Abstract
The serine/threonine kinase Akt, also known as protein kinase B (PKB), is a central node in cell signaling downstream of growth factors, cytokines, and other cellular stimuli. Aberrant loss or gain of Akt activation underlies the pathophysiological properties of a variety of complex diseases, including type-2 diabetes and cancer. Here, we review the molecular properties of Akt and the approaches used to characterize its true cellular targets. In addition, we discuss those Akt substrates that are most likely to contribute to the diverse cellular roles of Akt, which include cell survival, growth, proliferation, angiogenesis, metabolism, and migration.
The serine/threonine kinase Akt/PKB has emerged as a critical signaling node within all cells of higher eukaryotes and as one of the most important and versatile protein kinases at the core of human physiology and disease. Since its discovery as an oncogene within the mouse leukemia virus AKT8 (Bellacosa et al., 1991; Staal, 1987) and as a homolog of protein kinase C (Jones et al., 1991), there have been many exciting breakthroughs elucidating the mechanism of upstream regulation of Akt (summarized in Figure 1). Several excellent recent reviews have covered the molecular details of Akt regulation and its role in human disease (such as Bellacosa et al., 2005; Engelman et al., 2006). Here, we focus on signaling downstream of Akt, with an emphasis on direct phosphorylation targets of the kinase and its bona fide cellular functions.
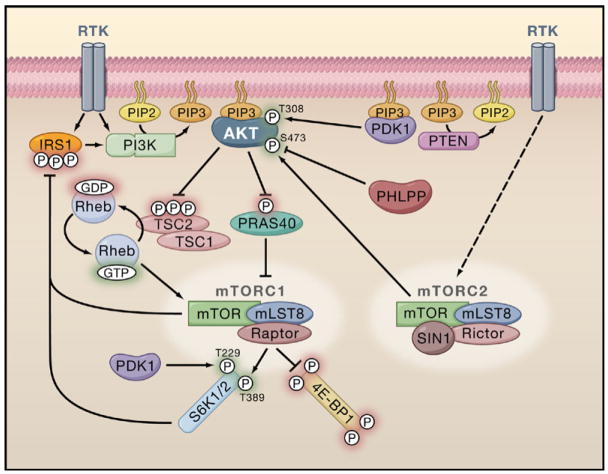
Figure 1. Upstream Activation of Akt by Growth Factors.
Also depicted is the complex relationship between Akt signaling and mTOR. Activated receptor tyrosine kinases (RTKs) activate class I phosphatidylinositol 3-kinase (PI3K) through direct binding or through tyrosine phosphorylation of scaffolding adaptors, such as IRS1, which then bind and activate PI3K. PI3K phosphorylates phosphatidylinositol-4,5-bisphosphate (PIP2) to generate phosphatidylinositol-3,4,5-trisphosphate (PIP3), in a reaction that can be reversed by the PIP3 phosphatase PTEN. AKT and PDK1 bind to PIP3 at the plasma membrane, and PDK1 phosphorylates the activation loop of AKT at T308. RTK signaling also activates mTOR complex 2 (mTORC2) through a currently unknown mechanism, and mTORC2 phosphorylates Akt on the hydrophobic motif S473, which can be dephosphorylated by the S473 phosphatase PHLPP. Akt activates mTOR complex 1 (mTORC1) through multisite phosphorylation of TSC2 within the TSC1-TSC2 complex, and this blocks the ability of TSC2 to act as a GTPase-activating protein (GAP) for Rheb, thereby allowing Rheb-GTP to accumulate. Rheb-GTP activates mTORC1, which phosphorylates downstream targets such as 4E-BP1 and the hydrophobic motif on the S6 kinases (S6Ks; T389 on S6K1). PDK1 phosphorylates the activation loop on the S6Ks (T229 on S6K1) in a reaction independent of PDK1 binding to PIP3. Akt can also activate mTORC1 by phosphorylating PRAS40, thereby relieving the PRAS40-mediated inhibition of mTORC1. Once active, both mTORC1 and S6K can phosphorylate serine residues on IRS1, which targets IRS1 for degradation, and this serves as a negative feedback mechanism to attenuate PI3K-Akt signaling. See text for references to recent reviews detailing Akt regulation and mTOR signaling.
Substrate Specificity
The three Akt isoforms (Akt1/PKBα, Akt2/PKBβ, and Akt3/PKBγ) have extensive homology to protein kinases A, G, and C within their kinase domains and are, therefore, members of the AGC kinase family. Following the identification of a residue on the kinase GSK3 as the first direct target of Akt in cells (Cross et al., 1995), ground-breaking experiments with peptides containing variants of this sequence defined the minimal recognition motif of Akt as R-X-R-X-X-S/T-B (Alessi et al., 1996b), where X represents any amino acid and B represents bulky hydrophobic residues. The critical requirement for R residues at both the −5 and −3 positions (that is, 5 and 3 residues, respectively, N-terminal to the phospho-acceptor site) on peptides efficiently phosphorylated by Akt distinguishes the substrate specificity of Akt from that of two other mitogen-stimulated AGC kinases, RSK (MAPKAP-K1) and S6K1 (p70S6K), which can better tolerate K at these positions. In these “peptide-bashing” experiments, more subtle Akt preferences were also uncovered for other residues surrounding the phosphorylation site (such as a preference for T at −2). Structural insights into the molecular interactions dictating the substrate selectivity of Akt have been provided by a high-resolution crystal structure of Akt bound to this GSK3 peptide substrate (Yang et al., 2002).
Further details of the preferred substrate specificity of Akt have been obtained using peptide library screening (Hutti et al., 2004; Obata et al., 2000), which provides an unbiased, systematic, and quantitative score for all 20 amino acids at each site within seven residues N-terminal and C-terminal to the phospho-acceptor site. This approach allows the identification of residues selected both for and against by the kinase of interest at each position surrounding the phosphorylation site. A bioinformatics program called Scansite (http://scansite.mit.edu; Yaffe et al., 2001) has provided the ability to search protein databases with the matrix of data obtained from such screens, rather than a single consensus sequence, and has been instrumental in identifying new Akt substrates and narrowing down target residues on suspected substrates. However, this approach needs to be used with caution, as it is only useful for identifying candidate phosphorylation sites. The sheer number of high-quality Akt sites within the proteome, in addition to the overlap in substrate specificity with other AGC kinases, illustrates the need for a rigorous demonstration of the direct in vivo phosphorylation of a given candidate target by Akt.
It remains possible that there are unknown sequence contexts or macromolecular interactions within cells that might allow Akt to phosphorylate motifs other than the minimally required R-X-R-X-X-S/T. Currently, however, there are no rigorously demonstrated and independently confirmed Akt substrates that fall into this category. Therefore, in the discussion of substrate characterization and the role of specific substrates below, we consider the presence of an R-X-R-X-X-S/T motif to be essential.
Defining Criteria for Bona Fide Akt Substrates
Here we review criteria that, in our view, should be met to define an in vivo Akt substrate. We recognize the limits of such standards, as it is possible that the same site phosphorylated by Akt in one cell type under one condition might be phosphorylated by additional kinases in other settings. However, given the increasing complexity of Akt signaling and its importance in human physiology and disease, it is worth evaluating the experimental paradigms currently used to define a direct downstream target of Akt. Our discussion is specific to Akt, but the criteria are similar to that put forward for other protein kinases (Frame and Cohen, 2001). Many approaches have been used to demonstrate phosphorylation of sites on target proteins within cells. However, no single method is sufficient; rather, both Akt loss- and gain-of-function approaches are needed. In the end, it is essential to demonstrate that phosphorylation of a candidate site on an endogenous substrate is induced by specific physiological stimuli that activate endogenous Akt and that the phosphorylation is lost upon Akt inactivation.
In Vitro Phosphorylation
Demonstration of direct in vitro phosphorylation of a candidate R-X-R-X-X-S/T site by Akt is a necessary step in defining an Akt substrate. Phosphorylation of the full-length protein of interest should be demonstrated, rather than fragments or motifs fused to other proteins (e.g., GST). Phosphate incorporation at the relevant site must be demonstrated. However, Akt is a somewhat promiscuous kinase in vitro and will phosphorylate most R-X-RX-X-S/T sites and some R-X-X-S/T sites if given enough time and substrate. Therefore, in vitro phosphorylation, although necessary, is not sufficient for defining a new phosphorylation site for Akt.
Phosphorylation-Site Readout
In order to confirm candidate phosphorylation sites detected using bioinformatics and/or in vitro approaches, one first needs a reliable and specific readout of the phosphorylation event in question. This can occasionally be detected as a shift to a slower migrating form on denaturing polyacrylamide gels. However, it is rare that phosphorylation of an Akt site causes such a shift, which is usually indicative of a proline-directed site (i.e., S/T-P), and Akt poorly phosphorylates such sites (Alessi et al., 1996b; Hutti et al., 2004). Mass spectrometry techniques and in vivo [32P]-orthophosphate labeling followed by two-dimensional phospho-peptide mapping are indispensable tools for identifying in vivo phosphorylation sites. However, due to their time-consuming and low-throughput nature, these approaches are somewhat less useful as an experimental readout when characterizing cellular conditions and kinases responsible for the phosphorylation. Antibodies specific for a phosphorylated motif (i.e., phospho-substrate antibodies) have been extremely valuable for identifying and characterizing new Akt substrates over the past five years (e.g., Kovacina et al., 2003; Manning et al., 2002; Sano et al., 2003). These antibodies are raised against degenerate phospho-peptides containing the phosphorylated minimal Akt substrate motif (R-X-R-X-X-pS/pT; Zhang et al., 2002). Although these antibodies are helpful in identifying candidate Akt sites, mutation analysis must be used to confirm that the antibody is recognizing the predicted site(s). As a cautionary note, at least some of the currently available Akt phospho-substrate antibodies can recognize R-X-X-pS/pT motifs in sequence contexts lacking an R at the −5 position (Zhang et al., 2002), and this is especially true when such a phospho-protein is overexpressed (B.D.M., unpublished data). Finally, once data are obtained suggesting the regulated phosphorylation of a specific site, the gold standard is the use of a phospho-specific antibody unique to the site of interest. Regardless of the readout for a given phosphorylation event, this approach must be followed by experiments that demonstrate a requirement for Akt.
