Abstract
Although many animals use the Earth’s magnetic field for orientation and navigation1,2, the precise biophysical mechanisms underlying magnetic sensing have been elusive. One theoretical model proposes that geomagnetic fields are perceived by chemical reactions involving specialized photoreceptors3. But the specific photoreceptor involved in such magnetoreception has not been demonstrated conclusively in any animal. Here we show that the UV-A/blue light photoreceptor CRYPTOCHROME (CRY) is necessary for light-dependent magnetosensitive responses in Drosophila melanogaster. In a binary-choice behavioural assay for magnetosensitivity, wild-type flies exhibit significant naïve and trained responses to a magnetic field under full-spectrum light (~300–700 nm) but do not respond to the field when wavelengths in the CRY-sensitive, UV-A/blue part of the spectrum (<420 nm) are blocked. Remarkably, CRY-deficient cry0 and cryb flies do not show either naïve or trained responses to a magnet field under full-spectrum light. Moreover, CRY-dependent magnetosensitivity does not require a functioning circadian clock. Our work provides the first genetic evidence for a CRY-based magnetosensitive system in any animal.
The ability of an animal to detect geomagnetic fields has substantial biological relevance as it is used by many invertebrate and vertebrate species for orientation and navigation purposes, including homing, building activity and long-distance migration2,4. Three general modes of magnetoreception have been proposed5. One mode is electromagnetic induction by the Earth’s magnetic field as may occur in electrosensitive marine fish, although there is scant evidence supporting such sensing. The two other modes, for which experimental evidence does exist, are a magnetite-based process6–8 and chemical-based reactions9,10 that are modulated by magnetic fields. One chemical model of magnetoreception proposes that magnetic information is transmitted to the nervous system through the light-induced product of magnetically sensitive radical-pair reactions in specialized photoreceptors3.
CRYs are flavoproteins that have been postulated to generate magnetosensitive radical pairs that could provide a photoinduced electron transfer reaction for the detection of magnetic fields3. CRY proteins are best known for their roles in the regulation of circadian clocks11,12 and can be categorized into two groups based on current phylogenetic and functional relationships13,14. Drosophila-like CRYs are sensitive to light in the UV-A/blue range15 and function primarily as photoreceptors that synchronize (entrain) circadian clocks. Vertebrate-like CRYs, which have also been found in every non-drosophilid insect so far examined14, do not appear to be directly light sensitive. Instead, vertebrate-like CRY proteins are potent repressors of the CLOCK and CYCLE/BMAL1 transcription factors, which as heterodimers, drive the intracellular transcriptional feedback loop of the circadian clock mechanism in all animals studied.
Although there is good behavioural evidence for the involvement of short-wavelength photoreceptors in the detection of a geomagnetic field5,16–18, an essential link between CRY and magnetoreception has not been established in any animal. Drosophila is ideally suited to investigate a role for CRY as a magnetoreceptor, because they only have the light-sensitive CRY14, whose action spectrum peaks in the UV-A range (350 – 400 nm) with a plateau in the near blue (430 – 450 nm)19,20. Importantly, flies that lack CRY21 or harbor the severely hypomorphic cryb mutation22,23 can be used to evaluate the role of CRY in magnetosensitive responses.
We initiated our studies by developing a novel behavioural assay for magnetosensitivity in Drosophila (Fig. 1a). In this illuminated apparatus, flies experience a magnetic field generated by an electric coil system and display their magnetosensitivity in a binary-choice T-maze. The two-coil system is ideal for behavioural studies of magnetosensitivity, because it produces a magnetic field on one side of the T-maze, while producing no field on the opposite side. This design eliminates non-magnetic differences such as heat generated by the electric coils between sides during test sessions24. Flies were tested either for their response to the magnetic field in the naïve state (naïve group) or following a training session pairing the field with sucrose reward (trained group).
Figure 1. Behavioural apparatus for magnetosensitivity and behavioural responses in different Drosophila strains.
