant to escape into the cytosol (25).
F. tularensis is transiently trafficked into acidified vacuoles during early stages of phagosome biogenesis.
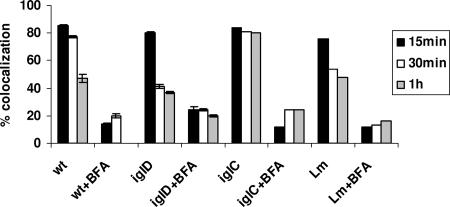
Direct measurements of the acidity of the region surrounding F. tularensis within macrophages at 4 h after infection have shown this region to be neutral and not to acquire the lysomotropic agent LysoTracker, suggesting equilibration of the phagosomal environment with that of the macrophage cytosol (8). However, more-recent studies have shown that the FCPs become disrupted within 30 to 60 min postinfection and that >90% of the FCPs are disrupted by 4 h postinfection (25). Importantly, phagosome acidification of internalized particles is exhibited within 15 to 30 min after formation of the phagosome. Therefore, we monitored the acidification of the FCP at 15 to 60 min by using the lysomotropic agent LysoTracker red DND-99, which concentrates in acidified vesicles and compartments. We used L. monocytogenes as our positive control since its phagosome acquires the vATPase pump (5, 21) to acidify the phagosome, resulting in activation of the pore-forming hemolysin listeriolysin O, which disrupts the phagosome to allow bacterial escape into the cytosol (22). Our results showed that ∼80% of the wild-type-strain FCPs colocalized with the LysoTracker red DND-99 dye at 15 and 30 min postinfection (Fig. 3 and 4). At 1 h after infection of hMDMs with the wild-type strain, most of the FCPs lost colocalization with the LysoTracker dye (Fig. 3 and 4), consistent with the gradual decline of colocalization with the vATPase pump (Fig. 1 and 2) and bacterial escape into the cytosol.
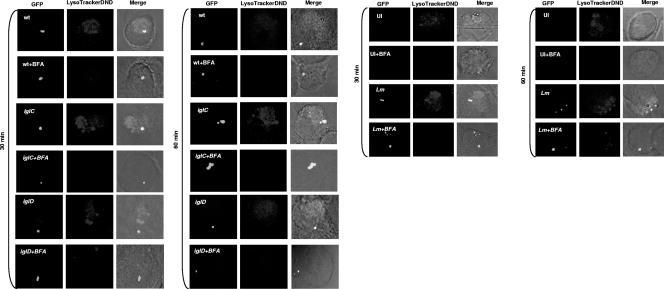
FIG. 3.
Representative confocal microscopy images of transient colocalization of the FCPs with the LysoTracker dye. Colocalization of the FCP with the LysoTracker DND-99 dye by the GFP-expressing wild-type (wt) strain and its isogenic iglC or iglD mutant or by the L. monocytogenes (Lm) positive control at 15, 30, and 60 min postinfection is shown. In some infections, the cells were treated with BFA. Uninfected (UI) cells were used as a negative control, while L. monocytogenes served as a positive control. The images are representatives of 100 infected cells examined from three different coverslips. The results shown are representative of three independent experiments.
FIG. 4.
F. tularensis resides transiently in acidified compartments within hMDMs during early time points of infection. Quantification of colocalization of the LysoTracker DND-99 dye with the FCPs of the wild-type (wt) strain and its isogenic iglC or iglD mutant or by the L. monocytogenes (Lm) positive control at 15, 30, and 60 min postinfection is shown. In some infections, the cells were treated with BFA. Analyses were based on examination of 100 infected cells from three different coverslips. The results shown are representative of three independent experiments, and the error bars represent standard deviations of triplicate samples.
BFA, a specific pharmacological inhibitor of the vATPase pump, was used to inhibit phagosomal acidification. Inhibition of the vATPase pump by BFA (5, 21) blocks phagosomal acidification and escape of L. monocytogenes from the phagosome into the cytosol of the host cell (4). Therefore, we examined whether BFA blocks acidification of the FCP, using L. monocytogenes as a control. Our data showed that treatment of hMDMs with BFA dramatically reduced colocalization of the LysoTracker red DND-99 dye with the FCP, similar to what was found for the L. monocytogenes control (Fig. 3 and 4). Therefore, the wild-type strain of F. tularensis resides transiently in acidified vacuoles during the first 15 to 30 min after infection and this acidification is blocked by BFA, which is a specific inhibitor of the vATPase pump.
