Abstract
Aging is a paradox of reduced immunity and chronic inflammation. Dendritic cells are central orchestrators of the immune response with a key role in the generation of immunity and maintenance of tolerance. The functions of DCs are compromised with age. There is no major effect on the numbers and phenotype of DC subsets in aged subjects; nevertheless, their capacity to phagocytose antigens and migrate is impaired with age. There is aberrant cytokine secretion by various DC subsets with CDCs secreting increased basal level of pro-inflammatory cytokines but the response on stimulation to foreign antigens is decreased. In contrast, the response to self antigens is increased suggesting erosion of peripheral self tolerance. PDC subset also secretes reduced IFN-alpha in response to viruses. The capacity of DCs to prime T cell responses is also affected. Aging thus has a profound affect on DC functions. Present review summarizes the effect of advancing age on DC functions in humans in the context of both immunity and tolerance.
Keywords: Dendritic cells, Aging, Tolerance, immunity
Introduction
DCs are potent antigen presenting cells that possess the unique capacity to stimulate naïve T cells and induce not only T cell immunity (Inaba et al. 1990; Banchereau and Steinman, 1998; Cella et al., 1997; Banchereau et al., 2000; Ueno et al, 2010) but also T cell tolerance (Steinman et al., 2001; Steinman et al., 2002; Hawiger et al., 2001; Steinman et al., 2003; Coquerelle and Moser; 2010). Uptake and ingestion of apoptotic cells by DCs is considered to be one of the major mechanisms employed by DCs in inducing peripheral self tolerance (Steinman et al., 2001; Steinman et al., 2002; Hawiger et al., 2001; Steinman et al., 2003; Coquerelle and Moser; 2010). Under steady state conditions immature dendritic cells continuously sample the self-antigens from apoptotic cells in the periphery, leading to T cell tolerance. The term “immature dendritic cell” signifies that it is not yet a fully functional antigen-presenting cell. This state of dendritic cells is characterized by high phagocytic capacity and low levels of expression of major histocompatibility complex (MHC) and costimulatory molecules such as CD80, 86 etc. The presentation of antigen by an immature dendritic cell causes tolerance because of lack of costimulation. However, upon stimulation with microbial products, inflammatory cytokines or CD40 ligation, DCs start to mature and migrate to the draining lymph nodes. Maturation of DCs is associated with phenotypic changes, including down regulation of phagocytic capacity, upregulation of costimulatory molecules, MHC and secretion of cytokines, transforming them into fully functional antigen presenting cells (APC) capable of priming naïve T cells.
DCs detect and respond to pathogens through the expression of pattern recognition receptors (PRRs) (Manicassamy and Pulendran; 2009; Takeuchi and Akira; 2010; Iwasaki and Medzhitov, 2010). PRRs can recognize conserved molecular components or patterns of the pathogens. Examples of PRRs include Toll-like receptors, RIG-I like receptors, and Nod-like receptors (Manicassamy and Pulendran, 2009; Takeuchi and Akira, 2010). In addition to these, a new class of PRRs, the C-type lectin receptor family, has also emerged as a major sensor of pathogens. C-type lectins recognize carbohydrate moieties on bacteria and fungi (Brown and Gordon, 2001; Reid et al, 2009; Goodridge et al, 2009; Geijtenbeek and Gringhuis, 2009). Exposure of DCs to ligands of all these PRRs results in production of cytokines that modulate the type of T cell response and functions (Manicassamy and Pulendran, 2009; Zhou et al, 2009; Iwasaki and Medzhitov, 2010). For example, IL12p70 secretion by DCs polarizes the Th cell response towards IFN-γ secreting, Th1 cells, (Maldonado-Lopez et al, 1999; Kapsenberg, 2003) while the production of IL23 along with IL1β from DCs leads to the generation of Th17 cells(Wilson et al, 2007; Acosta-Rodriguez et al, 2007). IL10 secretion by DCs on the other hand can lead to a Th2 type of T cells, particularly in mice ((Agrawal et al, 2006; Dillon et al, 2006). IL10 secretion along with low costimulation can also result in the induction of Treg (Zhou et., 2009). More recently it has been observed that IL-12p70 secretion by DCs also induces the generation of IL-21 secreting T follicular helper cells (Tfh) which can prime B cell antibody responses (Schmitt et al, 2009). DCs at mucosal surfaces such as in the lung and gut display several immunoregulatory features and secrete predominantly IL-10 (Lambrecht and Hammad, 2009; Strober, 2009; Grainger et al., 2010), because DCs in the mucosa have to maintain tolerance towards the commensal microflora. The nature of cytokine secretion by DCs is therefore influenced by the microbes as well as their anatomical location.