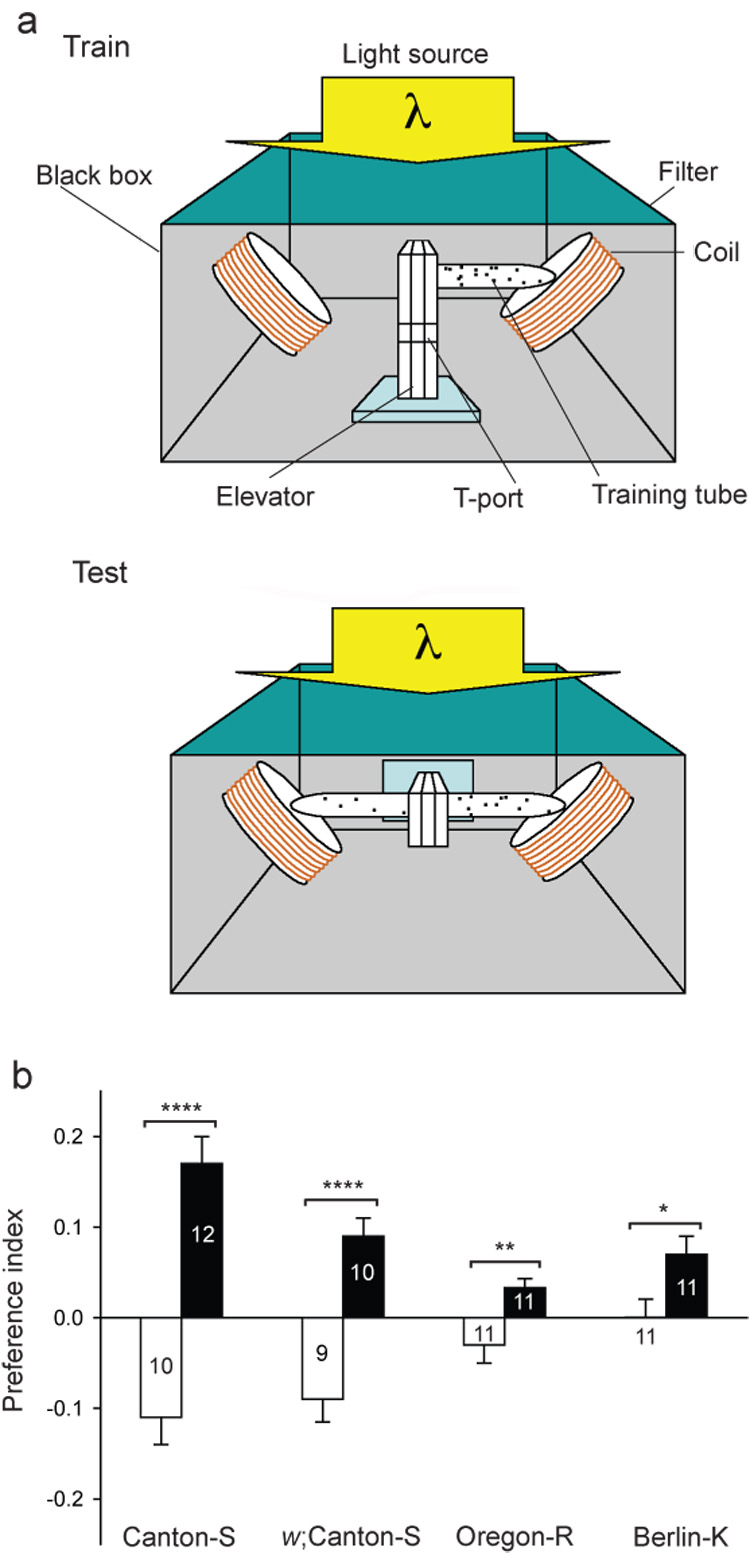
a, Behavioral apparatus for magnetosensitivity. Upper (Train), frontal view of the choice chamber apparatus positioned for training. The chamber apparatus consisted of a training tube, an elevator to transfer flies, and a duel-choice point (T-port). For training, the apparatus, with training tube only, was placed upright in an illuminated black box containing a two-coil system. A population of flies (dots) was loaded into the training tube with or without sucrose reinforcement and a magnetic field. Lower (Test), frontal view of the choice chamber apparatus positioned for testing. For testing, the apparatus, with tubes attached to the T-port (T-maze), was rotated to the horizontal, and flies were transferred from elevator section to the T-port. Wavelength-dependence was examined using long-wavelength pass filters.
b, Drosophila strains vary in their behavioural response to a magnetic field under full-spectrum light (Fig. 2a). Bars show preference index of the naïve (white) or trained (black) groups. Numbers are groups tested. Values are mean ± s.e.m. *, P<0.05; **, P<0.01; ****, P<0.0001.
Wild-type Canton-S, white-eyed w;Canton-S, Oregon-R-S, and Berlin-K strains all developed a learned preference for a magnetic field (Fig. 1b). The trained groups in the two Canton-S lines showed the greatest response to the field (P=0.002, one-way ANOVA) and were the only ones to show a naïve avoidance of the field (P<0.0001, one-sample t-test). Thus, Drosophila consistently show magnetosensitivity that varies in magnitude in a strain-dependent manner. The similarity of behavioural responses between red-eyed, wild-type Canton-S flies and white-eyed w;Canton-S flies shows that eye color does not substantially alter behavioural responses to the magnetic field.
Because wild-type Canton-S flies showed the most robust trained and naïve responses of the strains tested, we used them to determine whether the magnetic responses we observed were light-dependent. We assayed naïve and trained Canton-S flies under different long-wavelength pass filters that transmitted wavelengths of light at > 500 nm, > 420 nm, or > 400 nm (Fig. 2a). In contrast to flies assayed under full-spectrum light (Fig. 1b and Fig. 2a), flies did not exhibit either naïve or trained responses to the field when wavelengths <420 nm were blocked (Fig 2b). Because the filter which blocked light <420 nm, also caused a 13% decrease in total irradiance (Fig. 2c, red line), we examined whether the filter-induced lack of behavioural responses to the magnetic field was secondary to the decrement in irradiance. When Canton-S flies were studied under full-spectrum light, with a total irradiance level lower than that imposed by the filter (Fig. 2c, blue line), the flies still showed significant naïve (P=0.0005, one-sample t-test) and trained responses to the magnetic field (Fig. 2d). Thus, the filter-induced loss of behavioural responses to the magnetic field is due to the loss of short wavelength light.
Figure 2. Short-wavelength light is required for magnetosensitivity in Canton-S flies.