Persistent acidification of phagosomes harboring the iglC mutant in contrast with rapid loss of acidification of phagosomes harboring the iglD mutant.
Since phagosomes harboring the iglC and iglD mutants acquired the vATPase pump, which was rapidly lost by the iglD mutant-containing phagosomes, we examined whether the iglC mutant was trafficked into an acidified compartment while the iglD mutant rapidly escaped from the acidified compartment. The data showed that similar to that harboring the wild-type strain, the iglD mutant-containing phagosome colocalized with the LysoTracker red DND-99 dye at 15 min after infection but rapidly lost this colocalization at 30 to 60 min (Fig. 3 and 4). In contrast, most of the iglC mutant-containing phagosomes persistently acquired and retained the LysoTracker red DND-99 dye (colocalization, ∼80%) between the 15- and 60-min time points after infection (Fig. 3 and 4). These data show that while the iglC mutant FCPs are persistently acidified by 15 min postinfection, the iglD mutant FCPs are transiently acidified but the mutant bacteria rapidly escape this acidic compartment. These observations are consistent with the rapid escape of the iglD mutant from the phagosome into the macrophage cytosol (6, 25).
The proton vATPase pump is essential for bacterial escape into the cytosol.
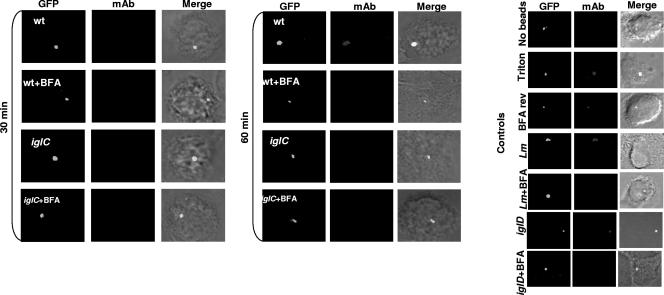
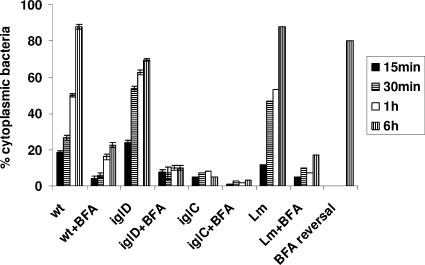
It has recently been shown that by 30 to 60 min after infection, the FCP becomes disrupted and its integrity is compromised, followed by bacterial escape into the cytosol (6, 25). Inhibition of the vATPase pump by the specific inhibitor BFA (5) blocks escape of L. monocytogenes from the phagosome into the cytosol of the host cell (4). Therefore, we examined whether acquisition of the vATPase pump for acidification of the FCP was required for the ability of F. tularensis to escape into the macrophage cytosol. We utilized BFA as the “gold standard” specific inhibitor of the proton vATPase pump (5) to block acidification of the late-endosomal and late-endosomal-like phagosomal compartments to determine the effect on bacterial escape into the macrophage cytosol. The hMDMs were pretreated with 25 nM BFA for 1 h, infected as described above, and examined for FCP integrity by using the glass bead loading technique. In this assay, the macrophage cytosol is loaded with specific antibacterial antibodies after selective permeabilization of the plasma membrane. The infected cells are examined by fluorescence microscopy for phagosome integrity (17), since bacteria in intact phagosomes do not bind the antibodies while cytosolic bacteria or bacteria in compromised phagosomes bind the antibodies (25). For a negative control, infected cells were treated with monoclonal antibody without permeabilization of the plasma membrane by the glass beads, which showed no binding of the antibody to intracellular bacteria for any of the strains tested (Fig. 5 and 6). For the positive control, the cells were permeabilized by Triton X-100, which allowed the antibodies to bind to all intracellular bacteria for all the strains (Fig. 5 and 6). L. monocytogenes was used as a control (Fig. 5 and 6). The data showed that BFA blocked escape of L. monocytogenes into the cytosol. In contrast to untreated cells, wild-type F. tularensis failed to escape rapidly from the phagosome in BFA-treated hMDMs, but 30% to 50% of the bacteria escaped by 6 to 12 h after BFA treatment (Fig. 5 and 6 and data not shown). Pretreatment of wild-type F. tularensis with BFA for 6 h had no effect on viability, escape from the FCP, or intracellular replication within untreated hMDMs (data not shown). Importantly, the effect of BFA was completely reversible (Fig. 5 and 6), since subsequent incubation of BFA-treated, infected hMDMs in BFA-free medium for 1 h reversed the effect of BFA on inhibition of bacterial escape, as evident