Pharmacological
The growth-factor-stimulated phosphorylation of an Akt site should be sensitive to pretreatment with phosphatidylinositol 3-kinase (PI3K) inhibitors, such as wortmannin (≤100 nM) or LY294002 (≤20 μM), as class I PI3Ks are upstream activators of Akt (Figure 1). Although a low dose of wortmannin is specific to PI3K, it inhibits all classes of PI3Ks, and there are likely to be PI3K-regulated kinases, other than Akt, that are affected by such inhibition. Resistance to highly specific inhibitors of the kinases RSK (e.g., BI-D1870; Sapkota et al., 2007) and mTORC1 (e.g., rapamycin) is also important for ruling out RSK and S6K, respectively, as candidate kinases for the in vivo phosphorylation event observed. Specificity issues exist with currently available Akt inhibitors, thereby limiting their use for defining Akt as the relevant kinase for a given phosphorylation event or cellular function. However, inhibitors that target regions of Akt outside of its highly conserved ATP-binding pocket are promising and should show increased specificity for Akt over other AGC kinases. As the specificity of AGC kinase inhibitors improves, they will certainly be valuable tools in identifying and verifying Akt substrates.
Loss of Function
The current limitations of pharmacological approaches dictate that Akt-specific loss-of-function approaches are needed. Although recently developed cell lines lacking Akt function provide the most stringent setting for confirming a substrate (see below), several types of dominant-negative Akt constructs are available, with variable success at inhibiting endogenous Akt. In our experience, Akt lacking its two activating phosphorylation sites (Akt-T308A/S473A) works more reproducibly as a dominant-negative than the kinase-dead (K179D) version. However, the molecular mechanisms underlying the dominant-negative effects of these various constructs are unknown, and potential effects on upstream kinases such as phosphoinositide-dependent kinase-1 (PDK1) and mTORC2 make this approach less desirable. Knockdown of Akt isoforms through small interfering (si)RNAs is becoming used more often to define Akt substrates and functions. Knowledge of the expression patterns of different Akt isoforms and the use of isoform-specific antibodies are important considerations here. It is likely that, in any given setting, knockdown of more than one isoform will be required. However, recent studies suggest that isoform-specific substrates and functions of Akt are more common than previously suspected (see below).
Gain of Function
Overexpression of exogenous Akt constructs invariably increases the basal and stimulated phosphorylation of its substrates. However, given the strict temporal and spatial regulation that accompany kinase-substrate interactions in cells, exogenously overexpressed constructs should never be used as the sole basis for defining an Akt substrate. Membrane-targeting versions of Akt, such as myristoylated PH domain-deleted Akt (myr-ΔPH-Akt)—which are brought to the plasma membrane independent of PI3K activity—have been the most widely used for determining the cellular effects of constitutive activation of Akt (Andjelkovic et al., 1997; Kohn et al., 1996). However, depending on the membrane-targeting construct used, differential effects on downstream signaling can be detected. An alternative is the phospho-mimetic mutant of Akt (Akt-T308D/S473D), which has lower activity than the myr-Akt derivatives but is constitutively active and PI3K independent (Alessi et al., 1996a). Overall, the general concerns regarding potential overexpression artifacts definitely apply to kinase-substrate interactions and phosphorylation events, and results obtained with such constructs should be interpreted with caution.
Genetics
Genetic systems have been instrumental in identifying and confirming kinase substrates. C. elegans and Drosophila genetics have been powerful tools for elucidating signaling pathways. An important example of using a genetic approach to elucidate Akt signaling was the finding that the forkhead box O (FOXO) transcription factor Daf-16 is downstream of the PI3K and Akt orthologs in C. elegans for control of the developmental and metabolic state referred to as dauer (Paradis and Ruvkun, 1998). Although genetic epistasis analysis is useful for positioning new candidate substrates downstream of a specific kinase and for functional insights into the effects of a kinase on the given target, more rigorous approaches are needed to demonstrate a direct phosphorylation. However, loss-of-function or deletion alleles of a kinase can be valuable for confirming the kinase specificity of an in vivo phosphorylation event. To this end, mouse genetic models have been increasingly important for defining bona fide targets of Akt. A variety of isoform-specific Akt knockout mice and combinations have now been made (reviewed in Dummler and Hemmings, 2007), and these can be used to demonstrate that phosphorylation of a given substrate requires a specific Akt isoform in a particular cell type of interest. Other powerful mouse models for the confirmation of in vivo Akt substrates are a pair of PDK1 knockin mutants generated by Alessi and colleagues. The first is a knockin of a PDK1-PH domain mutant (PKD1-RRR472-474LLL or PDK1-PHKI), which cannot bind to PI3K lipid products and is defective in the phosphorylation and activation of Akt isoforms, while maintaining the ability to phosphorylate the other AGC kinases (McManus et al., 2004). The phosphorylation of several proposed Akt substrates has been confirmed using PDK1-PHKI embryonic stem cells, including sites on FOXO1 and 3, TSC2, GSK3, PRAS40, and WNK1 (McManus et al., 2004). The other PDK1 knockin mutant is the PDK1 -PIF pocket mutant (PDK1-L155E), which can activate Akt but not the other AGC kinases (Collins et al., 2003). Embryonic stem cells expressing this mutant are useful in parallel to critically test in vivo phosphorylation sites for Akt specificity. Ultimately, the dependence on Akt activity for the phosphorylation of all candidate substrates and sites should be verified in such cell and mouse models.
Akt Substrates and Functions
A careful search of the literature finds over 100 reported nonredundant Akt substrates, of which approximately 25% do not contain the minimal requirements for an Akt site (e.g., R-X-R-X-X-S/T). Furthermore, many of those that do contain this motif have not been characterized further than in vitro kinase assays. Although we have not determined how all of the remaining published candidate Akt substrates measure up to the criteria outlined above, we have assessed the literature on 18 substrates for which there have been multiple independent published reports (Tables 1 and S1). Within this survey, we have included an analysis of evolutionary conservation (Table S1). The degree of conservation of an identified Akt phosphorylation site can be indicative of the relevance of the substrate toward the defined cellular functions of Akt. Most thoroughly studied and independently confirmed Akt phosphorylation sites are conserved amongst the orthologs from all mammals, and a handful are conserved down to invertebrate models such as Drosophila or C. elegans (Table S1, Metazoan). Conservation analysis is particularly important when using animal models to determine the role of Akt substrates in human disease. For instance, a study using the rat version of the protein Par-4/PAWR concluded that this protein is a direct target of Akt essential for Akt’s role in cancer cell survival (Goswami et al., 2005), but the proposed Akt site is not conserved in human, or even mouse, Par-4/PAWR. Finally, it should be stated that exclusion from the list in Table 1 or the discussion below does not rule out a published substrate as being a bona fide Akt target. We anxiously await further study on these other candidate substrates.
Table 1.
Characteristics and Experimental Evidence for a Subset of Akt Substratesa
| In Vivod |
|||||||
|---|---|---|---|---|---|---|---|
| Target | Human Site(s)b | In Vitroc | W/L | LOF | GOF | Genetic Evidencee | Regulatory Effect?f |
| FOXO1 | T24, S256, S319 | + | + | + | + | M, F, W | Inhibit |
| FOXO3A | T32, S253, S315 | + | + | + | + | M, F, W | Inhibit |
| FOXO4 | T32, S197, S262 | + | + | + | + | M, F, W | Inhibit |
| TSC2 | S939, T1462 | + | + | + | + | M, F | Inhibit |
| GSK3α/β | S21/S9 | + | + | + | + | M | Inhibit |
| RAF1 | S259 | + | + | + | + | − | Inhibit |
| PRAS40 | T246 | + | + | − | + | M | Inhibit |
| AS160 | S588, T642 | + | + | − | + | − | Inhibit |
| BAD | S99 | + | + | + | + | − | Inhibit |
| WNK1 | T60 | + | + | + | + | M | ? |
| MDM2 | S166, S186 | + | + | + | + | − | Activate |
| Chk1 | S280 | + | + | + | + | M | Inhibit |
| eNOS | S1177 | + | + | + | + | M | Activate |
| ASK1 | S83 | + | + | + | + | − | Inhibit |
| IKKα | T23 | + | − | − | + | − | Activate |
| P21CIP1 | T145 | + | + | + | + | − | Inhibit |
| p27KIP1 | T157 | + | + | + | + | − | Inhibit |
| Casp9 | S196 | + | − | + | + | − | Inhibit |
For an expanded version of this table see Table S1.
Human numbering of sites with strongest evidence of being phosphorylated by Akt in vivo.
Direct phosphorylation of the given site(s) with purified Akt and full-length substrate in vitro.
Evidence of Akt-dependent phosphorylation of the given site(s) within cells (− means no published evidence), including: W/L, sensitivity to PI3K inhibition using ≤ 100 nM wortmannin (W) and/or ≤ 20 μM LY294002 (L); LOF, loss-of-AKT-function evidence using dominant-negative mutants and/or RNAi approaches; GOF, gain-of-Akt-function evidence using overexpression and/or constitutively active mutants.
Genetic evidence in model organisms, including epistasis analyses in Drosophila (F) or C. elegans (W) or loss-of-phosphorylation in mouse mutants (M) lacking Akt function.
Functional consequence of Akt-mediated phosphorylation.
In this section, we focus on the known cellular functions of Akt and the direct downstream targets of Akt that are most likely to mediate these functions. This discussion illustrates the most challenging aspect of characterizing any kinase-substrate pair: defining the functional consequences of phosphorylation of a specific site or sites on a new substrate. To this end, phosphorylation-site mutants (S/T to A) are particularly useful if the substrate has a known function. Such a mutant should block the positive or negative effects of Akt on the activity of the substrate or a cellular process involving the substrate. Although it has been done for very few substrates, the generation of phosphorylation-site mutant knockin mice is an especially powerful approach for determining the physiological importance of phosphorylation of a given target by Akt (e.g., McManus et al., 2005). Phosphomimetic mutants (S/T to D/E) have a more limited usefulness. Changing the phospho-acceptor site to an acidic residue might mimic phosphorylation, but not in all cases. This is especially true when the Akt phosphorylation site creates a 14-3-3 binding motif, which is a common mode of regulation (see below), as D and E residues are poor substitutes for phospho-S/T in mediating 14-3-3 binding. One theme that is obvious from the discussion below is that the diverse cellular roles of Akt do not fall under a one-substrate-one-function paradigm; rather, each physiological response downstream of Akt appears to be mediated by multiple targets (Figure 2). Furthermore, some Akt substrates control more than one cellular function, and these functions may vary in a cell- and signal-context-dependent manner.