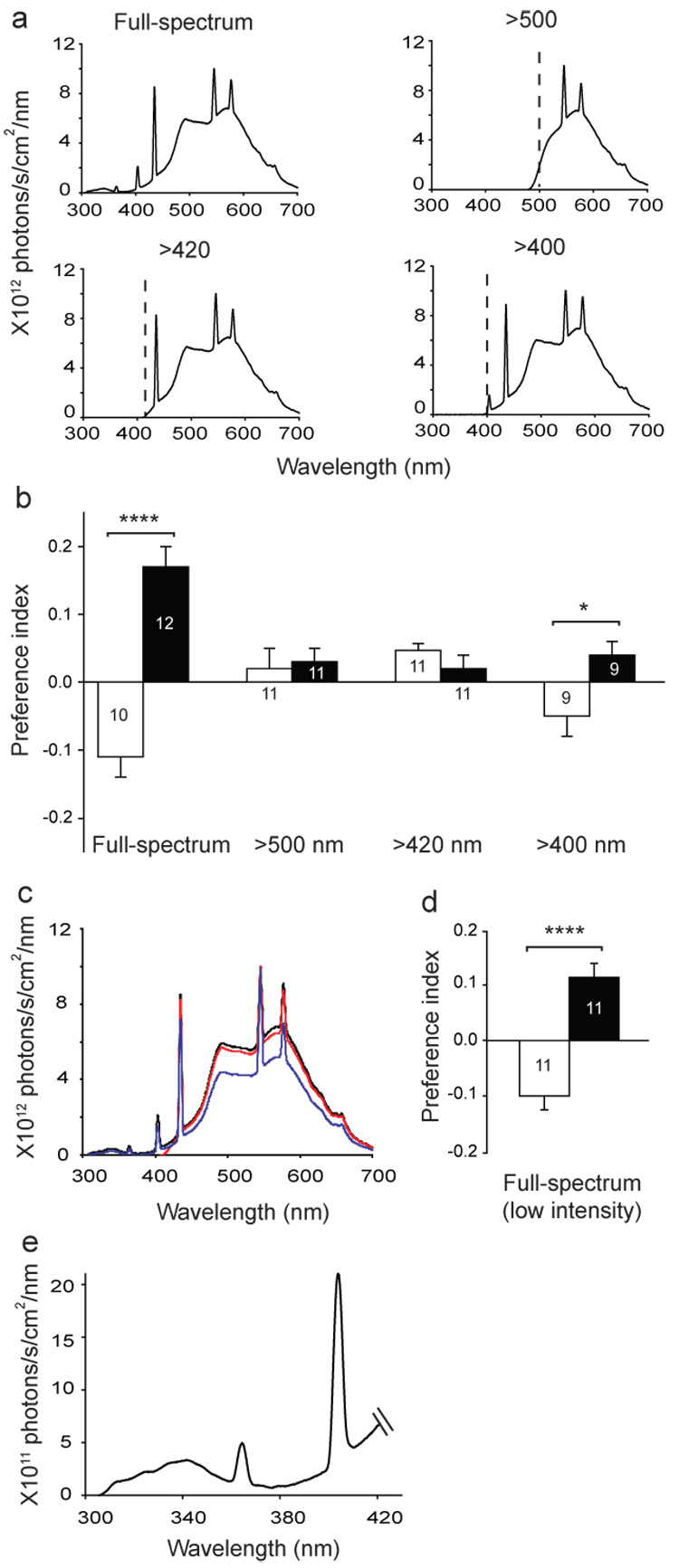
a, Irradiance curves for different light conditions. Light measurements were taken inside the training/test tube. Dashed lines, cut-off points of the blocking filters.
b, Wavelength-dependence of magnetic response. Bars show preference index of the naïve (white) or trained (black) groups. Full-spectrum data are from Figure 1b. Numbers are groups tested. *, P<0.05; ****, P<0.0001.
c, Irradiance curves depicting full-spectrum light (black line), light >420 nm (red line), and full-spectrum light with reduced total irradiance (full-spectrum, low intensity; blue line).
d, Canton-S flies still elicited significant responses to the magnetic field under full-spectrum, low intensity light. ****, P<0.0001.
e, Irradiance values from 300 – 420 nm. Data are expanded scale from full-spectrum pattern in panel a. The irradiance values in UV-A/blue in our studies (300 – 420 nm) are in line with those reported for Drosophila CRY function, using other biological responses19,20; that is, range of 1011 to 1012 photons/s/cm2/nm. Values from b, d, e are mean ± s.e.m.
Behavioural responses to the magnet were partially restored when 400–420 nm light was included (Fig. 2b), which is consistent with the action spectrum of Drosophila CRY tailing into the near blue19, and, as expected, the trained response was weaker than under full-spectrum light (full spectrum vs. > 400 nm, P<0.001, Student’s t-test). This wavelength-dependent effect of the magnetic field on behaviour suggests that Drosophila has a photoreceptor-based magnetosensitive system. Moreover, because the response to the magnetic field requires UV-A/blue light (<420 nm) (Fig. 2e), these data are consistent with the hypothesis that CRY can function as a magnetoreceptor in Drosophila.
We next used CRY-deficient cry0 mutant flies to directly examine whether CRY is required for magnetosensitive behaviour. We tested two of the newly generated cry0 fly lines, because in cry0 flies, the entire cry coding sequence has been replaced with mini-white+ by homologous recombination, ensuring that, unlike in the more commonly used CRY-defective cryb flies, there is no possibility of residual CRY activity21. In addition, the three cry0 fly lines (cry01 through cry03) were backcrossed independently into a w1118 background21. Thus, we were able to use the appropriate w1118 control flies to test the contribution of the cry gene in magnetosensitive behaviour.