by subsequent escape of F. tularensis from the FCP into the cytosol after removal of the BFA inhibitor (Fig. 5 and 6). Similar results for the BFA treatment were obtained using NH4Cl to neutralize the cytosolic vesicles and compartments during the first 60 min after infection of hMDMs (data not shown). Therefore, we conclude that functional activity of the proton vATPase pump for acidification the FCP within the first 15 min postinfection is essential for disruption of the FCP and subsequent rapid escape of F. tularensis from the phagosome into the cytosols of hMDMs. Future studies should determine the role of acidification in the biochemical mechanisms that trigger disruption of the phagosomal membrane and bacterial escape into the cytosol.
FIG. 5.
Transient acquisition of the proton vATPase pump by the FCP during early stages of infection is essential for disruption of the FCP and bacterial escape into the macrophage cytosol. Shown are representative confocal microscopy images of the phagosome integrity assay for determining differential binding of specific antibacterial antibodies loaded into the cytosols of live, uninfected hMDMs or hMDMs infected by the wild-type (wt) strain and its isogenic iglC or iglD mutant or by L. monocytogenes (Lm), which was used as a positive control. After 15 min of infection at an MOI 10, the cytosols of live hMDMs were loaded with specific antibacterial monoclonal antibodies (mAbs; red) after 15, 30, and 60 min and 6 h of incubation following the 15-min synchronized infection by GFP-expressing bacteria. Inhibition of the vATPase pump was accomplished by pretreating the cells for 1 h with BFA, which was maintained during infection. For the BFA reversal, the infected, treated cells were incubated for 1 h after removal of BFA, and then bacteria were labeled and examined after 6 h of total incubation. The images are representatives of 100 infected cells examined from three different coverslips. The results shown are representative of three independent experiments.
FIG. 6.
Quantitative analyses for the role of the vATPase proton pump in escape of F. tularensis from the phagosome into the cytosols of hMDMs. The fluorescence microscopy-based phagosome integrity assay was used to differentiate between vacuolar and cytosolic bacteria in untreated cells or cells treated with BFA and infected by the wild-type (wt) strain and its isogenic iglC or iglD mutant or by the L. monocytogenes (Lm) positive control. The cells were examined at 15, 30, and 60 min and 6 h of incubation following the 15-min synchronized infection. In some BFA-treated, infected cells, BFA was removed and the cells were incubated for 1 h to examine whether the effect of BFA treatment on the blocking of bacterial escape from the phagosome was reversible (BFA reversal). Quantification was based on examination of 100 infected cells from three different coverslips for accessibility of intracellular bacteria to the antibodies loaded into the macrophage cytosol. The error bars represent standard deviations of triplicate samples, and the results shown are representative of three independent experiments.
Inhibition of the vATPase proton pump blocks rapid escape of the iglD mutant into the macrophage cytosol.
Since the iglD mutant escapes from the FCP faster than the parental strain but fails to replicate in the cytosols of hMDMs (25), we examined whether the rapid escape of the iglD mutant from the phagosome was blocked by inhibiting the vATPase proton pump by BFA. Since the iglC mutant does not escape from the phagosome, it was included as a control. The data showed that inhibition of the vATPase proton pump by BFA blocked rapid escape of the iglD mutant into the cytosols of hMDMs (Fig. 5 and 6). As expected, there was no detectable effect of inhibition of the vATPase proton pump on the failure of the iglC mutant control to escape from the phagosome (Fig. 5 and 6).