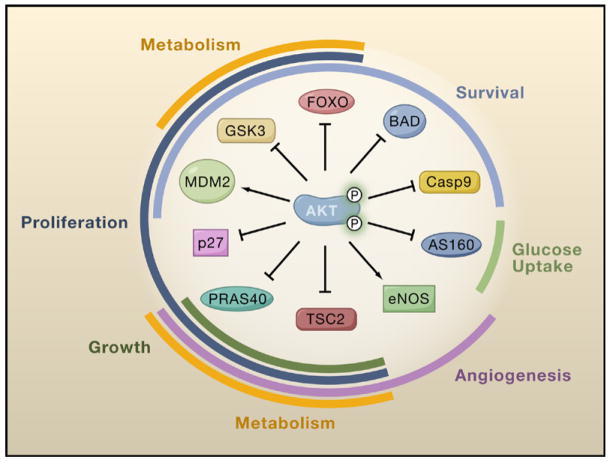
Figure 2. Cellular Functions of Ten Akt Substrates.
Akt-mediated phosphorylation of these proteins leads to their activation (arrows) or inhibition (blocking arrows). Regulation of these substrates by Akt contributes to activation of the various cellular processes shown (i.e., survival, growth, proliferation, glucose uptake, metabolism, and angiogenesis). As illustrated by these ten targets, a high degree of functional versatility and overlap exists amongst Akt substrates. See text for detailed descriptions of substrates and functions.
Cell Survival
Even before the relevant substrates were identified, several groups independently demonstrated a critical role for Akt in promoting cell survival downstream of growth factors, oncogenes, and cell stress (reviewed in Marte and Downward, 1997). Akt enhances the survival of cells by blocking the function of proapoptotic proteins and processes. Akt negatively regulates the function or expression of several Bcl-2 homology domain 3 (BH3)-only proteins, which exert their proapoptotic effects by binding to and inactivating prosurvival Bcl-2 family members. For instance, Akt directly phosphorylates and inhibits the BH3-only protein BAD (Datta et al., 1997; delPeso et al., 1997). Survival factors stimulate Akt-mediated phosphorylation of BAD on S136, and this creates a binding site for 14-3-3 proteins, which triggers release of BAD from its target proteins (Datta et al., 2000). The ability to phosphorylate S136 on BAD is important for the survival effects of Akt on neurons and other cell types (Datta et al., 1997, 2002). Akt also inhibits the expression of BH3-only proteins through effects on transcription factors, such as FOXO and p53. Akt phosphorylates FOXO1 on T24, S256, and S319, and it phosphorylates FOXO3a and FOXO4 on three equivalent sites (reviewed in Tran et al., 2003). Akt’s phosphorylation of FOXO proteins occurs in the nucleus, and, as with BAD, phosphorylated T24 and S256 are bound by 14-3-3 proteins, which displace FOXO transcription factors from target genes and trigger their export from the nucleus. Through this mechanism, Akt blocks FOXO-mediated transcription of target genes that promote apoptosis, cell-cycle arrest, and metabolic processes (see below). An important proapoptotic target of FOXO proteins is the BH3-only protein BIM, which stimulates cell death in hematopoietic lineages upon cytokine withdrawal (Dijkers et al., 2002). In addition, when unphosphorylated and active, FOXO factors can induce the expression of the proapoptotic cytokine Fas ligand (FasL; Brunet et al., 1999). A third target of Akt that promotes survival by inhibiting BH3-only proteins is MDM2 (or HDM2 in humans), an E3 ubiquitin ligase that triggers p53 degradation. Akt phosphorylates MDM2 on S166 and S186, and this promotes translocation of MDM2 to the nucleus, where it negatively regulates p53 function (Mayo and Donner, 2001; Zhou et al., 2001b). Two transcriptional targets of p53 are the BH3-only proteins Puma and Noxa, which appear to be the essential targets in p53-induced apoptosis (Villunger et al., 2003). However, the relative importance of downregulation of these p53 targets to Akt-mediated cell survival has not been thoroughly examined.
The importance of other direct targets of Akt toward inhibiting apoptosis is less well understood. Akt phosphorylates GSK3 isoforms on a highly conserved N-terminal regulatory site (GSK3α-S21, GSK3β-S9), and this phosphorylation inactivates the kinase (Cross et al., 1995). Although GSK3 has many substrates that could explain a proapoptotic role for this Ser/Thr kinase (reviewed in Frame and Cohen, 2001), the prosurvival Bcl-2 family member MCL-1 has recently been found to be a direct target inhibited by GSK3 (Maurer et al., 2006). Downstream of the Bcl-2 family members within the mitochondrial pathway of apoptosis are a cascade of caspase-family proteases initiated by caspase-9. Caspase-9 is proteolytically processed and activated from its procaspase form upon cytochrome c release from the mitochondria. Therefore, Akt activation prevents the processing of procaspase-9 through the effects on Bcl2 family members described above. Akt has also been found to directly phosphorylate S196 on human procaspase-9, and this phosphorylation correlates with a decrease in the protease activity of caspase-9 in vitro (Cardone et al., 1998). A procaspase-9-S196A mutant is more efficient than wild-type caspase-9 at inducing apoptosis when co-overexpressed with activated Akt, suggesting a role for caspase-9 phosphorylation in the antiapoptotic effects of Akt. However, the sequence surrounding caspase-9-S196 is unusual for an Akt substrate in that the +1 position is a P, and such proline-directed sites are strongly selected against by Akt in peptide phosphorylation experiments (Alessi et al., 1996b; Hutti et al., 2004). Furthermore, despite the highly conserved role of Akt in blocking apoptosis, this Akt phosphorylation site is not found in all mammals and appears to be restricted to caspase-9 in primates. Additional studies are required to determine the physiological relevance of caspase-9 relative to other proapoptotic proteins inhibited by Akt.
It is also possible that Akt exerts some of its cell-survival effects through crosstalk with other pathways or through effects on nutrient uptake and metabolism. For instance, it has been reported that, under some conditions, the PI3K-Akt pathway activates NFκB survival signaling or inhibits JNK/p38 apoptotic signaling (e.g., Kim et al., 2001; Ozes et al., 1999; Romashkova and Makarov, 1999). An important, but more indirect, effect of Akt signaling on cell survival is through its roles in nutrient uptake, metabolism, and the maintenance of mitochondrial membrane potential (reviewed in Plas and Thompson, 2005; Robey and Hay, 2006). Both signaling crosstalk and effects of Akt on cellular metabolism are discussed in detail below.
Cell Growth
One of the best-conserved functions of Akt is its role in promoting cell growth (i.e., an increase in cell mass). The predominant mechanism appears to be through activation of mTOR complex 1 (mTORC1 or the mTOR-raptor complex), which is regulated by both nutrients and growth factor signaling. MTORC1 is a critical regulator of translation initiation and ribosome biogenesis and plays an evolutionarily conserved role in cell growth control (reviewed in Wullschleger et al., 2006). The enhanced sensitivity of cancer cells and mouse tumor models exhibiting oncogenic activation of the PI3K-Akt pathway to mTORC1 inhibitors, such as rapamycin, illustrates the importance of mTORC1 activation downstream of Akt (reviewed in Sabatini, 2006). Although Akt activation has been known for some time to effect downstream substrates of mTORC1, such as S6K1 and eukaryotic initiation factor 4E (eIF4E)-binding protein 1 (4E-BP1), the mechanism by which Akt regulates mTORC1 signaling to these targets was more difficult to ascertain. Akt has been suggested to directly phosphorylate mTOR on S2448, which often correlates with mTORC1 activation, but mutational analysis has failed to demonstrate any functional significance for this phosphorylation (Scott et al., 1998; Sekulic et al., 2000). Moreover, S6K1 rather than Akt has recently been shown to be responsible for phosphorylation of this site (Chiang and Abraham, 2005; Holz and Blenis, 2005).
Complementary approaches by several independent laboratories using Drosophila genetics and mammalian cell biology found that the tuberous sclerosis complex 2 (TSC2; also known as tuberin) tumor suppressor is a critical negative regulator of mTORC1 signaling (Gao and Pan, 2001; Goncharova et al., 2002; Tapon et al., 2001) and that Akt-mediated phosphorylation inhibits TSC2 function (Inoki et al., 2002; Manning et al., 2002; Potter et al., 2002). Akt has been shown to directly phosphorylate two sites on TSC2 (S939 and T1462 on the full-length human protein), which are conserved and phosphorylated in Drosophila TSC2, and is likely to phosphorylate two or three additional sites (S981 and S1130/S1132). The relative importance of these distinct sites for Akt’s regulation of TSC2 downstream of different stimuli has yet to be determined. However, various phosphorylation-site mutants (S/T to A) of TSC2 can dominantly block Akt-mediated activation of mTORC1 signaling (Cai et al., 2006; Inoki et al., 2002; Manning et al., 2002). TSC2, in a complex with its binding partner TSC1 (also known as hamartin), acts as a GTPase-activating protein (GAP) for the Ras-related small G protein Rheb, which strongly activates mTORC1 when in its GTP-bound active form (Figure 1; reviewed in Manning and Cantley, 2003). Therefore, Akt activates mTORC1 indirectly by inhibiting TSC2, thereby allowing Rheb-GTP to activate mTORC1 signaling.
More recently, a second Akt substrate has been found to be involved in mTORC1 regulation. Like TSC2 (Manning et al., 2002), the proline-rich Akt substrate of 40 kDa (PRAS40) was identified using the Akt phospho-substrate antibody and was found to be a major protein bound to 14-3-3 in response to insulin (Kovacina et al., 2003). PRAS40 was independently identified (dubbed p39) for its ability to bind to 14-3-3 proteins in a growth-factor- and nutrient-regulated manner (Harthill et al., 2002). Although the function of the protein was unknown, Akt was shown to directly phosphorylate PRAS40 on T246 (Kovacina et al., 2003), and this phosphorylation was later shown to be important for 14-3-3 binding (Vander Haar et al., 2007). It should be noted that T246 has the minimal requirements for an Akt phosphorylation site, yet it scores significantly low relative to other Akt substrates (Table S1), suggesting that additional kinases might also phosphorylate this site. Importantly, PRAS40 has been found to associate with mTORC1 and appears to negatively regulate mTORC1 signaling (Sancak et al., 2007; Vander Haar et al., 2007). Overexpression of a PRAS40T246A mutant can block Akt activation of S6K1, suggesting that Akt-mediated phoshorylation of PRAS40 at T246 stimulates mTORC1 signaling. In addition, there is a weak homolog of PRAS40 in Drosophila called Lobe, which contains a site with similarity to T246, and Lobe appears to behave like PRAS40 in Drosophila S2 cells (Sancak et al., 2007). A requirement for Lobe phosphorylation in parallel to TSC2 phosphorylation might explain the lack of an obvious developmental phenotype in Drosophila expressing an Akt phosphorylation-site mutant of TSC2 (Dong and Pan, 2004). Therefore, there appears to be two parallel and, perhaps, functionally redundant mechanisms by which Akt activates mTORC1 (Figure 1). It will be interesting to determine the relative importance of TSC2 and PRAS40 for Akt-mediated mTORC1 activation under different physiological conditions.