Control w1118 flies exhibited a clear naïve preference for, rather than avoidance of, the magnetic field (Fig. 3a). The difference in the direction of the naïve response to the magnetic field between Canton-S flies and the w1118 line re-emphasizes the importance of controlling for genetic background for studies of magnetosensitivity in flies. Nonetheless, like Canton-S flies, the naïve response of w1118 flies to the magnetic field was light dependent; the naïve preference for the magnetic field was abolished in the absence of UV-A/blue light (<420 nm) (Fig. 3a).
Figure 3. Drosophila CRY mediates magnetosensitivity.
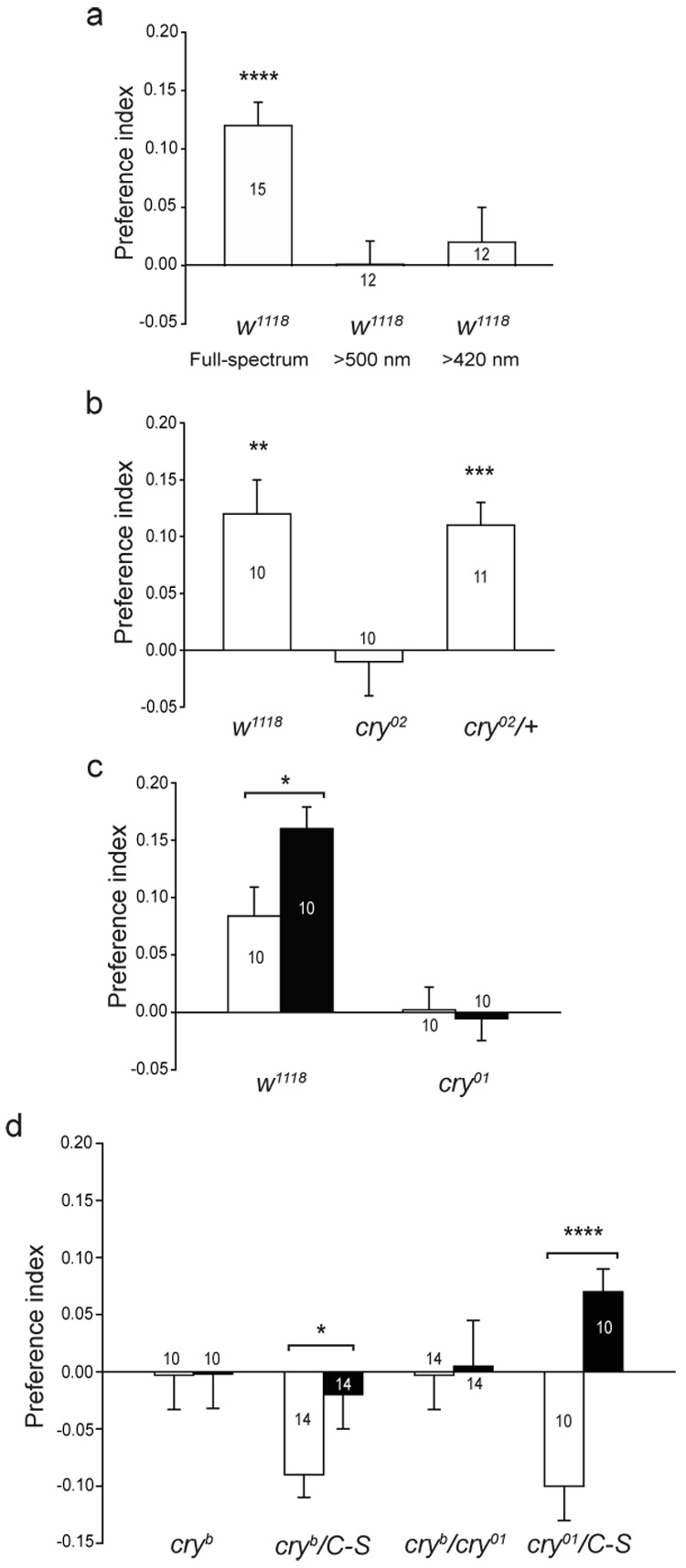
a, Magnetosensitivity in w1118 flies depends on UV-A/blue light. Bars show PI for naïve responses under full-spectrum light, and light > 500 nm and > 420 nm. Numbers are groups tested. ****, P<0.0001.
b, Naïve response to a magnetic field is impaired in CRY-deficient cry02 flies, but not in cry02/+ flies. Bars show preference index values for naïve responses. **, P<0.01; ***, P<0.001.
c, Naïve and trained responses to a magnetic field are impaired in CRY-deficient cry01 flies. Bars are preference index values for naïve (white) and trained (black) groups. *, P<0.05.
d, Naïve and trained responses to a magnetic field are impaired in homozygous cryb and transheterozygous cryb/cry01 flies. Bars show preference index values for naïve (white) or trained (black) groups. C-S, Canton-S. *, P<0.05; ****, P<0.0001. Values from a–d are mean s.e.m.
Homozygous cry02 flies lacking CRY did not show a naïve response to the magnet under full-spectrum light, in contrast to the significant naïve responses manifested by both w1118 and heterozygous cry02/+ flies (Fig. 3b). Training control w1118 flies to prefer the magnetic field under full-spectrum light significantly enhanced their naïve preference for the field (Fig. 3c). In contrast, homozygous cry01 flies did not show either a naïve preference for the field (like cry02 flies) or an enhanced preference for the field after training (Fig. 3c). The loss of the response to the magnetic field in the CRY-deficient flies resembled the behaviour when w1118 flies are deprived of UV-A/blue light (Fig. 3a), which is consistent with CRY being the relevant light sensor. These data using two cry null strains strongly suggest that both naïve and trained responses to the magnetic field in Drosophila require CRY function.