Taken together, these data indicate that the functional activity of the vATPase proton pump for acidification of the FCP is essential for rapid escape of F. tularensis into the macrophage cytosol.
Rab7 is recruited transiently to the FCP.
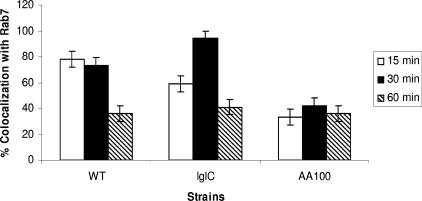
The main regulator of fusion of late endosomes to lysosomes is the Rab7 GTPase. Whether the limited fusion of the FCP to lysosomes is due to failure to recruit Ra7 is not known. We utilized confocal microscopy using anti-Rab7 antiserum to examine whether Rab7 was recruited to the FCP prior to bacterial release to the cytoplasm. Our data show that similar to the kinetics of recruitment of other late-endosomal markers, such as LAMP1 and LAMP2, Rab7 was transiently recruited to the FCP at 15 to 30 min and was lost by 60 min postinfection, which coincides with bacterial escape to the cytosol (Fig. 7). The FCP harboring the iglC mutant positive control, which fuses to lysosomes, acquired Rab7 at a much higher frequency (>90%) by 30 min postinfection, but the marker was lost by 60 min postinfection, consistent with fusion to the lysosomes that do not have Rab7 (Fig. 7). Phagosomes harboring the L. pneumophila strain AA100 negative control showed minimal acquisition of Rab7 (Fig. 7).
FIG. 7.
Quantitative analyses of Rab7 recruitment to the FCP within hMDMs. Colocalization of the FCP with Rab7 was determined by labeling with anti-Rab7 rabbit antisera at 15 to 60 min after infection of hMDMs at an MOI of 10. Quantification was based on examination of 100 infected cells from three different coverslips. WT, wild type.
Disruption of the FCP was thought to occur by 4 to 6 h postinfection when examined in earlier ultrastructural studies that could detect only major structural alterations in the integrity of the phagosome (8, 13, 24, 27). Therefore, the lysomotropic agent LysoTracker has been found not to concentrate around F. tularensis within macrophages at 4 h after infection, but earlier stages of the infection were not examined in these reported studies (8). However, acidification of the phagosome is expected to occur within the first 15 to 30 min after biogenesis of the phagosome from the plasma membrane. Data from two independent groups utilizing fluorescence microscopy-based assays for differential labeling of vacuolar versus cytosolic bacteria have shown that the FCP becomes disrupted by 30 to 60 min postinfection (6, 25). Therefore, the earlier study (8) that has determined the status of acidification of the FCP presumed the FCP to be intact at 4 h postinfection, while the recent, more accurate studies have shown that the disrupted phagosome equilibrates with the cytosol within 30 to 60 min postinfection (6, 25). Our current data show that within the first 15 min postinfection, the FCPs acquire the proton vATPase pump transiently and colocalize with the LysoTracker dye, which concentrates in acidic compartments. Both markers are gradually lost from the FCP by 30 to 60 min postinfection, which coincides with disruption of the FCPs and bacterial egress into the cytosol within 30 to 60 min postinfection. Inhibition of the proton vATPase pump by the specific inhibitor BFA abolishes the ability of F. tularensis to escape into the cytosols of hMDMs, but 30% to 50% of the bacteria gradually and slowly escape by 6 to 12 h after BFA treatment. Thus, acquisition of the proton vATPase pump and acidification of the FCP are essential for disruption of the FCP and escape of F. tularensis into the macrophage cytosol. Our data also show that the limited fusion of the FCP to lysosomes is not due to failure to acquire the Rab7 GTPase, which controls fusion of late endosomes to lysosomes. We speculate that the FCP does not block fusion to the lysosomes but, rather, most of the organisms escape into the cytosol upon phagosome acidification and prior to lysosomal fusion. Our data suggest that at the cellular level, trafficking of F. tularensis and its subsequent escape into the cytosol may resemble that of L. monocytogenes, but the biochemical mechanisms involved are likely to be different. Interestingly, genomic analyses of F. tularensis have indicated the absence of potential hydrolytic enzymes involved in lysis of eukaryotic membranes. Whether IglC and/or IglD is directly involved in lysis of the FCP membrane is not known. We speculate that the proton vATPase pump is essential for activation of a bacterial protein that disrupts the FCP or that the acidic environment within the FCP is essential for triggering specific bacterial gene expression involved in disruption of the FCP. Future studies are directed at dissecting the molecular and biochemical mechanisms involved in disruption of the acidified FCP.