DCs are unique among antigen presenting cells because they can cross present antigens (Burgdorf et al., 2008; Amigorena and Savina, 2010). Cross presentation is the ability to present phagocytosed or endocytosed antigens to CD8+ T cells. It is important in eliciting CTL responses against tumors and viruses in humans. Cross-presentation of antigens from dying target cells may be important in the pathogenesis of a CTL-mediated autoimmune disease like type 1 diabetes (Blachere et al, 2005). Recent reports suggest that cross presentation to MHCII in DCs may also be feasible and is facilitated by autophagy which allows cytosolic antigens to access class II MHC molecules (Crotzer and Blum, 2009). DCs are also important in launching humoral immunity partly because of their capacity to directly interact with B cells (Jego et al., 2005; Qi et al., 2006; Ueno et al., 2010). DCs can route antigens into non-degradative recycling compartments, which allow presentation of unprocessed antigens to B cells (Bergtold et al., 2005; Batista and Harwood, 2009).
DCs thus play a critical role in immune defenses via linkage between innate and adaptive immunity, and any alteration in DC functions with age would compromise both immunity and tolerance.
Aging is associated with a paradox of progressive immunodeficiency, inflammation, and autoimmunity, resulting in an increased susceptibility to infections, and impaired response to vaccines in aged individuals (Boren and Gershwin, 2004; Hainz et al., 2005; Kaml et al., 2006; Grubeck-Loebenstein et al. 2009; Dorshkind et al., 2009; McElhaney and Effros, 2009). This suggests a decrease in the protective immune responses to exogenous and infectious agents, and an increase in reactivity to endogenous danger signals in older individuals, which may be due to several factors, including deficiencies in immune tolerance with age, progressive age-associated loss of tissue integrity yielding neo-self antigens, and an autoimmune memory response through molecular mimicry (Weynaud et al., 2003; Ramos-Carlos et al., 2003; Ginaldi et al., 2004). The present review focuses on the age-associated changes in human dendritic cells (DC) and consequences on immunity and tolerance.
1. Dendritic cells and immunity to infections
DCs comprise of two major subsets, the DC of myeloid origin, including conventional DCs (CDCs) in the blood, interstitial DCs in tissues, Langerhan cells in the skin and monocyte-derived DCs (MODCs), and DCs of lymphoid origin, the plasmacytoid DCs (PDCs). The major function of CDCs is to sense and capture microbes at the entry portals such as skin, mucosa etc., and migrate to lymphoid organs to prime naïve T cells, and regulate B cell and NK cell responses. CDCs secrete a variety of cytokines and chemokines but are generally known for their capacity to secrete IL-12 which is essential to prime T cells to produce IFN-γ. The PDCs are considered crucial in defense against viral infections because of their capacity to secrete very high amounts of type I-IFN. In humans, most of the studies have focused on circulating CDCs and MODCs. Advantages and disadvantages of using various DC subsets to study aging have been outlined in Table 1. Age-associated alterations in DC functions have been summarized in Table 2.
Table 1.
Advantages and disadvantages of using various DC subsets for experiments in aging.
| Human DC subsets | Methods of generation | Advantages | Disadvantages |
|---|---|---|---|
| Conventional DC (CDC) |
Purified from Blood using BDCA- 3 magnetic beads or Stained directly in the blood |
differentiated in vivo Behave in a similar manner to CDCs. Can obtain sufficient numbers to allow mechanistic studies including signaling. Can also be manipulated for gene silencing studies |
Numbers obtained too few to perform mechanistic studies |
| Monocyte derived DCs (MODCs) |
Generated by culturing purified monocytes in the presence of GMCSF and lL-4 |
Final stage generation from monocytes to DCs is in vitro so some affects of aged microenvironment may be lost. |
|
| Plasmacytoid DC (PDC) |
Purified from Blood using BDCA- 2 magnetic beads or Stained directly in the blood |
differentiated in vivo | Numbers obtained too few to perform mechanistic studies |
| Mouse DC subsets | Methods of generation | Advantages | Disadvantages |
| Bone marrow derived DCs(BMDC) |
Generated by culturing CD34 progenitor cells from bone marrow in the presence of GMCSF and lL-4 |
Behave in a similar manner to CDCs. Can obtain sufficient numbers to allow mechanistic studies including signaling. Can also be manipulated for gene silencing studies |
Generated in vitro therefore the effect of aged microenvironment may not be apparent |
| Splenic DC | Purified from spleen using CD11c magnetic beads |
Differentiated in vivo | Numbers obtained too few to perform mechanistic studies |
| Splenic PDC | Purified from spleen using PDCA- 1 magnetic beads |
Differentiated in vivo | Numbers obtained too few to perform mechanistic studies |
| Bone marrow PDCs | Purified from bone marrow using PDCA-1 magnetic beads |
Differentiated in vivo. Can obtain sufficient numbers to allow mechanistic studies including signaling |
Bone marrow may have newly generated PDCs which may not display the effect of aged microenvironment. |
Table 2.