Aside from the ability of mTORC1 to enhance protein synthesis through its downstream targets, additional anabolic processes through which Akt stimulates an increase in cell size are poorly defined. Both mTORCI-dependent and -independent functions of Akt in nutrient uptake and metabolism are likely to contribute to increases in cell growth (e.g., Edinger and Thompson, 2002; Plas and Thompson, 2005). In addition to increasing protein content, in order for a cell to gain size it must increase membrane biosynthesis. One suggested target of Akt that would contribute to the synthesis on new lipids is ATP citrate lyase (ACL; Berwick et al., 2002), which converts citrate to cytosolic acetyl CoA thereby providing the essential building block for lipid biosynthesis. However, the proposed Akt phosphorylation site on ACL (S454; P-S-R-T-A-S-F) lacks the critical −5 R. It will be important to examine the phosphorylation of this site in the Akt loss-of-function mouse models described above.
Cell Proliferation
Although Akt is best known for promoting cell survival and growth through pathways parallel to Erk’s control over cell proliferation, Akt activation can also stimulate proliferation through multiple downstream targets impinging on cell-cycle regulation. Several groups have independently found that Akt phosphorylates the p27Kip1 cyclin-dependent kinase inhibitor on T157 (Liang et al., 2002; Shin et al., 2002; Viglietto et al., 2002), and this leads to cytosolic sequestration via 14-3-3 binding (Sekimoto et al., 2004). Preventing p27 localization to the nucleus attenuates its cell-cycle inhibitory effects. Expression of a phosphorylation-site mutant of p27 (p27-T157A) blocks the proliferative effects of various constitutively active Akt derivatives (Liang et al., 2002; Shin et al., 2002; Viglietto et al., 2002). However, Akt-mediated phosphorylation of p27 is not required for its effects on proliferation in all systems, as T157 is not conserved in rodent versions of p27. Akt also inhibits p27 expression through phosphorylation and inhibition of the FOXO transcription factors (Medema et al., 2000). Akt has also been found to phosphorylate the cyclin-dependent kinase inhibitor p21Cip1/WAF1 on T145, which is a low-scoring Akt site on Scansite, and, like p27, this phosphorylation leads to p21 cytosolic localization (Zhou et al., 2001a). Interestingly, it has been suggested that Akt1, but not Akt2, phosphorylates and inhibits p21, whereas Akt2 appears to bind and stabilize p21, thereby blocking cell-cycle progression (Heron-Milhavet et al., 2006). Akt might also inhibit p21 expression through its phosphorylation and activation of MDM2 and subsequent down-regulation of p53-mediated transcription of p21 (Mayo and Donner, 2001; Zhou et al., 2001b).
Akt-dependent phosphorylation of targets already discussed, such as GSK3, TSC2, and PRAS40, is also likely to drive cell proliferation through regulation of the stability and synthesis of proteins involved in cell-cycle entry. GSK3-mediated phosphorylation of the G1 cyclins cyclin D and cyclin E and the transcripton factors c-jun and c-myc, which all play a central role in the G1-to-S-phase cell-cycle transition, targets them for proteasomal degradation (Diehl et al., 1998; Wei et al., 2005; Welcker et al., 2003; Yeh et al., 2004). Therefore, phosphorylation and inhibition of GSK3 by Akt should enhance the stability of these proteins. Akt signaling also controls the translation of proteins important for cell-cycle progression, predominantly through phosphorylation of TSC2 and PRAS40 and subsequent activation of mTORC1. Although best known for its role in promoting cell growth, mTORC1 is a critical regulator of cell proliferation. The predominant cellular effect of mTORC1 inhibitors, such as rapamycin, is to cause a G1 cell-cycle arrest, and Akt-mediated cell proliferation and oncogenic transformation has recently been shown to be dependent on mTORC1 activation (Skeen et al., 2006). Akt signaling leads to activation of eIF4E through mTORC1’s inhibition of 4E-BP1, and eIF4E promotes the cap-dependent translation of many target mRNAs, including those encoding cyclin D1 and c-Myc (reviewed in Mamane et al., 2004). Therefore, as with cell survival and growth, Akt controls cell proliferation through multiple complementary downstream pathways.
Whether PI3K-Akt signaling is important in the transition through other phases of the cell cycle is less clear. In some cell lines, Akt activity has been shown to be elevated during the G2/M phase of the cell cycle, and Akt activation promotes progression through mitosis, even in the presence of DNA damage (Kandel et al., 2002; Shtivelman et al., 2002). One mechanism explaining this observation is that Akt directly phosphorylates the DNA damage checkpoint kinase Chk1 on S280 (King et al., 2004; Puc et al., 2005). S280 phosphorylation blocks checkpoint function by stimulating Chk1 translocation to the cytosol, where it is sequestered from the DNA damage-sensing kinases ATM and ATR (Puc et al., 2005). Therefore, loss of the PTEN tumor suppressor causes a checkpoint defect due to constitutive Akt activation and phosphorylation of Chk1, and this might contribute to genomic instability in this setting.
Angiogenesis
Akt plays important roles in both physiological and pathological angiogenesis through effects in both endothelial cells and cells producing angiogenic signals, such as tumor cells. In endothelial cells, the PI3K-Akt pathway is robustly activated by vascular endothelial growth factor (VEGF; reviewed in Olsson et al., 2006), and phosphorylation of the Akt targets discussed above is likely to contribute to endothelial cell survival, growth, and proliferation. In addition, Akt activates endothelial nitric oxide (NO) synthase (eNOS) through direct phosphorylation of S1177 (Dimmeler et al., 1999; Fulton et al., 1999). The release of NO produced by activated eNOS can stimulate vasodilation, vascular remodeling, and angiogenesis (reviewed in Morbidelli et al., 2003). Akt signaling also leads to an increased production of the hypoxia-inducible factor α (HIF1α and HIF2α) transcription factors, at least in part, through mTORC1-dependent translation (reviewed in Gordan and Simon, 2007; Semenza, 2003). HIFα activation in endothelial cells and other cells leads to expression and subsequent secretion of VEGF and other angiogenic factors, thereby stimulating angiogenesis through both autocrine and paracrine signaling. Finally, Akt1, the primary isoform in endothelial cells, is required for proper endothelial cell migration (Ackah et al., 2005), although the relevant downstream targets have not been defined.
Cellular Metabolism
In response to growth factors, Akt signaling regulates nutrient uptake and metabolism in a cell-intrinsic and cell-type-specfic manner through a variety of downstream targets. One of the most important physiological functions of Akt is to acutely stimulate glucose uptake in response to insulin. Akt2, the primary isoform in insulin-responsive tissues, has been found to associate with glucose transporter 4 (Glut4)-containing vesicles upon insulin stimulation of adipocytes (Calera et al., 1998), and Akt activation leads to Glut4 translocation to the plasma membrane (Kohn et al., 1996). Although the precise molecular mechanisms by which Akt stimulates Glut4 translocation are still being intensely studied, the Rab-GAP AS160 (also known as TBC1 domain family member 4; TBC1D4) has emerged as an important direct target of Akt involved in this process (Eguez et al., 2005; Sano et al., 2003). Five putative Akt sites are phosphorylated on AS160 in response to insulin, of which S588 and T642 score the highest using the Scansite program. Importantly, mutation of these two sites to alanine significantly blocks insulin-stimulated Glut4 translocation (Sano et al., 2003). The current model is that Akt-mediated phosphorylation of some combination of the sites on AS160 inhibits its GAP activity, thereby allowing a Rab-family GTPase to become GTP loaded and stimulate Glut4 vesicle translocation. However, recent studies have suggested AS160-independent mechanisms of regulation of this process (Bai et al., 2007), and other candidate Akt substrates involved in various steps of Glut4 translocation have been identified, including PIKfyve (Berwick et al., 2004). Glut1 is the main glucose transporter in most cell types, and unlike Glut4, it appears to be regulated primarily through alterations in expression levels. Activation of mTORC1, through Akt-mediated phosphorylation of TSC2 and PRAS40, can contribute to both HIFα-dependent transcription of the Glut1 gene and cap-dependent translation of Glut1 mRNA (e.g., Taha et al., 1999; Zelzer et al., 1998). The frequent occurrence of PI3K-Akt pathway activation and HIFα accumulation in human cancers is likely to explain the high levels of Glut1 and enhanced glucose uptake observed in tumors (reviewed in Majumder and Sellers, 2005; Semenza, 2003). In addition to glucose uptake, Akt has also been suggested to regulate the cell surface expression of transporters for other nutrients, such as amino acids, in an mTORC1-dependent manner (Edinger and Thompson, 2002). However, the mechanism and broader applicability of this effect of Akt and mTORC1 are currently unclear.
Akt activation can also alter glucose and lipid metabolism within cells. Upon entry into the cell, glucose is converted to its active form glucose 6-phosphate through the action of hexokinases. Akt has been demonstrated to stimulate the association of hexokinase isoforms with the mitochondria, where they more readily phosphorylate glucose, but the direct target of Akt responsible is currently unknown (reviewed in Robey and Hay, 2006). Glucose 6-phosphate can be stored by conversion to glycogen or catabolized to produce cellular energy through glycolysis, and Akt signaling can regulate both of these processes. Particularly important in muscle and liver, Akt-mediated phosphorylation and inhibition of GSK3 prevents GSK3 from phosphorylating and inhibiting its name-sake substrate glycogen synthase, thereby stimulating glycogen synthesis. Akt activation also increases the rate of glycolysis (Elstrom et al., 2004), and this is probably a major factor contributing to the highly glycolytic nature of tumor cells. Akt’s ability to enhance the rate of glycolysis is due, at least in part, to its ability to promote the expression of glycolytic enzymes through HIFα (e.g., Lum et al., 2007; Majumder et al., 2004; Semenza et al., 1994). In a cell-context-dependent manner, Akt-mediated phosphorylation and inhibition of FOXO1 also contribute to glucose homeostasis, as FOXO1 promotes hepatic glucose production and regulates the differentiation of cells involved in metabolic control (reviewed in Accili and Arden, 2004). In hepatocytes, Akt can also inhibit gluconeogenesis and fatty acid oxidation through direct phosphorylation of S570 on PGC-1α (Li et al., 2007), which is a coactivator that can coregulate genes with FOXO1 and other transcription factors. Akt signaling regulates lipid metabolism through phosphorylation and inhibition of GSK3. As described above, phosphorylation of substrates by GSK3 often targets them for proteasomal degradation, and GSK3 has been shown to promote degradation of the sterol regulatory element-binding proteins (SREBPs), which are transcription factors that turn on the expression of genes involved in cholesterol and fatty acid biosynthesis (Sundqvist et al., 2005). Therefore, Akt-mediated inhibition of GSK3 promotes SREBP stability and enhances lipid production. As discussed above, Akt has also been proposed to directly activate ACL (Berwick et al., 2002), with the caveat that the relevant phosphorylation site on this enzyme does not meet the minimal consensus for an Akt site. As intense interest in the role of the PI3K-Akt pathway in metabolic diseases and tumor metabolism continues, it is likely that Akt signaling will be found to impinge on many other areas of central metabolism.