The CRY-defective cryb mutant flies are also unable to respond to the magnetic field; cryb is a chemically-induced missense mutation that renders CRYB essentially non-functional22,23. Because the genetic background of cryb mutant flies is not well-defined, we compared behavioural responses to the magnetic field between homozygous cryb flies and heterozygous cryb/Canton-S flies. Whereas homozygous cryb flies did not show either naïve or trained responses to the magnetic field under full-spectrum light, heterozygous cryb/Canton-S flies showed significant naïve (P=0.0004, one-sample t-test) and trained responses (Fig. 3d); the trained response in the heterozygotes was less than that of wild-type Canton-S flies (Fig. 1b) and likely results from differences in genetic background.
To rule out non-cry mutations as the reason for the lack of magnetic responses in cryb mutants, we showed that the cryb mutation fails to complement the cry01 null mutation. Transheterozygous cryb/cry01 flies did not show significant naïve or trained responses to the magnet, while heterozygous cry01/Canton-S and cryb/Canton-S flies did (naïve response, P=0.006, one-sample t-test; Fig. 3d). Taken together, these data indicate that the cry locus is necessary for light-dependent magnetosensitivity in Drosophila. Furthermore, the lack of a trained response in both cry01 and cryb mutant flies is consistent with CRY being an essential component of the magnetosensitive sensory input pathway and perhaps the magnetoreceptor itself.
Because light-activated CRY interacts with the critical circadian clock protein TIMELESS to reset the circadian clock mechanism25, we examined whether an intact circadian system is necessary for the CRY-dependent magnetosensitive responses in wild-type Canton-S flies. Circadian arrhythmicity was induced by constant light (LL) which disrupts circadian clock function in CRY-containing cells by causing the constant degradation of not only CRY, but also TIMELESS and then PERIOD25. We subsequently tested behavioural responses to the magnetic field after at least five days in LL when the flies were shown to exhibit arrhythmic locomotor behaviour (Fig. 4a), disrupted PERIOD abundance rhythms (Fig. 4b), and to express constant low levels of CRY (Fig. 4c). Strikingly, these arrhythmic flies continued to show significant naïve (P=0.004, one-sample t-test) and trained responses to the magnetic field (Fig. 4d). Thus, the continuous activation of CRY by light does not disrupt its ability to sense the magnet, and an intact circadian system is not required for the magnetoreception mechanism to operate.
Figure 4. Constant light disrupts circadian function but not CRY-mediated magnetosensitivity in Canton-S flies.
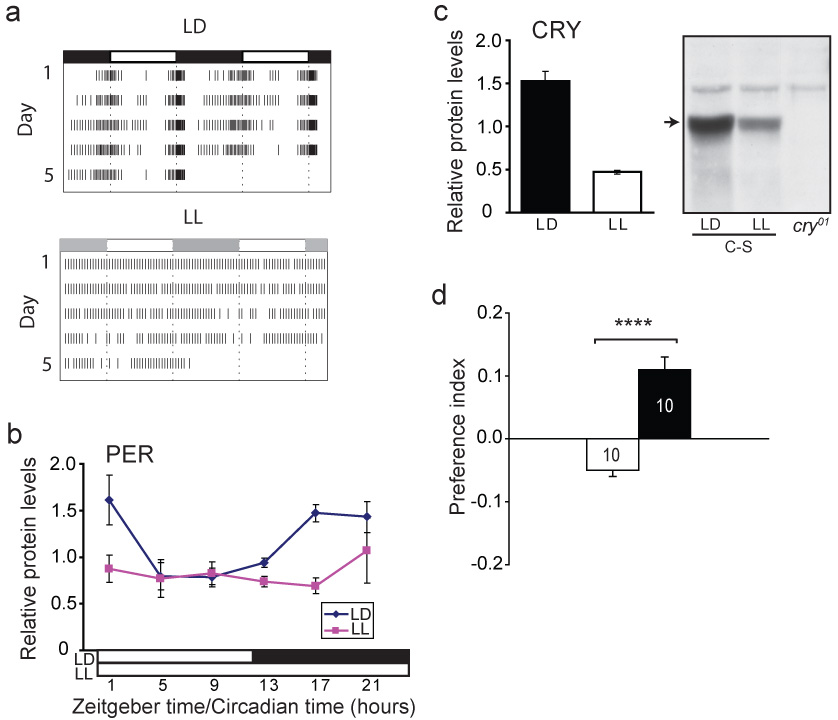
a, Mean activity records in LD (upper) or LL (lower) in double-plotted format (n = 62 for each group). The lighting conditions were identical to those used for housing flies tested for responses to magnet; light irradiance, 1.5×1015 photons/s/cm2/nm. For LD, 94% expressed circadian rhythms when released in constant darkness (period, 24.6 ± 0.03 hours). All the LL flies were arrhythmic.
b, Temporal profiles of PERIOD in heads. Protein abundance was rhythmic in LD (p< 0.01, one-way ANOVA), but not in LL. Head extracts were analyzed by western blot30 and normalized against α-tubulin. Values are mean ± s.e.m. from three sets of heads.
c, CRY abundance is decreased in LL. Values are mean ± s.e.m. from three sets of heads collected over 24-hours in LD or LL. Right, western blot probed for CRY30 showing presence (arrow) in LD or LL in Canton-S (C-S) heads, and absence in cry01 heads.
d, Flies in LL elicit behavioural responses to the magnetic field. Values are mean ± s.e.m. ****, p < 0.0001.