Comparison of functions of various subsets of DCs from aged and young.
| Humans | CDC | MODC | PDC |
|---|---|---|---|
| Numbers | Comparable in healthy aged except for 1 report |
Comparable | No consensus, both comparable and reduced numbers reported Decreased TLR 7 and 9 expression reported in few studies. Other markers not done comparable influenza uptake |
| Phenotype | Comparable in healthy aged except 1–2 reports of HLADR down regulation |
Comparable | |
| Phagocytosis | not done | decreased | |
| Migration | not done | decreased | not done |
| Cytokine secretion |
increased basal level of TNF-α and IL-6 secretion but reduced secretion of cytokines on activation with Pam3Cys, lipoteioic acid, flagellin, poly IC and R848 |
comparable or higher | decreased IFN-alpha production |
| T cell proliferation |
not done | comparable or decreased | not done |
| Tolerance | not done but studies report increased basal level of activation |
Increased reactivity to self antigens | not done |
| Mice | CDC | BMDC | PDC |
| Numbers | comparable | comparable | comparable |
| Phenotype | comparable | comparable | comparable |
| Phagocytosis | not done | reduced uptake of tumor anitigens | not done |
| Migration | reduced | not done reduced TNF and IL-23 secretion −1 |
not done |
| Cytokine secretion |
comparable | report each, comparable in other reports |
reduced IFN-alpha production |
| T cell proliferation |
comparable | reduced | not done |
| Tolerance | not done | not done | not done |
1.1 DC numbers and phenotype
Numerous studies have reported age-associated alterations in the functions of both CDCs and PDCs. Numbers or percentage of circulating DC and their phenotype have been measured by all groups using flow cytometry. The staining strategy utilized for identification of DC subsets is also similar (Lineage-HLADR+ CD11c+ are CDC and Lineage-HLADR+ CD123+ are PDC). Despite this, the results obtained by different groups are conflicting, Most studies find that the percentage of CDCs in the blood of the healthy aged subjects are comparable to young subjects (Agrawal et al., 2007; Jing et al., 2009) however, Della bella (Della bella et al., 2007) reported a decline in CDC percentage and absolute numbers in the blood of aged subjects. Even though all studies have been performed on healthy aged donors, only Della bella adhered stringently to SENEIUR protocol for subject selection. Variability in the donor population amongst different regions may also account for the different results. In contrast to healthy aged, a decline in CDC population has been reported in nursing home or frail aged subjects (Jing et al., 2009). There is consensus regarding MODCs numbers and all studies reported no alteration between aged and young subjects (Steger et al., 1996; Lung et al., 2000). We also observed comparable numbers of MODC between the two populations (Agrawal et al., 2007). The reports of the number of PDCs in aged subjects are conflicting; both normal and decreased numbers of PDCs have been observed (Shodell and Siegel et al, 2002; Perez-Cabeza et al., 2007; Jing et al., 2009; Canaday et al., 2010). In our own study with healthy aged, we did not observe any significant change in the frequency of CDCs or PDCs with age (Agrawal et al., 2007). Since all studies used similar methods for PDC enumeration, variability amongst the donor population seems to be logical to explain the observed differences.
There is a paucity of information regarding the status of age-associated changes in tissue DC populations which may play an important role in local pathology. A decrease in human Langerhans cell (LC) densities has been observed in the epidermis of the skin of aged subjects (Grewe, 2001). A similar decrease has also been reported in earlier studies with mice (Choi and Sauder, 1987; Sprecher et al., 1990). Bodineau et al (Bodineau et al., 2007; Bodineau et al., 2009) report an age-associated decrease in intraepithelial LC with chronic periodontitis. The dendritic processes are also smaller (reduced surface area) in the LCs from aged which may lead to reduced antigen presentation to T cells. The authors however, reported an upregulation of LAMP (Lysosomal-associated membrane protein) and CD83 (maturation marker for DCs) in the LCs from elderly. Such studies may be useful with DCs from other tissues or blood. In contrast to intraepithelial LCs, Bodineau et al observed an increased number of LCs in the upper epithelial connective tissue. Except for a study by Della bella (Della Bella et al., 2007) where an increased expression of CD83 and CD86 was observed on circulating DCs, the majority of studies (Steger et al., 1996; Lung et al., 2000; Agrawal et al., 2007) of circulating DCs or MODCs have reported comparable expression of costimulatory (CD40, CD80, CD86) and antigen presenting molecules (MHC-II) on DCs from aged and young subjects. Others (Haruna et al., 1995) have reported a decrease in HLA-DR expression on peripheral blood DCs and DCs from a strain of senescence-accelerated mouse (SAMP-1). Recently it was reported that the number of T regs were increased in lymph nodes of old mice (Zhao et al., 2007; Chiu et al., 2007). These T reg cells suppressed the expression of CD40 and CD86 co-stimulatory molecule expression in lymph node DCs and the expression could be restored to levels of young mice by inactivating Treg cells with anti-CD25 monoclonal antibodies. In summary, the majority of studies do not observe significant age-associated changes in CDC numbers and phenotype in human blood. Nevertheless, the picture may be entirely different for tissue specific DCs. PDC numbers on the contrary are generally observed to show a decrease with advancing age.