Cell Migration and Invasion
Until recently, a role for Akt in the control of cell migration, invasion of the extracellular matrix, and ultimately metastasis has been difficult to ascertain. Strikingly, activation of Akt1 has been found to decrease mammary epithelial cell migration, and Akt1 prevents an epithelial-to-mesenchymal transition that resembles events required for metastasis (Irie et al., 2005; Yoeli-Lerner et al., 2005). Two independent mechanisms for this surprising Akt function have been explored. The first found that the inhibitory effect of Akt1 on the in vitro migration and invasion properties of breast cancer cell lines involved a pathway leading to degradation of the nuclear factor of activated T cells (NFAT) transcription factors (Yoeli-Lerner et al., 2005). However, the molecular mechanism of Akt1-mediated degradation of NFAT is currently unknown. A second group found that siRNA knockdown of Akt1, but not Akt2, led to an increase in the migration of mammary epithelial cells (Irie et al., 2005). Loss of Akt1, specifically, led to an increase in the activation of Erk1 and Erk2, which was found to be required for the enhanced migration. Again, the mechanism by which Akt1, but not Akt2, inhibits Erk signaling in this system remains unknown. Interestingly, mouse tumor models have also suggested that Akt1 inhibits metastases (Hutchinson et al., 2004), whereas Akt2 promotes metastases (Arboleda et al., 2003). However, these differential effects of Akt1 and Akt2 on epithelial cell migration may not translate to other cell types. In fact, studies on cell migration using mouse embryonic fibroblasts deficient of specific Akt isoforms have suggested opposite effects on fibroblast migration, with Akt1 promoting migration and with Akt2 inhibiting it (Zhou et al., 2006). These studies demonstrate both the importance of crosstalk between the PI3K-Akt pathway and other pathways and the emerging recognition that the three isoforms of Akt can have distinct cellular functions.
Crosstalk with Other Pathways
Extensive research on the wiring of signal transduction pathways over the past ten years has uncovered a level of complexity dictating that no single pathway operates in isolation and that pathways are small parts of fully integrated networks within the cell. This is certainly true of the PI3K-Akt pathway, and we discuss just three examples here. Several studies have demonstrated that Akt signaling can activate the NF-κB transcription factor downstream of a variety of stimuli, such as tumor necrosis factor (TNFα), and platelet-derived growth factor (PDGF; e.g., Ozes et al., 1999; Romashkova and Makarov, 1999). Although there are likely to be multiple levels of crosstalk between the PI3K-Akt and NF-κB pathways, one mechanism has been attributed to direct phosphorylation of the amino acid residue T23 on IκB kinase α (IKKα) by Akt, thereby leading to activation of this kinase upstream of NF-κB (Ozes et al., 1999). However, to date this connection between Akt and IKKα has not been verified conclusively in vivo. Akt also blocks Erk signaling through inhibition of c-Raf (Raf1; Rommel et al., 1999; Zimmermann and Moelling, 1999). Akt can directly phosphorylate c-Raf on T259, and this can lead to an inhibitory effect of Akt on the Erk pathway (Zimmermann and Moelling, 1999). As with NF-κB, this crosstalk between Akt and Erk signaling is not ubiquitous and appears to occur only in specific settings. In addition to Erk, the stress-activated MAPKs JNK and p38 have also been shown to be inhibited by Akt signaling. Akt can directly phosphorylate the amino acid residue S83 on apoptosis signal-regulated kinase 1 (ASK1, also known as MAPKKK5), which is an upstream activating kinase within the JNK and p38 pathways (Kim et al., 2001). Akt blocks the apoptotic stimulus-induced activation of ASK1 in a manner dependent on S83. Therefore, a balance between PI3K-Akt survival signaling and JNK/p38 apoptotic signaling can be established through such crosstalk.
Substrate Selectivity between Akt Isoforms and Other AGC Kinases
From the discussion above, it should be obvious that Akt phosphorylates lots of different targets involved in a large variety of cellular processes and that it is unlikely that Akt hits all of these targets in every setting. Aside from differences in substrate expression under various conditions, Akt is also likely to have stimulus-specific differences in its substrate utilization. Although there are many possible explanations, including temporal considerations, subcellular localization, and degree of kinase activation, the mechanisms dictating substrate selectivity are poorly understood. Recent observations using mouse embryonic fibroblasts lacking components of the Akt-activating kinase mTORC2 have suggested that phosphorylation of Akt at S473 is required for its ability to properly phosphorylate the N-terminal site on the FOXO proteins, but phosphorylation of Akt-S473 does not appear to be required for Akt-mediated phosphorylation of the other FOXO sites or the sites on TSC2 and GSK3 (Guertin et al., 2006; Jacinto et al., 2006). Therefore, it has been argued that S473 phosphorylation can dictate differential substrate utilization (Jacinto et al., 2006). Another viable explanation is that different substrates, and even different sites on a single substrate, require different levels of Akt activity to reach saturation or to be phosphorylated to an extent that we can detect experimentally (i.e., using available phospho-specific antibodies). To this end, it is has been known for some time that S473 phoshorylation is not essential for growth-factor stimulation of Akt, but it does result in a 5- to 10-fold increase in Akt activity (Alessi et al., 1996a). However, to our knowledge, there are no physiological stimuli that result in T308 phosphorylation in the absence of S473 phosphorylation. Although it is an intriguing possibility, further work is clearly required to support a model in which S473 dictates a substrate-switching mechanism.
An exciting area of Akt research is the increasing number of studies demonstrating isoform-specific functions for Akt. The three Akt isoforms are very similar, especially within their kinase domains, and it is unlikely that they have distinguishable substrate specificities in vitro. Furthermore, Akt1 and Akt2 are expressed ubiquitously, and many cell lines express all three isoforms. However, mouse knockouts have uncovered distinct physiological functions for the three Akt isoforms: Akt1-deficient mice display developmental defects, Akt2-deficient mice have defects in glucose homeostasis, and Akt3-deficient mice show defects in brain development (reviewed in Dummler and Hemmings, 2007). Although combined knockouts have illustrated that functional redundancy exists between the three isoforms, the distinct phenotypes resulting from loss of each individually suggests that there are likely to be functional differences at the cellular level. The first clear example of this was the role of Akt in stimulating Glut4 translocation in adipocytes, where it was found that Akt2 rather than Akt1 was the primary isoform responsible (e.g., Katome et al., 2003). A second example is the one discussed above regarding the differential effects of Akt1 and Akt2 on cell migration (Irie et al., 2005; Zhou et al., 2006). In both instances, there must be an isoform-specific substrate to which the other isoforms have less access or ability to recognize. A recent study on the role of two Akt-S473 phosphatases, PHLPP1 and PHLPP2, which differentially regulate the three Akt isoforms, has emphasized the extent to which Akt substrates are isoform specific (Brognard et al., 2007). A survey of the phosphorylation status of six known Akt substrates following siRNA-mediated knockdown of each of the three Akt isoforms beautifully demonstrated that each isoform has both unique and overlapping substrate specificities. More work is required to determine if the specificities observed pertain to all cell lines and, most importantly, what dictates this specificity.
A further complicating factor in the study of Akt signaling is that other AGC family members can, in certain settings, phosphorylate sites previously characterized to be bona fide Akt targets. This often occurs in situations where a stimulus or genetic lesion strongly activates an AGC kinase in the absence of Akt activation. For instance, S6K1 can phosphorylate GSK3 on its Akt site under conditions of elevated mTORC1-S6K1 signaling, which triggers a negative feedback mechanism blocking growth-factor activation of Akt (Zhang et al., 2006). However, other AGC kinases can also be redundant with Akt under circumstances where Akt remains active and capable of phosphorylating a specific site. This appears to be the case for T32 on FOXO3a, where both Akt and the closely related AGC kinase SGK can phosphorylate this site in response to PI3K-activating stimuli (Brunet et al., 2001). How widespread such compensatory and redundant mechanisms are in Akt signaling is not known, but the potential for alternative regulation should be kept in mind when examining any seemingly well-characterized target in a novel setting.
The Future of Akt Research
Incredible insight into the regulation and function of Akt has been gained over the past ten years. However, the clear recognition that defects in Akt signaling underlie a diverse array of human diseases makes the clarification of downstream targets and functions of central importance to our understanding and treatment of such diseases. As our ability to knockout, knockdown, or pharmacologically inhibit specific Akt isoforms and related AGC kinases has significantly improved, previously identified substrates should be more rigorously confirmed. More advanced animal models, such as knockins encoding catalytically inactive mutants of the three Akt isoforms–which should be less susceptible to functional compensation than complete knockouts–will likely provide a more accurate picture of isoform-specific substrates and functions. Knockin mutants encoding substrates lacking Akt phosphorylation sites will allow a critical determination of the in vivo relevance and contribution of defined phosphorylation events to specific physiological and pathological states. With respect to determinants of Akt substrate specificity within cells, many difficult questions remain. How do the three Akt isoforms differ in their regulation, localization, and substrate utilization? What is the degree of functional redundancy between the Akt isoforms and other AGC kinases? Which substrates are subjected to such redundancy, and how is this dictated? Do Akt isoforms use docking sites separate from phosphorylation sites to recognize substrates? How do different Akt-activating stimuli impinge on substrate selectivity? It will also be important to further define the role of crosstalk between distinct downstream branches of the PI3K-Akt pathway, as well as with other signaling pathways, in the ultimate physiological outcome of Akt activation. Our ability to grasp the complex circuitry of Akt signaling and to predict the consequences of altering specific components of the larger network will require systems biological approaches as a complement to genetics and biochemistry. Although the past ten years have been spent assembling a parts list, the challenging work of piecing it all together and figuring out how it really works lies ahead.