There are two other published reports of magnetosensitivity in Drosophila26,27. One describes behavioural evidence that male wild-type Oregon-R flies exhibit a light-dependent magnetic compass response in a radial maze whereas female flies did not respond to the magnet27. Additionally, male flies responded in opposite directions when tested under either 365 nm or 500 nm light. In our studies, both male and female flies showed a magnetic response. Regardless of experimental differences, both the previous study27 and ours demonstrate that fruit flies can respond to a magnetic field in a wavelength-dependent manner.
Our results extend substantially the presence of a light-dependent magnetic sense in Drosophila by showing the necessity of CRY. We cannot distinguish unequivocally whether fly CRY functions as the actual magnetoreceptor or is an essential component downstream of the receptor. CRY is necessary for both the naïve and trained responses to the magnetic field which is consistent with the notion that CRY is in the input pathway of magnetic sensing. In addition, the continued behavioural responses to the magnet in LL, in which the known CRY signalling components are being constantly degraded and the circadian clock is rendered non-functional, is also consistent with an input function. The most compelling evidence supporting a magnetoreceptor role for CRY is that the CRY-dependent behavioural responses to the magnetic field require UV-A/blue light, which matches the action spectrum of Drosophila CRY19,20.
Our behavioural assay for magnetosensitivity does not currently have a pure directional component, and therefore it is difficult to directly relate our findings to the use of geomagnetic fields for animal orientation and navigation. Nevertheless, it is likely that the response we have identified is the prototype for CRY’s involvement in chemical-based magnetic sensing. Thus, our findings open new avenues of investigation into the cellular and molecular basis of chemical-based magnetic sensing in animals. The powerful genetics of Drosophila will facilitate an understanding of the precise mechanism of action of CRY in magnetosensitivity, such as the actual involvement of magnetosensitive radical pairs produced by photoinduced electron transfer reactions28. Our data further show that the biological functions of Drosophila CRY extend beyond those in circadian clocks.
Methods Summary
Fly stocks were raised on standard cornmeal/agar medium at 25°C and 60% relative humidity under a 12 h light:12 h dark lighting cycle. The w1118;; cry 0 flies (cry01 through cry03) were a gift from Jeffrey Hall and are described in21. The w1118 stock used in our experiments was the same stock used to create w1118;;cry0 flies21. The cryb line was a gift from Patrick Emery22. Our choice apparatus was based on the olfactory conditioning apparatus described in Tully and Quinn29. Our two-coil system was based on the double-wrapped coil system described in24. We adjusted the current flowing through the coils so that the magnetic field intensity was no more than 5 G in any area along the tube. Coils were positioned 45° to the horizontal for experiments involving Canton-S, w;Canton-S, Berlin-K and Oregon-R-S flies. Coils were positioned parallel to the horizontal for all other experiments, because it produced a more robust response and eliminated a polar gradient; that is, there was no horizontal magnetic gradient, as the field was perpendicular to the T-port tubes. To assess the magnetoresponse of flies, we used a simple choice paradigm. Flies were placed in a glass vial containing moistened Whatman paper and starved for 22 h prior to training. All experiments were performed between 0800 – 1200 hours EST. For each population of flies tested, we calculated a Preference Index (PI) value based on the following equation: (PM−0.5)/[(PM+0.5)−(2* PM *0.5)], where PM is the proportion of flies on the magnetic field side of the T-port. To test if flies responded to the experimental magnetic field, we either used a Student’s t-test to compare PI values between trained and naïve groups or a one-sample t-test to compare PI values to zero (i.e., PI value expected with no response to the magnetic field).
Methods
Fly Strains
Oregon-R-S, Berlin-K and w1118 stocks were provided by the Bloomington Drosophila Stock Center (#6326, #4269, and #8522, respectively).
Behavioural apparatus
The main chamber consisted of a training tube, a center elevator section to transfer flies, and a two-tube choice point or T-port for testing the relative response of flies to a magnetic field (Fig. 1a). The training and T-port tubes were round-bottom polystyrene tubes and could be removed from the main section of the apparatus. The magnetic stimulus was delivered to the training and T-port tubes by placing the choice chamber apparatus inside an opaque housing box that contained the magnetic coil system. The box was constructed such that the main chamber could be placed between the two coils in either an upright position (as shown in Fig. 1a, upper panel) or a horizontal position (as shown in Fig. 1a, lower panel). The upright position of the chamber apparatus was used for training so that the tube could be placed in the center of one coil, and the horizontal position of the apparatus was used to suspend the two tubes of the T-maze in the same area of the coil during test sessions. In this way, flies were subjected to the same light conditions and intensity of magnetic field during both training and test sessions.
Each of the two coils was wrapped with two wires; the wires were wrapped in one direction on one coil and in the opposite direction on the other. Current flow through the coils produced a magnetic field in one coil (parallel current flow) but not the other (opposite current flow). A double-pole, double-throw switch reversed the current flow in one wire loop but not the other, which allowed us to easily move the magnetic field from the right to the left side of the T-maze. A DC power supply with current and voltage controls was used so that we could change the intensity of the magnetic field produced by the coils. Each coil was mounted on a plastic track so that it could be positioned either directly under the tubes (field perpendicular to the tubes), at the end of the tubes at a 45° angle (as shown in Fig. 1a), or at the end of the tubes (field parallel to the tubes). We used a magnetic field intensity of 5 G for our experiments, because it gave the most consistent naïve response; decreasing to 1 G increased variability to the point that responses were no longer significant.