Acknowledgments
Y.A.K. is supported by Public Health Service Awards R01AI065974 and R01AI069321 from NIAID and by the commonwealth of Kentucky Research Challenge Trust Fund. M.S. is supported by the Ministry of Science, Education and Sports of the Republic of Croatia (062-0621273-0950).
Editor: A. J. Bäumler
Footnotes
Published ahead of print on 7 April 2008.
REFERENCES
- 1.Abd, H., T. Johansson, I. Golovliov, G. Sandstrom, and M. Forsman. 2003. Survival and growth of Francisella tularensis in Acanthamoeba castellanii. Appl. Environ. Microbiol. 69600-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abram, M., and M. Doric. 1997. Primary Listeria monocytogenes infection in gestating mice. Folia Microbiol. (Praha) 4265-71. [DOI] [PubMed] [Google Scholar]
- 3.Asare, R., and Y. Abu Kwaik. 2007. Early trafficking and intracellular replication of Legionella longbeachaea within an ER-derived late endosome-like phagosome. Cell. Microbiol. 91571-1587. [DOI] [PubMed] [Google Scholar]
- 4.Beauregard, K. E., K. D. Lee, R. J. Collier, and J. A. Swanson. 1997. pH-dependent perforation of macrophage phagosomes by listeriolysin O from Listeria monocytogenes. J. Exp. Med. 1861159-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bowman, E. J., A. Siebers, and K. Altendorf. 1988. Bafilomycins: a class of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells. Proc. Natl. Acad. Sci. USA 857972-7976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Checroun, C., T. D. Wehrly, E. R. Fischer, S. F. Hayes, and J. Celli. 2006. Autophagy-mediated reentry of Francisella tularensis into the endocytic compartment after cytoplasmic replication. Proc. Natl. Acad. Sci. USA 10314578-14583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi, E. 2002. Tularemia and Q fever. Med. Clin. N. Am. 86393-416. [DOI] [PubMed] [Google Scholar]
- 8.Clemens, D. L., B. Y. Lee, and M. A. Horwitz. 2004. Virulent and avirulent strains of Francisella tularensis prevent acidification and maturation of their phagosomes and escape into the cytoplasm in human macrophages. Infect. Immun. 723204-3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duclos, S., and M. Desjardins. 2000. Subversion of a young phagosome: the survival strategies of intracellular pathogens. Cell. Microbiol. 2365-377. [DOI] [PubMed] [Google Scholar]
- 10.Ellis, J., P. C. Oyston, M. Green, and R. W. Titball. 2002. Tularemia. Clin. Microbiol. Rev. 15631-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forsman, M., G. Sandstrom, and B. Jaurin. 1990. Identification of Francisella species and discrimination of type A and type B strains of F. tularensis by 16S rRNA analysis. Appl. Environ. Microbiol. 56949-955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forsman, M., G. Sandstrom, and A. Sjostedt. 1994. Analysis of 16S ribosomal DNA sequences of Francisella strains and utilization for determination of the phylogeny of the genus and for identification of strains by PCR. Int. J. Syst. Bacteriol. 4438-46. [DOI] [PubMed] [Google Scholar]
- 13.Golovliov, I., V. Baranov, Z. Krocova, H. Kovarova, and A. Sjostedt. 2003. An attenuated strain of the facultative intracellular bacterium Francisella tularensis can escape the phagosome of monocytic cells. Infect. Immun. 715940-5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hackstadt, T. 2000. Redirection of host vesicle trafficking pathways by intracellular parasites. Traffic 193-99. [DOI] [PubMed] [Google Scholar]