Supplementary Material
Acknowledgments
Work on Akt signaling in the Manning and Cantley laboratories is supported by the American Diabetes Association (B.D.M.) and NIH grants CA122617 (B.D.M.), P01-CA120964 (B.D.M.), GM41890 (L.C.C.), and P01-CA089021 (L.C.C.).
Footnotes
Supplemental Data include one table and can be found with this article online at http://www.cell.com/cgi/content/full/129/7/1261/DC1/.
References
- Accili D, Arden KC. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell. 2004;117:421–426. doi: 10.1016/s0092-8674(04)00452-0. [DOI] [PubMed] [Google Scholar]
- Ackah E, Yu J, Zoellner S, Iwakiri Y, Skurk C, Shibata R, Ouchi N, Easton RM, Galasso G, Birnbaum MJ, et al. Akt1/protein kinase Balpha is critical for ischemic and VEGF-mediated angiogenesis. J Clin Invest. 2005;115:2119–2127. doi: 10.1172/JCI24726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessi DR, Andjekovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings BA. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996a;15:6541–6551. [PMC free article] [PubMed] [Google Scholar]
- Alessi DR, Caudwell FB, Andejelkovic M, Hemmings BA, Cohen P. Molecular basis for the substrate specificity of protein kinase B; comparison with MAPKAP kinase-1 and p70 S6 kinase. FEBS Lett. 1996b;399:333–338. doi: 10.1016/s0014-5793(96)01370-1. [DOI] [PubMed] [Google Scholar]
- Andjelkovic M, Alessi D, Meier R, Fernandez A, Lamb NJC, Frech M, Cron P, Cohen P, Lucocq JM, Hemmings BA. Role of translocation in the activation and function of protein kinase B. J Biol Chem. 1997;272:31515–31524. doi: 10.1074/jbc.272.50.31515. [DOI] [PubMed] [Google Scholar]
- Arboleda MJ, Lyons JF, Kabbinavar FF, Bray MR, Snow BE, Ayala R, Danino M, Karlan BY, Slamon DJ. Overexpression of AKT2/protein kinase Bbeta leads to up-regulation of beta1 integrins, increased invasion, and metastasis of human breast and ovarian cancer cells. Cancer Res. 2003;63:196–206. [PubMed] [Google Scholar]
- Bai L, Wang Y, Fan J, Chen Y, Ji W, Qu A, Xu P, James DE, Xu T. Dissecting multiple steps of GLUT4 trafficking and identifying the sites of insulin action. Cell Metab. 2007;5:47–57. doi: 10.1016/j.cmet.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Bellacosa A, Testa JR, Staal SP, Tsichlis PN. A retroviral oncogene, akt, encoding a serine-threonine kinase containing an SH2-like region. Science. 1991;254:274–277. doi: 10.1126/science.254.5029.274. [DOI] [PubMed] [Google Scholar]
- Bellacosa A, Kumar CC, Di Cristofano A, Testa JR. Activation of AKT kinases in cancer: implications for therapeutic targeting. Adv Cancer Res. 2005;94:29–86. doi: 10.1016/S0065-230X(05)94002-5. [DOI] [PubMed] [Google Scholar]
- Berwick DC, Hers I, Heesom KJ, Moule SK, Tavare JM. The identification of ATP-citrate lyase as a protein kinase B (Akt) substrate in primary adipocytes. J Biol Chem. 2002;277:33895–33900. doi: 10.1074/jbc.M204681200. [DOI] [PubMed] [Google Scholar]
- Berwick DC, Dell GC, Welsh GI, Heesom KJ, Hers I, Fletcher LM, Cooke FT, Tavare JM. Protein kinase B phosphorylation of PIKfyve regulates the trafficking of GLUT4 vesicles. J Cell Sci. 2004;117:5985–5993. doi: 10.1242/jcs.01517. [DOI] [PubMed] [Google Scholar]
- Brognard J, Sierecki E, Gao T, Newton AC. PHLPP and a second isoform, PHLPP2, differentially attenuate the amplitude of Akt signaling by regulating distinct Akt isoforms. Mol Cell. 2007;25:917–931. doi: 10.1016/j.molcel.2007.02.017. [DOI] [PubMed] [Google Scholar]
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- Brunet A, Park J, Tran H, Hu LS, Hemmings BA, Greenberg ME. Protein kinase SGK mediates survival signals by phosphorylating the forkhead transcription factor FKHRL1 (FOXO3a) Mol Cell Biol. 2001;21:952–965. doi: 10.1128/MCB.21.3.952-965.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai SL, Tee AR, Short JD, Bergeron JM, Kim J, Shen J, Guo R, Johnson CL, Kiguchi K, Walker CL. Activity of TSC2 is inhibited by AKT-mediated phosphorylation and membrane partitioning. J Cell Biol. 2006;173:279–289. doi: 10.1083/jcb.200507119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calera MR, Martinez C, Liu H, Jack AK, Birnbaum MJ, Pilch PF. Insulin increases the association of Akt-2 with Glut4-containing vesicles. J Biol Chem. 1998;273:7201–7204. doi: 10.1074/jbc.273.13.7201. [DOI] [PubMed] [Google Scholar]
- Cardone MH, Roy N, Stennicke HR, Salvasen GS, Franke TF, Stanbridge E, Frisch S, Reed JC. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- Chiang GG, Abraham RT. Phosphorylation of mammalian target of rapamycin (mTOR) at Ser-2448 is mediated by p70S6 kinase. J Biol Chem. 2005;280:25485–25490. doi: 10.1074/jbc.M501707200. [DOI] [PubMed] [Google Scholar]
- Collins BJ, Deak M, Arthur JS, Armit LJ, Alessi DR. In vivo role of the PIF-binding docking site of PDK1 defined by knock-in mutation. EMBO J. 2003;22:4202–4211. doi: 10.1093/emboj/cdg407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature . 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- Datta SR, Katsov A, Hu L, Petros A, Fesik SW, Yaffe MB, Greenberg ME. 14–3-3 proteins and survival kinases cooperate to inactivate BAD by BH3 domain phosphorylation. Mol Cell. 2000;6:41–51. [PubMed] [Google Scholar]
- Datta SR, Ranger AM, Lin MZ, Sturgill JF, Ma YC, Cowan CW, Dikkes P, Korsmeyer SJ, Greenberg ME. Survival factor-mediated BAD phosphorylation raises the mitochondrial threshold for apoptosis. Dev Cell. 2002;3:631–643. doi: 10.1016/s1534-5807(02)00326-x. [DOI] [PubMed] [Google Scholar]
- delPeso L, Gonzalez-Garcia M, Page C, Herrera R, Nunez G. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science. 1997;278:687–689. doi: 10.1126/science.278.5338.687. [DOI] [PubMed] [Google Scholar]
- Diehl JA, Cheng M, Roussel MF, Sherr CJ. Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 1998;12:3499–3511. doi: 10.1101/gad.12.22.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkers PF, Birkenkamp KU, Lam EW, Thomas NS, Lammers JW, Koenderman L, Coffer PJ. FKHR-L1 can act as a critical effector of cell death induced by cytokine withdrawal: protein kinase B-enhanced cell survival through maintenance of mitochondrial integrity. J Cell Biol. 2002;156:531–542. doi: 10.1083/jcb.200108084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- Dong J, Pan D. Tsc2 is not a critical target of Akt during normal Drosophila development. Genes Dev. 2004;18:2479–2484. doi: 10.1101/gad.1240504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dummler B, Hemmings BA. Physiological roles of PKB/Akt isoforms in development and disease. Biochem Soc Trans. 2007;35:231–235. doi: 10.1042/BST0350231. [DOI] [PubMed] [Google Scholar]
- Edinger AL, Thompson CB. Akt maintains cell size and survival by increasing mTOR-dependent nutrient uptake. Mol Biol Cell. 2002;13:2276–2288. doi: 10.1091/mbc.01-12-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguez L, Lee A, Chavez JA, Miinea CP, Kane S, Lienhard GE, McGraw TE. Full intracellular retention of GLUT4 requires AS160 Rab GTPase activating protein. Cell Metab. 2005;2:263–272. doi: 10.1016/j.cmet.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Elstrom RL, Bauer DE, Buzzai M, Karnauskas R, Harris MH, Plas DR, Zhuang H, Cinalli RM, Alavi A, Rudin CM, Thompson CB. Akt stimulates aerobic glycolysis in cancer cells. Cancer Res. 2004;64:3892–3899. doi: 10.1158/0008-5472.CAN-03-2904. [DOI] [PubMed] [Google Scholar]
- Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- Frame S, Cohen P. GSK3 takes centre stage more than 20 years after its discovery. Biochem J. 2001;359:1–16. doi: 10.1042/0264-6021:3590001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton D, Gratton J, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Pan D. TSC1 and TSC2 tumor suppressors antagonize insulin signaling in cell growth. Genes Dev. 2001;15:1383–1392. doi: 10.1101/gad.901101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncharova EA, Goncharov DA, Eszterhas A, Hunter DS, Glassberg MK, Yeung RS, Walker CL, Noonan D, Kwiatkowski DJ, Chou MM, et al. Tuberin regulates p70 S6 kinase activation and ribosomal protein S6 phosphorylation: a role for the TSC2 tumor suppressor gene in pulmonary lymphangioleiomyomatosis (LAM) J Biochem (Tokyo) 2002;277:30958–30967. doi: 10.1074/jbc.M202678200. [DOI] [PubMed] [Google Scholar]