The housing box for the test or choice chamber was open on the top so that the chamber, regardless of position, could be illuminated by one ZooMed Reptisun 10.0 UVB fluorescent tube (F20T12) and one Agrobrite full spectrum fluorescent grow tube. Wavelengths entering the box were restricted by covering the top of the box with a long-wavelength filter that transmitted wavelengths of light >500 nm (Edmund Optics, Barrington NJ) or > 420 nm or >400nm (E400 and E420 from Gentex, Carbondale PA). Irradiance measurements (from an Ocean Optics USB 2000 fiber optic spectrometer, Dunedin FL) were taken from inside one arm of the T-maze portion of the choice chamber apparatus; thus, lighting conditions represent those experienced by flies while either being trained or assayed for sensitivity to a magnetic field.
Experimental procedure
For the Training Group, a population of 100–150 flies was loaded into the elevator section of the choice apparatus with an empty training tube facing one of the coils (Fig. 1a, upper panel). Flies were transferred to the training tube for 2 minutes and then transferred back to the elevator and held for a 1-minute rest period. The empty training tube was next replaced with a tube containing sucrose reinforcement and flies were allowed to feed for 2 minutes in the presence of a magnetic field. Flies were then transferred back to the elevator and held for 1 minute, while the coil system was turned off. During this time, the training tube was also removed, and two empty tubes were added to form the two arms of the T-port. The choice chamber was then positioned horizontally in the box (Fig. 1a, lower panel). The coil system was turned on, and flies were transferred to the T-port, in which they were allowed to choose between the sides with or without a magnetic field. After 2 minutes, the two arms of the T-port were blocked and flies from each side were collected into separate empty vials and counted.
For the Naïve Group, a second population of 100–150 flies was immediately loaded into the elevator section of the horizontally-placed choice chamber and the coil system was turned on (Fig. 1b). After 1 minute, flies were transferred directly to the T-port for 2 minutes.
Trained and Naïve Groups were tested consecutively and with the magnetic field on the same side. This was done to control for the possibility that the choice behaviour of flies reflected a preference for one arm of the T-port and not a response to the magnetic field. As an additional control for side preferences independent of magnetic stimuli, we alternated the side of the T-port containing the field after each consecutive set of trained and naïve flies (i.e., trained and naïve with magnet on the left side followed by trained and naïve with magnet on the right).
Acknowedgements
We thank Haisun Zhu for the protein work in Figure 4b and 4c, Lauren Foley for assistance, Jeffrey C. Hall for the cry0 flies, Patrick Emery for PER and CRY antibodies, and Patrick Emery, Paola Perrat, Benjamin Leung, Shamik DasGupta, Michael Krashes, and Haisun Zhu for discussions. This work was supported by grants from the NIH.
References
- 1.Lohmann KJ, Lohmann CMF, Putman NF. Magnetic maps in animals: nature's GPS. Journal of Experimental Biology. 2007;210:3697–3705. doi: 10.1242/jeb.001313. [DOI] [PubMed] [Google Scholar]
- 2.Wiltschko W, Wiltschko R. Magnetic orientation and magnetoreception in birds and other animals. Journal of Comparative Physiology a-Neuroethology Sensory Neural and Behavioral Physiology. 2005;191:675–693. doi: 10.1007/s00359-005-0627-7. [DOI] [PubMed] [Google Scholar]
- 3.Ritz T, Adem S, Schulten K. A model for photoreceptor-based magnetoreception in birds. Biophysical Journal. 2000;78:707–718. doi: 10.1016/S0006-3495(00)76629-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luschi P, et al. Marine turtles use geomagnetic cues during open-sea homing. Current Biology. 2007;17:126–133. doi: 10.1016/j.cub.2006.11.062. [DOI] [PubMed] [Google Scholar]
- 5.Johnsen S, Lohmann KJ. The physics and neurobiology of magnetoreception. Nature Reviews Neuroscience. 2005;6:703–712. doi: 10.1038/nrn1745. [DOI] [PubMed] [Google Scholar]
- 6.Kirschvink JL, Gould JL. Biogenic Magnetite as a Basis for Magnetic-Field Detection in Animals. Biosystems. 1981;13:181–201. doi: 10.1016/0303-2647(81)90060-5. [DOI] [PubMed] [Google Scholar]
- 7.Walker MM. A model for encoding of magnetic field intensity by magnetite-based magnetoreceptor cells. Journal of Theoretical Biology. 2008;250:85–91. doi: 10.1016/j.jtbi.2007.09.030. [DOI] [PubMed] [Google Scholar]
- 8.Kirschvink JL, Walker MM, Diebel CE. Magnetite-based magnetoreception. Current Opinion in Neurobiology. 2001;11:462–467. doi: 10.1016/s0959-4388(00)00235-x. [DOI] [PubMed] [Google Scholar]