- 15.Kahn, R. A., H. Fu, and C. R. Roy. 2002. Cellular hijacking: a common strategy for microbial infection. Trends Biochem. Sci. 27308-314. [DOI] [PubMed] [Google Scholar]
- 16.Lauriano, C. M., J. R. Barker, F. E. Nano, B. P. Arulanandam, and K. E. Klose. 2003. Allelic exchange in Francisella tularensis using PCR products. FEMS Microbiol. Lett. 229195-202. [DOI] [PubMed] [Google Scholar]
- 17.McNeil, P. L., and E. Warder. 1987. Glass beads load macromolecules into living cells. J. Cell Sci. 88669-678. [DOI] [PubMed] [Google Scholar]
- 18.Molmeret, M., D. Bitar, L. Han, and Y. Abu Kwaik. 2004. Disruption of the phagosomal membrane and egress of Legionella pneumophila into the cytoplasm during late stages of the intracellular infection of macrophages and Acanthamoeba polyphaga. Infect. Immun. 724040-4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Molmeret, M., M. Santic, R. Asare, R. A. Carabeo, and Y. Abu Kwaik. 2007. Rapid escape of the dot/icm mutants of Legionella pneumophila into the cytosol of mammalian and protozoan cells. Infect. Immun. 753290-3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nano, F. E., N. Zhang, S. C. Cowley, K. E. Klose, K. K. Cheung, M. J. Roberts, J. S. Ludu, G. W. Letendre, A. I. Meierovics, G. Stephens, and K. L. Elkins. 2004. A Francisella tularensis pathogenicity island required for intramacrophage growth. J. Bacteriol. 1866430-6436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poole, B., and S. Ohkuma. 1981. Effect of weak bases on the intralysosomal pH in mouse peritoneal macrophages. J. Cell Biol. 90665-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Portnoy, D. A., T. Chakraborty, W. Goebel, and P. Cossart. 1992. Molecular deteminants of Listeria monocytogenes pathogenesis. Infect. Immun. 601263-1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reddy, A., E. V. Caler, and N. W. Andrews. 2001. Plasma membrane repair is mediated by Ca(2+)-regulated exocytosis of lysosomes. Cell 106157-169. [DOI] [PubMed] [Google Scholar]
- 24.Santic, M., M. Molmeret, and Y. Abu Kwaik. 2005. Modulation of biogenesis of the Francisella tularensis subsp. novicida-containing phagosome in quiescent human macrophages and its maturation into a phagolysosome upon actiavtion by IFN-gamma. Cell. Microbiol. 7957-967. [DOI] [PubMed] [Google Scholar]
- 25.Santic, M., M. Molmeret, J. R. Barker, K. E. Klose, A. Dekanic, M. Doric, and Y. Abu Kwaik. 2007. A Francisella tularensis pathogenicity island protein essential for bacterial proliferation within the host cell cytosol. Cell. Microbiol. 92391-2403. [DOI] [PubMed] [Google Scholar]
- 26.Santic, M., M. Molmeret, K. E. Klose, and Y. Abu Kwaik. 2006. Francisella tularensis travels a novel, twisted road within macrophages. Trends Microbiol. 1437-44. [DOI] [PubMed] [Google Scholar]
- 27.Santic, M., M. Molmeret, K. E. Klose, S. Jones, and Y. Abu Kwaik. 2005. The Francisella tularensis pathogenicity island protein IglC and its regulator MglA are essential for modulating phagosome biogenesis and subsequent bacterial escape into the cytoplasm. Cell. Microbiol. 7969-979. [DOI] [PubMed] [Google Scholar]
- 28.Saslaw, S., H. T. Eigelsbach, J. A. Prior, H. E. Wilson, and S. Carhart. 1961. Tularemia vaccine study. II. Respiratory challenge. Arch. Intern. Med. 107134-146. [DOI] [PubMed] [Google Scholar]
- 29.Tarnvik, A., M. Eriksson, G. Sandstrom, and A. Sjostedt. 1992. Francisella tularensis—a model for studies of the immune response to intracellular bacteria in man. Immunology 76349-354. [PMC free article] [PubMed] [Google Scholar]