1.2 Expression of Pattern Recognition Receptors (PRR)
DCs sense and capture pathogens via the expression of pathogen recognition receptors (PRR) such as TLRs (Toll-like receptors), NLRs (NOD like receptors) and CLRs (C-type lectin receptors). Studies in aged have focused mainly on the expression and function of TLRs. Majority of investigators did not observe any age-related change in the expression of TLRs in MODCs. We also did not find any significant change in TLR4 expression in MODCs from aged (Agrawal et al., 2007). Recently, Panda et al (Panda et al., 2010) reported decreased expression of TLR1, 3 and 8 in CDCs from aged individuals; however, TLR2 levels were unchanged. They also observed reduced expression of TLR7 but normal expression of TLR9 in aged PDCs. Jing et al (Jing et al., 2009), on the other hand, reported a decreased expression of both TLR7 and TLR9 in PDCs from aged. In contrast to these studies, we did not observe any significant change in the expression of TLRs 7 and 9 in PDCs from aged subjects (unpublished observations). Most mouse studies also do not report an age-associated decline in TLR expression in CDC and PDCs (Tesar et al., 2007; Stout-Delgado et al., 2008).
Except for a study by Grollieu-Julius et al (Grolleau-Julius et al., 2008) who observed a decrease in DC-SIGN expression in bone marrow-derived DCs (BMDCs) from aged mice there is no information regarding the expression non-TLR PRRs in DCs in aging. This is an area of pivotal interest as an increasing number of non-TLR PRRs are being implicated in various pathological conditions. Furthermore, a number of new-generation vaccine adjuvants also target CLRs and NLRs (Ishii and Akira, 2007; Warshakoon et al., 2009; Ferwerda et al., 2010; Williams et al., 2010).
1.3 Phagocytosis or capture of antigens
Sensing of pathogens via PRRs leads to their capture and uptake by DCs resulting in DC maturation, activation, and subsequent T cell priming. Efficient antigen capture is thus central to generate effective immunity. Our observations demonstrate that both the phagocytosis of dextran beads and pinocytosis of the dye Lucifer Yellow are impaired in MODCs from aged subjects when compared to young (Agrawal et al., 2007). This suggests that MODCs from aged displayed reduced capacity to capture antigen via both receptor-dependent and receptor-independent mechanisms of antigen uptake. Furthermore, we identified that reduced activation of AKT kinase in the PI3-kinase signaling pathway may be responsible for the reduced antigen capture by aging DCs. Uptake and clearance of apoptotic cells by DCs plays an important role in the maintenance of peripheral self tolerance which is discussed in section (2.1). A recent study(Grolleau-Julius et al., 2006) in mice using BMDCs did not find reduction in the uptake of tumor antigens in old mice, though tumor antigen specific T cell stimulation was significantly reduced. However, these differences in mice and humans may be due to differences in the species.
1.4 Migration of DCs
Following activation, DCs migrate to T cell areas to induce effective cellular immune responses. Stimulation of immature DCs with TLR ligands results in the down-regulation of CCR6 and upregulation of CCR7, which enhances their ability to migrate from the peripheral tissues to the draining lymph node (Gunn, 2003). CCL21 and CCL19 both bind to the CCR7 receptor and are potent chemo attractants for mature DCs (Kellerman et al., 1999). Mice deficient in CCR7 are unable to mount an effective T cell immunity (Forster et al., 1999). Relatively few studies have addressed the question of migration of DCs in aging. We determined the migration of DCs from aged and young human subjects using a transwell system where the MODCs in the upper chamber migrate through a transwell of defined pore size to the lower chamber in response to a chemokine gradient (Agrawal et al., 2007). We observed that MODCs from aged subjects were impaired in their capacity to migrate in response to CCL19 and CXCL12 compared to MODCs from young, which was independent of CCR7 or CXCR4 expression since they were normally expressed. We also did not observe any significant difference in the basal level of the chemokines from MODCs in aged and young individuals. In contrast, Pietschmann et al (Pietschmann et al., 2000) observed normal trans-endothelial migration of peripheral blood myeloid-enriched lymphocyte-depleted cells in elderly subjects which could be due to the difference in the model system. Bhushan et al (Bhushan et al., 2002) also observed significantly impaired migration of LCs in response to TNF-α in elderly subjects, and aged mice. Choi and Sauder (Choi and Sauder, 1987) reported decreased LCs mobilization and the subsequent accumulation of DCs in the regional lymph nodes in aged mice in response to topical challenge with a chemical agent; however, contact hypersensitivity responses were not compromised. Linton et al (Linton et al., 2005) in a TCR transgenic mice model, have reported in vivo impaired migration of DCs from aged mice to the draining lymph nodes, which they suggested to be due to both intrinsic defect of DCs and aged microenvironment. A recent study by Toapanta et al (Toapanta et al., 2009) observed reduced CDC migration in the lungs of aged mice after infection with influenza. In summary, all studies (humans and mice) report impaired migration of aged DCs except for one study which utilized enriched DC population which may be contaminated by the presence of other cells. Since the migration of DCs to lymph nodes is pivotal to the establishment of the immune response, reduced migration may contribute to age-associated immune dysfunction.