- 9.Leask MJM. Physicochemical Mechanism for Magnetic-Field Detection by Migratory Birds and Homing Pigeons. Nature. 1977;267:144–145. doi: 10.1038/267144a0. [DOI] [PubMed] [Google Scholar]
- 10.Schulten K, Swenberg CE, Weller A. Biomagnetic Sensory Mechanism Based on Magnetic-Field Modulated Coherent Electron-Spin Motion. Zeitschrift Fur Physikalische Chemie-Frankfurt. 1978;111:1–5. [Google Scholar]
- 11.Cashmore AR. Cryptochromes: Enabling plants and animals to determine circadian time. Cell. 2003;114:537–543. [PubMed] [Google Scholar]
- 12.Partch CL, Sancar A. Photochemistry and photobiology of cryptochrome blue-light photopigments: The search for a photocycle. Photochemistry and Photobiology. 2005;81:1291–1304. doi: 10.1562/2005-07-08-IR-607. [DOI] [PubMed] [Google Scholar]
- 13.Zhu HS, et al. The two CRYs of the butterfly. Current Biology. 2005;15:R953–R954. doi: 10.1016/j.cub.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 14.Yuan Q, Metterville D, Briscoe AD, Reppert SM. Insect cryptochromes: Gene duplication and loss define diverse ways to construct insect circadian clocks. Molecular Biology and Evolution. 2007;24:948–955. doi: 10.1093/molbev/msm011. [DOI] [PubMed] [Google Scholar]
- 15.Oeztuerk N, Song SH, Selby CP, Sancar A. Animal type 1 cryptochromes - Analysis of the redox state of the flavin cofactor by site-directed mutagenesis. Journal of Biological Chemistry. 2008;283:3256–3263. doi: 10.1074/jbc.M708612200. [DOI] [PubMed] [Google Scholar]
- 16.Phillips JB, Borland SC. Behavioral Evidence for Use of a Light-Dependent Magnetoreception Mechanism by a Vertebrate. Nature. 1992;359:142–144. [Google Scholar]
- 17.Ritz T, Dommer DH, Phillips JB. Shedding light on vertebrate magnetoreception. Neuron. 2002;34:503–506. doi: 10.1016/s0896-6273(02)00707-9. [DOI] [PubMed] [Google Scholar]
- 18.Wiltschko R, Wiltschko W. Magnetoreception. Bioessays. 2006;28:157–168. doi: 10.1002/bies.20363. [DOI] [PubMed] [Google Scholar]
- 19.vanVickle-Chavez SJ, van Gelder RN. Action spectrum of Drosophila cryptochrome. Journal of Biological Chemistry. 2007;282:10561–10566. doi: 10.1074/jbc.M609314200. [DOI] [PubMed] [Google Scholar]
- 20.Helfrich-Forster C, et al. The extraretinal eyelet of Drosophila: Development, ultrastructure, and putative circadian function. Journal of Neuroscience. 2002;22:9255–9266. doi: 10.1523/JNEUROSCI.22-21-09255.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dolezelova E, Dolezel D, Hall JC. Rhythm defects caused by newly engineered null mutations in Drosophila's cryptochrome gene. Genetics. 2007;177:329–345. doi: 10.1534/genetics.107.076513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Emery P, et al. CRY, a Drosophila clock and light-regulated cryptochrome, is a major contributor to circadian rhythm resetting and photosensitivity. Cell. 1998;95:669–679. doi: 10.1016/s0092-8674(00)81637-2. [DOI] [PubMed] [Google Scholar]
- 23.Stanewsky R, et al. The cry(b) mutation identifies cryptochrome as a circadian photoreceptor in Drosophila. Cell. 1998;95:681–692. doi: 10.1016/s0092-8674(00)81638-4. [DOI] [PubMed] [Google Scholar]
- 24.Kirschvink JL. Uniform Magnetic-Fields and Double-Wrapped Coil Systems - Improved Techniques for the Design of Bioelectromagnetic Experiments. Bioelectromagnetics. 1992;13:401–411. doi: 10.1002/bem.2250130507. [DOI] [PubMed] [Google Scholar]
- 25.Stanewsky R. Genetic analysis of the circadian system in Drosophila melanogaster and mammals. Journal of Neurobiology. 2003;54:111–147. doi: 10.1002/neu.10164. [DOI] [PubMed] [Google Scholar]
- 26.Wehner R, Labhart T. Perception of Geomagnetic Field in Fly Drosophila-Melanogaster. Experientia. 1970;26:967-&. doi: 10.1007/BF02114135. [DOI] [PubMed] [Google Scholar]
- 27.Phillips JB, Sayeed O. Wavelength-Dependent Effects of Light on Magnetic Compass Orientation in Drosophila-Melanogaster. Journal of Comparative Physiology a-Sensory Neural and Behavioral Physiology. 1993;172:303–308. doi: 10.1007/BF00216612. [DOI] [PubMed] [Google Scholar]
- 28.Maeda K, et al. Chemical compass model of avian magnetoreception. Nature. 2008;453:387–390. doi: 10.1038/nature06834. [DOI] [PubMed] [Google Scholar]
- 29.Tully T, Quinn WG. Classical-Conditioning and Retention in Normal and Mutant Drosophila-Melanogaster. Journal of Comparative Physiology a-Sensory Neural and Behavioral Physiology. 1985;157:263–277. doi: 10.1007/BF01350033. [DOI] [PubMed] [Google Scholar]
- 30.Rush BL, Murad A, Emery P, Giebultowicz JM. Ectopic CRYPTOCHROME renders TIM light sensitive in the Drosophila ovary. Journal of Biological Rhythms. 2006;21:272–278. doi: 10.1177/0748730406290416. [DOI] [PubMed] [Google Scholar]