- Gordan JD, Simon MC. Hypoxia-inducible factors: central regulators of the tumor phenotype. Curr Opin Genet Dev. 2007;17:71–77. doi: 10.1016/j.gde.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami A, Burikhanov R, de Thonel A, Fujita N, Goswami M, Zhao Y, Eriksson JE, Tsuruo T, Rangnekar VM. Binding and phosphorylation of par-4 by akt is essential for cancer cell survival. Mol Cell. 2005;20:33–44. doi: 10.1016/j.molcel.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Guertin DA, Stevens DM, Thoreen CC, Burds AA, Kalaany NY, Moffat J, Brown M, Fitzgerald KJ, Sabatini DM. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PK-Calpha, but not S6K1. Dev Cell. 2006;11:859–871. doi: 10.1016/j.devcel.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Harthill JE, Pozuelo Rubio M, Milne FC, MacKintosh C. Regulation of the 14–3-3-binding protein p39 by growth factors and nutrients in rat PC12 pheochromocytoma cells. Biochem J. 2002;368:565–572. doi: 10.1042/BJ20020838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heron-Milhavet L, Franckhauser C, Rana V, Berthenet C, Fisher D, Hemmings BA, Fernandez A, Lamb NJ. Only Akt1 is required for proliferation, while Akt2 promotes cell cycle exit through p21 binding. Mol Cell Biol. 2006;26:8267–8280. doi: 10.1128/MCB.00201-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz MK, Blenis J. Identification of S6 kinase 1 as a novel mammalian target of rapamycin (mTOR)-phosphorylating kinase. J Biol Chem. 2005;280:26089–26093. doi: 10.1074/jbc.M504045200. [DOI] [PubMed] [Google Scholar]
- Hutchinson JN, Jin J, Cardiff RD, Woodgett JR, Muller WJ. Activation of Akt-1 (PKB-alpha) can accelerate ErbB-2-mediated mammary tumorigenesis but suppresses tumor invasion. Cancer Res. 2004;64:3171–3178. doi: 10.1158/0008-5472.can-03-3465. [DOI] [PubMed] [Google Scholar]
- Hutti JE, Jarrell ET, Chang JD, Abbott DW, Storz P, Toker A, Cantley LC, Turk BE. A rapid method for determining protein kinase phosphorylation specificity. Nat Methods. 2004;1:27–29. doi: 10.1038/nmeth708. [DOI] [PubMed] [Google Scholar]
- Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol. 2002;4:648–657. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- Irie HY, Pearline RV, Grueneberg D, Hsia M, Ravichandran P, Kothari N, Natesan S, Brugge JS. Distinct roles of Akt1 and Akt2 in regulating cell migration and epithelial-mesenchymal transition. J Cell Biol. 2005;171:1023–1034. doi: 10.1083/jcb.200505087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacinto E, Facchinetti V, Liu D, Soto N, Wei S, Jung SY, Huang Q, Qin J, Su B. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell. 2006;127:125–137. doi: 10.1016/j.cell.2006.08.033. [DOI] [PubMed] [Google Scholar]
- Jones PF, Jakubowicz T, Pitossi FJ, Maurer F, Hemmings BA. Molecular cloning and identification of a serine/threonine protein kinase of the second-messenger subfamily. Proc Natl Acad Sci USA. 1991;88:4171–4175. doi: 10.1073/pnas.88.10.4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel ES, Skeen J, Majewski N, Di Cristofano A, Pandolfi PP, Feliciano CS, Gartel A, Hay N. Activation of Akt/protein kinase B overcomes a G(2)/m cell cycle checkpoint induced by DNA damage. Mol Cell Biol. 2002;22:7831–7841. doi: 10.1128/MCB.22.22.7831-7841.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katome T, Obata T, Matsushima R, Masuyama N, Cantley LC, Gotoh Y, Kishi K, Shiota H, Ebina Y. Use of RNA interference-mediated gene silencing and adenoviral overexpression to elucidate the roles of AKT/protein kinase B isoforms in insulin actions. J Biol Chem. 2003;278:28312–28323. doi: 10.1074/jbc.M302094200. [DOI] [PubMed] [Google Scholar]
- Kim AH, Khursigara G, Sun X, Franke TF, Chao MV. Akt phosphorylates and negatively regulates apoptosis signal-regulating kinase 1. Mol Cell Biol. 2001;21:893–901. doi: 10.1128/MCB.21.3.893-901.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King FW, Skeen J, Hay N, Shtivelman E. Inhibition of Chk1 by activated PKB/Akt. Cell Cycle. 2004;3:634–637. [PubMed] [Google Scholar]
- Kohn AD, Summers SA, Birnbaum MJ, Roth RA. Expression of a constitutively active Akt Ser/Thr kinase in 3T3–L1 adipocytes stimulates glucose uptake and glucose transporter 4 translocation. J Biol Chem. 1996;271:31372–31378. doi: 10.1074/jbc.271.49.31372. [DOI] [PubMed] [Google Scholar]
- Kovacina KS, Park GY, Bae SS, Guzzetta AW, Schaefer E, Birnbaum MJ, Roth RA. Identification of a proline-rich Akt substrate as a 14–3-3 binding partner. J Biol Chem. 2003;278:10189–10194. doi: 10.1074/jbc.M210837200. [DOI] [PubMed] [Google Scholar]
- Li X, Monks B, Ge Q, Birnbaum MJ. Akt/PKB regulates hepatic metabolism by directly inhibiting PGC-1α transcription coactivator. Nature. 2007 doi: 10.1038/nature05861. in press Published online June 6, 2007. [DOI] [PubMed] [Google Scholar]
- Liang J, Zubovitz J, Petrocelli T, Kotchetkov R, Connor MK, Han K, Lee JH, Ciarallo S, Catzavelos C, Beniston R, et al. PKB/Akt phosphorylates p27, impairs nuclear import of p27 and opposes p27-mediated G1 arrest. Nat Med. 2002;8:1153–1160. doi: 10.1038/nm761. [DOI] [PubMed] [Google Scholar]
- Lum JJ, Bui T, Gruber M, Gordan JD, DeBerardinis RJ, Covello KL, Simon MC, Thompson CB. The transcription factor HIF-1alpha plays a critical role in the growth factor-dependent regulation of both aerobic and anaerobic glycolysis. Genes Dev. 2007;21:1037–1049. doi: 10.1101/gad.1529107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumder PK, Sellers WR. Akt-regulated pathways in prostate cancer. Oncogene. 2005;24:7465–7474. doi: 10.1038/sj.onc.1209096. [DOI] [PubMed] [Google Scholar]
- Majumder PK, Febbo PG, Bikoff R, Berger R, Xue Q, McMahon LM, Manola J, Brugarolas J, McDonnell TJ, Golub TR, et al. mTOR inhibition reverses Akt-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nat Med. 2004;10:594–601. doi: 10.1038/nm1052. [DOI] [PubMed] [Google Scholar]
- Mamane Y, Petroulakis E, Rong L, Yoshida K, Ler LW, Sonenberg N. eIF4E–from translation to transformation. Oncogene. 2004;23:3172–3179. doi: 10.1038/sj.onc.1207549. [DOI] [PubMed] [Google Scholar]
- Manning BD, Cantley LC. Rheb fills a GAP between TSC and TOR. Trends Biochem Sci. 2003;28:573–576. doi: 10.1016/j.tibs.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Manning BD, Tee AR, Logsdon MN, Blenis J, Cantley LC. Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/Akt pathway. Mol Cell. 2002;10:151–162. doi: 10.1016/s1097-2765(02)00568-3. [DOI] [PubMed] [Google Scholar]
- Marte BM, Downward J. PKB/Akt: connecting phosphoinositide 3-kinase to cell survival and beyond. Trends Biochem Sci. 1997;22:355–358. doi: 10.1016/s0968-0004(97)01097-9. [DOI] [PubMed] [Google Scholar]
- Maurer U, Charvet C, Wagman AS, Dejardin E, Green DR. Glycogen synthase kinase-3 regulates mitochondrial outer membrane permeabilization and apoptosis by destabilization of MCL-1. Mol Cell. 2006;27:749–760. doi: 10.1016/j.molcel.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Mayo LD, Donner DB. A phosphatidylinositol 3-kinase/Akt pathway promotes translocation of Mdm2 from the cytoplasm to the nucleus. Proc Natl Acad Sci USA. 2001;98:11598–11603. doi: 10.1073/pnas.181181198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus EJ, Collins BJ, Ashby PR, Prescott AR, Murray-Tait V, Armit LJ, Arthur JS, Alessi DR. The in vivo role of PtdIns(3,4,5)P(3) binding to PDK1 PH domain defined by knockin mutation. EMBO J. 2004;23:2071–2082. doi: 10.1038/sj.emboj.7600218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus EJ, Sakamoto K, Armit LJ, Ronaldson L, Shpiro N, Marquez R, Alessi DR. Role that phosphorylation of GSK3 plays in insulin and Wnt signalling defined by knockin analysis. EMBO J. 2005;24:1571–1583. doi: 10.1038/sj.emboj.7600633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medema RH, Kops GJ, Bos JL, Burgering BM. AFX-like Forkhead transcription factors mediate cell-cycle regulation by Ras and PKB through p27kip1. Nature. 2000;404:782–787. doi: 10.1038/35008115. [DOI] [PubMed] [Google Scholar]
- Morbidelli L, Donnini S, Ziche M. Role of nitric oxide in the modulation of angiogenesis. Curr Pharm Des. 2003;9:521–530. doi: 10.2174/1381612033391405. [DOI] [PubMed] [Google Scholar]
- Obata T, Yaffe MB, Leparc GG, Piro ET, Maegawa H, Kashiwagi A, Kikkawa R, Cantley L. Use of peptide and protein library screening to define optimal substrate motifs for Akt/PKB. J Biol Chem. 2000;275:108–115. doi: 10.1074/jbc.M005497200. [DOI] [PubMed] [Google Scholar]
- Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB. NF-κB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature. 1999;401:82–85. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- Paradis S, Ruvkun G. Caenorhabditis elegans Akt/PKB transduces insulin receptor-like signals from AGE-1 PI3 kinase to the DAF-16 transcription factor. Genes Dev. 1998;12:2488–2498. doi: 10.1101/gad.12.16.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plas DR, Thompson CB. Akt-dependent transformation: there is more to growth than just surviving. Oncogene. 2005;24:7435–7442. doi: 10.1038/sj.onc.1209097. [DOI] [PubMed] [Google Scholar]
- Potter CJ, Pedraza LG, Xu T. Akt regulates growth by directly phosphorylating Tsc2. Nat Cell Biol. 2002;4:658–665. doi: 10.1038/ncb840. [DOI] [PubMed] [Google Scholar]
- Puc J, Keniry M, Li HS, Pandita TK, Choudhury AD, Memeo L, Mansukhani M, Murty VV, Gaciong Z, Meek SE, et al. Lack of PTEN sequesters CHK1 and initiates genetic instability. Cancer Cell. 2005;7:193–204. doi: 10.1016/j.ccr.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Robey RB, Hay N. Mitochondrial hexokinases, novel mediators of the antiapoptotic effects of growth factors and Akt. Oncogene. 2006;25:4683–4696. doi: 10.1038/sj.onc.1209595. [DOI] [PubMed] [Google Scholar]