1.5 DC Cytokine secretion
1.5.1. CDC/MODC cytokine secretion
One of the primary functions of DCs is to secrete cytokines on activation which aid in priming T cell responses. In addition to priming, the nature of cytokines secreted by DCs also dictates the polarization of Th cell responses. Furthermore, cytokine secretion by DCs also enhances inflammation. Therefore, it is important to understand age-associated changes in cytokine secretion by DCs in order to determine the effect on T cell priming, Th polarization and inflammation. The majority of earlier information on cytokine secretion by DCs in humans is from studies with MODCs because of the ease to obtain sufficient numbers of such DCs, allowing performance of functional studies. Lung et al (Lung et al., 2000) had reported comparable levels of cytokine secretion from MODCs of aged and young individuals. In contrast, we (Agrawal et al., 2007) observed increased secretion of pro-inflammatory cytokines, TNF-a and IL-6 by LPS-stimulated MODCs from aged subjects. In the last few years investigators have determined cytokine secretion by CDCs in the blood. Della bella et al (Della bella et al., 2007) reported decreased IL-12 secretion from LPS stimulated circulating CDCs in aged humans. Panda et al (Panda et al., 2010) also found decreased TNF-α, IL-6, and IL-12p40 by aged CDCs following stimulation with various TLR agonists including Pam3Cys, lipoteioic acid, flagellin, poly IC and R848. Earlier studies have also reported an age-associated defect in LPS-induced IL-12 production in CDCs from aged subjects (Mezayen et al., 2009). Reduced IL-12 secretion by DCs may be responsible for the reduced secretion of IFN-γ from T cells observed in the aged subjects. In contrast to decreased cytokine secretion by aged CDCs in response to TLR stimulation, Panda et al (Panda et al., 2010) observed increased basal level of these cytokines from aged CDCs. This is in agreement with our and Della bella observations where we observed that MODC from aged are in a semi activated state which may lead to cytokine secretion even at the basal level. In contrast, Jing et al. (Jing et al., 2009) did not observe any decrease in cytokine secretion from influenza virus-stimulated CDCs. These conflicting observations could be due to differences in stimuli and techniques of detecting cytokines, intracellular cytokine verses ELISA. Comparable levels of most cytokines have been reported from BMDCs in aged and young mice. However, Grollieu-Julius (Grollieu-Julius et al., 2006) did find a decrease in TNF-α secretion in BMDCs. Reduced TNF secretion was also observed by Wong et al (Wong et al., 2010) in mouse splenic DCs. More recently, Mezayen et al (Mezayen et al., 2009) observed an increase in IL-23 secretion by BMDCs. There are no reports regarding IL-23 production from human DCs. It would be of particular interest since IL-23 participates in the induction of Th17 cells and, therefore may explain age-associated inflammation.
1.5.2 PDC cytokine secretion
In contrast to CDCs, there is agreement amongst PDC cytokine secretion with almost all studies documenting a decline in IFN-α secretion from aged PDCs. Panda et al (Panda et al., 2010) reported decreased IFN-α (intracellular) in response to TLR7 and TLR9 ligands. Jing et al (Jing et al., 2009) also observed decreased IFN-α production from purified PDCs in response to both influenza virus and CpG. We have also observed decreased IFN-α secretion by aged pDCs in response to CpG and influenza virus (unpublished data). Canaday et al (Canaday et al., 2010) on the other hand observed that geriatric PBMCs produced significantly less IFN-α in response to live or inactivated influenza but responded normally to CpGODN (TLR9) and Guardiquimod (TLR7). They used whole PBMCs instead of purified PDCs for measuring IFN-α production which may account for the difference. Decreased IFN-α production has also been reported after stimulation of aged PBMCs with herpes simplex virus (Shodell and Siegel, 2002). In mice, Stout-Delgado et al (Stout-Delgado et al., 2008) reported decreased IFN-α production by aged PDCs in bone marrow in response to CPG stimulation. In contrast, Wong et al (Wong et al., 2010) observed that CpG-stimulated PDCs from spleen from aged mice had similar IFN-α production as that from young mice. The reason for such discrepancy may be due to the different anatomical locations of PDC studied, bone marrow vs. spleen. In summary, although there is consensus on reduced IFN-α secretion in response to viral stimulation, the results with CpG stimulation are contradictory both in humans and in mice. One possibility is that in contrast to CpG which signals only through TLR9, viruses such as influenza signal through multiple receptors including TLRs and cytosolic receptors such RIG-1 to induce IFN-α (Opitz et al., 2007), therefore the defect in IFN-α production may be due to an impairment in the combined function of these receptors plus TLRs. Nevertheless, it is suggested that reduced IFN-α secretion from aged PDCs may be one of the key players in increased susceptibility of aged subjects to infections such as influenza and respiratory synctial virus (RSV). Various mechanisms have been suggested for the observed decrease in IFN-α production by humans, including decreased numbers of PDC, and decreased expression of TLR7 and TLR9. Reduced age-associated activation of the key transcription factor, IRF-7 (Interferon Regulatory Factor −7) has been shown to be responsible for the decreased IFN-α secretion in PDCs in mice (Stout-Delgado et al., 2008). Our unpublished studies (manuscript in preparation) suggest that impaired IRF-7 activation may also be responsible for the decreased IFN-α in humans.
Recent studies document the secretion of another cytokine, IFN-III (IL28/IL29) from PDCs on stimulation with viruses (Kotenko et al., 2003; Dumoutier et al., 2004; Meager et al., 2005; Ank et al., 2006; Onoguchi et al., 2007; Pagliaccetti et al., 2008; Khaitov et al., 2009). The induction pathway of IFN-III is similar to IFN-I, though it exerts its effect on other cell types via a different receptor. Type III IFNs signal through a receptor complex composed of a unique IFN-λR1 chain and a shared IL-10R2 chain that is also the second subunit of the IL-10, IL-22, and IL-26 receptor complexes. Although type I IFN receptors are expressed in most cell types, IFN-λR1 expression is limited to mucosal and epithelial tissues. This underscores the importance of IFN-III in fighting infections such as influenza, RSV and pneumonia; all these are infections of the lung mucosa and considered the primary culprit for age associated morbidities and mortalities. It would be interesting to determine the effect of age on IFN-III. The information may prove useful in designing novel therapies for fighting viral infections as well as to increase the response to influenza vaccination.
1.6 T cell priming by DCs
DCs are unique APCs because of their capacity to prime naïve T cell responses. DCs therefore function as initiators of T cell immunity. DCs can prime or tolerize T cells by virtue of their costimulatory molecule expression. DCs orchestrate the nature of T cell responses via two mechanisms: 1) via costimulation, and 2) via cytokine secretion. The sub-optimal costimulation of DCs leads to T cell anergy or generation of T regulatory cells. The expression of inhibitory costimulatory molecules such as PDL-1 (Programmed cell death 1 ligand-1), PDL-2 (Programmed cell death 1 ligand-2), ICOS L, (inducible T-cell co-stimulatory molecule), ILT-3 (Immunoglobulin-like transcript 3), PIR-B (Paired immunoglobulin-like receptor B), and DIgR2 (dendritic cell-derived immunoglobulin receptor 2) on DCs also impairs T cell priming. Cytokine secretion by DCs is dependent on the type of receptor being activated by a pathogen. For example, engagement of TLR4 and 5 on DCs induces IL-12 secretion (Agrawal et al., 2003), while engagement of TLR2/dectin induces T regulatory cells (Agrawal et al., 2006). The cytokines secreted by DCs aid in T cell priming and proliferation, and are now considered the third signal required for efficient T cell activation. In addition to priming, the nature of cytokines secreted by DCs in response to a pathogen dictates the Th0 polarization to Th1/Th2/Th17/ T regulatory.