- Romashkova JA, Makarov SS. NF-kappaB is a target of AKT in anti-apoptotic PDGF signalling. Nature. 1999;401:86–90. doi: 10.1038/43474. [DOI] [PubMed] [Google Scholar]
- Rommel C, Clarke BA, Zimmermann S, Nunez L, Rossman R, Reid K, Moelling K, Yancopoulos GD, Glass DJ. Differentiation stage-specific inhibition of the Raf-MEK-ERK pathway by Akt. Science. 1999;286:1738–1741. doi: 10.1126/science.286.5445.1738. [DOI] [PubMed] [Google Scholar]
- Sabatini DM. mTOR and cancer: insights into a complex relationship. Nat Rev Cancer. 2006;6:729–734. doi: 10.1038/nrc1974. [DOI] [PubMed] [Google Scholar]
- Sancak Y, Thoreen CC, Peterson TR, Lindquist RA, Kang SA, Spooner E, Carr SA, Sabatini DM. PRAS40 is an insulin-regulated inhibitor of the mTORC1 protein kinase. Mol Cell. 2007;25:903–915. doi: 10.1016/j.molcel.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Sano H, Kane S, Sano E, Miinea CP, Asara JM, Lane WS, Garner CW, Lienhard GE. Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. J Biol Chem. 2003;278:14599–14602. doi: 10.1074/jbc.C300063200. [DOI] [PubMed] [Google Scholar]
- Sapkota GP, Cummings L, Newell FS, Armstrong C, Bain J, Frodin M, Grauert M, Hoffmann M, Schnapp G, Steegmaier M, et al. BI-D1870 is a specific inhibitor of the p90 RSK (ribosomal S6 kinase) isoforms in vitro and in vivo. Biochem J. 2007;401:29–38. doi: 10.1042/BJ20061088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott PH, Brunn GJ, Kohn AD, Roth RA, Lawrence JC. Evidence of insulin-stimulated phosphorylation and activation of the mammalian target of rapamycin mediated by a protein kinase B signaling pathway. Proc Natl Acad Sci USA. 1998;95:7772–7777. doi: 10.1073/pnas.95.13.7772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekimoto T, Fukumoto M, Yoneda Y. 14–3-3 suppresses the nuclear localization of threonine 157-phosphorylated p27(Kip1) EMBO J. 2004;23:1934–1942. doi: 10.1038/sj.emboj.7600198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekulic A, Hudson CC, Homme JL, Yin P, Otterness DM, Karnitz LM, Abraham RT. A direct linkage between the phosphoinositide 3-kinase-AKT signaling pathway and the mammalian target of rapamycin in mitogen-stimulated and transformed cells. Cancer Res. 2000;60:3504–3513. [PubMed] [Google Scholar]
- Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- Semenza GL, Roth PH, Fang HM, Wang GL. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J Biol Chem. 1994;269:23757–23763. [PubMed] [Google Scholar]
- Shin I, Yakes FM, Rojo F, Shin NY, Bakin AV, Baselga J, Arteaga CL. PKB/Akt mediates cell-cycle progression by phosphorylation of p27(Kip1) at threonine 157 and modulation of its cellular localization. Nat Med. 2002;8:1145–1152. doi: 10.1038/nm759. [DOI] [PubMed] [Google Scholar]
- Shtivelman E, Sussman J, Stokoe D. A role for PI 3-kinase and PKB activity in the G2/M phase of the cell cycle. Curr Biol. 2002;12:919–924. doi: 10.1016/s0960-9822(02)00843-6. [DOI] [PubMed] [Google Scholar]
- Skeen JE, Bhaskar PT, Chen CC, Chen WS, Peng XD, Nogueira V, Hahn-Windgassen A, Kiyokawa H, Hay N. Akt deficiency impairs normal cell proliferation and suppresses oncogenesis in a p53-independent and mTORC1-dependent manner. Cancer Cell. 2006;10:269–280. doi: 10.1016/j.ccr.2006.08.022. [DOI] [PubMed] [Google Scholar]
- Staal SP. Molecular cloning of the akt oncogene and its human homologues AKT1 and AKT2: amplification of AKT1 in a primary human gastric adenocarcinoma. Proc Natl Acad Sci USA. 1987;84:5034–5037. doi: 10.1073/pnas.84.14.5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundqvist A, Bengoechea-Alonso MT, Ye X, Lukiyanchuk V, Jin J, Harper JW, Ericsson J. Control of lipid metabolism by phosphorylation-dependent degradation of the SREBP family of transcription factors by SCF-FBW7. Cell Metab. 2005;1:379–391. doi: 10.1016/j.cmet.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Taha C, Liu Z, Jin J, Al-Hasani H, Sonenberg N, Klip A. Opposite translational control of GLUT1 and GLUT4 glucose transporter mRNAs in response to insulin. Role of mammalian target of rapamycin, protein kinase b, and phosphatidylinositol 3-kinase in GLUT1 mRNA translation. J Biol Chem. 1999;274:33085–33091. doi: 10.1074/jbc.274.46.33085. [DOI] [PubMed] [Google Scholar]
- Tapon N, Ito N, Dickson BJ, Treisman JE, Hariharan IK. The Drosophila tuberous sclerosis complex gene homologs restrict cell growth and cell proliferation. Cell. 2001;105:345–355. doi: 10.1016/s0092-8674(01)00332-4. [DOI] [PubMed] [Google Scholar]
- Tran H, Brunet A, Griffith EC, Greenberg ME. The many forks in FOXO’s road. Sci STKE . 2003;2003:RE5. doi: 10.1126/stke.2003.172.re5. [DOI] [PubMed] [Google Scholar]
- Vander Haar E, Lee SI, Bandhakavi S, Griffin TJ, Kim DH. Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. Nat Cell Biol. 2007;9:316–323. doi: 10.1038/ncb1547. [DOI] [PubMed] [Google Scholar]
- Viglietto G, Motti ML, Bruni P, Melillo RM, D’Alessio A, Califano D, Vinci F, Chiappetta G, Tsichlis P, Bellacosa A, et al. Cytoplasmic relocalization and inhibition of the cyclin-dependent kinase inhibitor p27(Kip1) by PKB/Akt-mediated phosphorylation in breast cancer. Nat Med. 2002;8:1136–1144. doi: 10.1038/nm762. [DOI] [PubMed] [Google Scholar]
- Villunger A, Michalak EM, Coultas L, Mullauer F, Bock G, Ausserlechner MJ, Adams JM, Strasser A. p53- and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science. 2003;302:1036–1038. doi: 10.1126/science.1090072. [DOI] [PubMed] [Google Scholar]
- Wei W, Jin J, Schlisio S, Harper JW, Kaelin WG., Jr The v-Jun point mutation allows c-Jun to escape GSK3-dependent recognition and destruction by the Fbw7 ubiquitin ligase. Cancer Cell. 2005;8:25–33. doi: 10.1016/j.ccr.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Welcker M, Singer J, Loeb KR, Grim J, Bloecher A, Gurien-West M, Clurman BE, Roberts JM. Multisite phosphorylation by Cdk2 and GSK3 controls cyclin E degradation. Mol Cell. 2003;12:381–392. doi: 10.1016/s1097-2765(03)00287-9. [DOI] [PubMed] [Google Scholar]
- Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Yaffe MB, Leparc GG, Lai J, Obata T, Volinia S, Cantley LC. A motif-based profile scanning approach for genome-wide prediction of signaling pathways. Nat Biotechnol. 2001;19:348–353. doi: 10.1038/86737. [DOI] [PubMed] [Google Scholar]
- Yang J, Cron P, Good VM, Thompson V, Hemmings BA, Barford D. Crystal structure of an activated Akt/protein kinase B ternary complex with GSK3-peptide and AMP-PNP. Nat Struct Biol. 2002;9:940–944. doi: 10.1038/nsb870. [DOI] [PubMed] [Google Scholar]
- Yeh E, Cunningham M, Arnold H, Chasse D, Monteith T, Ivaldi G, Hahn WC, Stukenberg PT, Shenolikar S, Uchida T, et al. A signalling pathway controlling c-Myc degradation that impacts oncogenic transformation of human cells. Nat Cell Biol. 2004;6:308–318. doi: 10.1038/ncb1110. [DOI] [PubMed] [Google Scholar]
- Yoeli-Lerner M, Yiu GK, Rabinovitz I, Erhardt P, Jauliac S, Toker A. Akt blocks breast cancer cell motility and invasion through the transcription factor NFAT. Mol Cell. 2005;20:539–550. doi: 10.1016/j.molcel.2005.10.033. [DOI] [PubMed] [Google Scholar]
- Zelzer E, Levy Y, Kahana C, Shilo BZ, Rubinstein M, Cohen B. Insulin induces transcription of target genes through the hypoxia-inducible factor HIF-1alpha/ARNT. EMBO J. 1998;17:5085–5094. doi: 10.1093/emboj/17.17.5085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Zha X, Tan Y, Hornbeck PV, Mastrangelo AJ, Alessi DR, Polakievicz RD, Comb MJ. Phosphoprotein analysis using antibodies broadly reactive against phosphorylated motifs. J Biol Chem. 2002;277:39379–39387. doi: 10.1074/jbc.M206399200. [DOI] [PubMed] [Google Scholar]
- Zhang HH, Lipovsky AI, Dibble CC, Sahin M, Manning BD. S6K1 regulates GSK3 under conditions of mTOR-dependent feedback inhibition of Akt. Mol Cell. 2006;24:185–197. doi: 10.1016/j.molcel.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou BP, Liao Y, Xia W, Spohn B, Lee M, Hung M. Cytoplasmic localization of p21Cip1/WAF1 by Akt-induced phosphorylation in HER-2/neu-overexpressing cells. Nat Cell Biol. 2001a;3:245–252. doi: 10.1038/35060032. [DOI] [PubMed] [Google Scholar]
- Zhou BP, Liao Y, Xia W, Zou Y, Spohn B, Hung MC. HER-2/neu induces p53 ubiquitination via Akt-mediated MDM2 phosphorylation. Nat Cell Biol. 2001b;3:973–982. doi: 10.1038/ncb1101-973. [DOI] [PubMed] [Google Scholar]
- Zhou GL, Tucker DF, Bae SS, Bhatheja K, Birnbaum MJ, Field J. Opposing roles for Akt1 and Akt2 in Rac/Pak signaling and cell migration. J Biol Chem. 2006;281:36443–36453. doi: 10.1074/jbc.M600788200. [DOI] [PubMed] [Google Scholar]
- Zimmermann S, Moelling K. Phosphorylation and regulation of Raf by Akt (protein kinase B) Science. 1999;286:1741–1744. doi: 10.1126/science.286.5445.1741. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.