Studies on T cell priming by DCs in humans are few, and generally report no significant difference in T cell priming by DCs in aged subjects. Steger et al (Steger et al., 1999) and Grewe et al (Grewe, 2001) reported that MODCs from young and aged subjects have similar stimulatory capacity to induce proliferation of T cell lines developed in long-term cultures. Our preliminary studies (unpublished) with MODCs show reduced proliferation of young T cells when co-cultured with aged DCs. These results may be different during an infection where decreased IFN-γ producing cells were observed in response to MODCs infected with respiratory syncytial virus (but not with influenza virus) from old individuals (Looney et al., 2002). Earlier studies in aged mice demonstrated a decrease in antigen presentation and T cell priming capacity of DCs in the lymph nodes (Donnini et al., 2002). However, data from two recent studies in mice are conflicting. A study by Tesar et al (Tesar et al., 2007) suggests an intrinsic defect in T cells during aging is responsible for the age-associated reduction in T cell functions. They observed that aged and young BMDCs stimulated with specific TLR ligands, petidoglycan (TLR 2/6), poly I:C (TLR 3), LPS (TLR 4), flagellin (TLR 5), and CpG) TLR 9) were not impaired in their capacity to upregulate co-stimulatory molecules CD40 and CD86, chemokine receptor-CCR7 or the production of pro-inflammatory cytokines IL-6 and TNF-α. Employing allogeneic and virus specific systems (LCMV), the authors determined the capacity of aged and young BMDCs to prime naïve T cells. Again they found that the capacity of both aged and young DCs to prime naïve T cells was comparable. However, when young DCs were cultured with aged and young T cells in the same model, they observed impaired T cell proliferation in aged T cells compared to young T cells suggesting that aged T cells are intrinsically defective. Wong et al (Wong et al., 2010) observed comparable levels of T cell stimulation by splenic DCs from aged and young mice. Grollaeu-julius (Grollaeu-julius et al., 2006) on the other hand reported that old bone marrow- derived DCs are less effective than young DCs in stimulating syngeneic, ova–specific CD4+ T-cell proliferation. They also reported a decrease in tumor regression in mice treated with the ovalbumin peptide-pulsed aged DCs than with ovalbumin peptide-pulsed young DCs. The discrepancy between the two studies may be due to differences in type of DCs; splenic verses BMDC.
PDCs are also capable of priming T cell responses. There is no information regarding their capacity to prime T cells in aging. This may be important since IFN-α secreted by PDC, which is reduced with age, is a critical factor in virus specific CD8 T cell responses.
2. Dendritic cells and maintenance of tolerance
2.1 Peripheral self Tolerance
The immune system undergoes continuous morphologic and functional changes throughout the years, characterized by a gradual decline in its function with age. Paradoxically, this decline in immune functions is associated with increased reactivity towards self or endogenous antigens, as evident by an increased frequency and increased titers of auto-antibodies with age (Franceschi et al., 1995; Lee and Weksler, 2001; Weksler and Goodhardt, 2002). Gebe et al (Gebe et al., 2006) have identified an age-dependent spontaneous loss of tolerance to two self-antigenic epitopes derived from putative diabetes-associated antigens glutamic acid decarboxylase (GAD65) and glial fibrillary acidic protein (GFAP) in humanized murine model, RIP-B7/DRB1*0404 HLA transgenic mice. They observed that diabetic and older non-diabetic mice exhibited a proliferative response to an immunodominant epitope from GAD65 and GFAP. The response to both of these self-antigens was not observed in young mice. A similar loss of oral tolerance to ova has been observed in aged mice. Faria et al (Faria et al., 1998) observed that oral tolerance to OVA could be induced in young mice at 20 weeks of age; however, oral tolerance induction was completely abolished in mice by 38 weeks of age. In general, it is believed that oral tolerance established in early age can be maintained despite aging, whereas the induction of oral tolerance to new antigens is impaired in aged mice (Faria et al., 1993; Wakabayashi et al., 1999; Kato et al., 2003; Fujihashi and Kiyono, 2009).
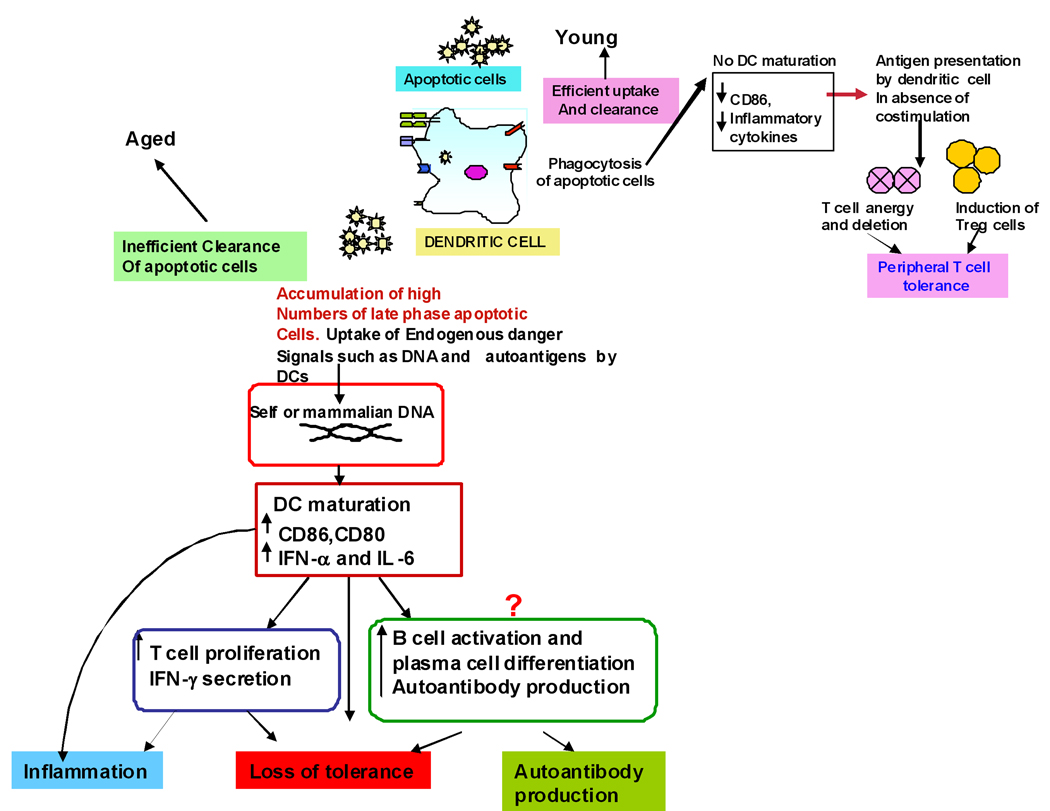
Most of our knowledge regarding maintenance of tolerance in aging is obtained from studies in mice; only few investigators, including ourselves, have studied tolerance in aged humans (Agrawal et al., 2009).. Apoptosis is a complex physiological programmed cell-death process, which plays an important role in certain effector functions, and immune homeostasis. One of the critical steps in apoptosis is rapid and efficient removal of apoptotic bodies and apoptotic cells by neighboring phagocytic cells, including DCs (Savill et al., 2002; Gaipl et al., 2003; Luckashenak et al., 2008; Mueller, 2010). Increasing evidence suggests that apoptosis is involved in many pathological conditions (Mevorach et al., 1998; Hermann et al., 1998; Cohen et al., 2002; Hardin, 2003; Shosan and Mevorach, 2004; Tisch R, Wang B, 2008; Ohnmacht et al., 2009). Uptake and ingestion of apoptotic cells by dendritic cells is considered to be one of the major mechanisms employed by DCs in inducing peripheral self tolerance. Under steady state, immature dendritic cells continuously sample self-antigens from apoptotic cells resulting in peripheral T cell tolerance. We have shown that DCs from aged subjects are impaired in their capacity to phagocytose antigens (section 1.3) including apoptotic cells. Apoptotic cell death and clearance of apoptotic cells and apoptotic bodies is of vital importance in developing and maintaining the normal tissue homeostasis and resolution of inflammation (Savill et al., 2002; Gaipl et al., 2003). The quick and efficient clearance of apoptotic cells is essential to prevent secondary necrosis in tissues (Sauter et al., 2000). This is of particular importance because the late phase of apoptotic cells is associated with additional proteolytic degradation of specific auto-antigens, which may release endogenous danger signals like nuclear structures clustered in apoptotic blebs (chromatin and dsDNA) and proteins such as heat-shock proteins (HSPs), resulting in maturation of dendritic cells, and T cell immunity to self and stimulating autoantibody responses (Okabe et al., 2005). Impaired clearance of apoptotic cells has been implicated in various autoimmune disorders like lupus and rheumatoid arthritis (Mevorach et al., 1998; Hermann et al., 1998; Cohen et al., 2002; Hardin, 2003; Shosan and Mevorach, 2004). To demonstrate that DCs from aged humans show increased reactivity to self antigens released during apoptosis, we purified DNA from human blood, delivered it intracellularly and compared the activation and cytokine secretion of aged and young DCs. MODCs from aged secrete increased quantities of IL-6 and IFN-α compared to MODCs from young subjects. A similar increase in cytokines was observed from aged DCs in response to late apoptotic cells. Furthermore, DNA primed DCs from aged induced increased T cell proliferation compared to young subjects. We further demonstrated that DCs from aged humans display increased reactivity to self antigens because there is increased basal level of NFκB activation which leads to increased IRF3 activation and subsequent IFN-α secretion. Increased basal level of NFκB suggests that DCs from aged exist in a semi-mature state causing increased reactivity to self antigens resulting in elevated cytokine levels even at the basal level, which contributes to loss of peripheral self tolerance and chronic inflammation observed during aging (Figure 1). Other investigators have also reported increased secretion of pro-inflammatory cytokines from unactivated CDCs of aged subjects (Della bella et al., 2007; Panda et al., 2010). Furthermore, a study by Benito et al (Benito et al., 2008) reports that renal graft from young but not from aged donors seem to induce DCs of a tolerogenic phenotype, both in aged and young recipients, suggesting that donor age may have consequences in terms of tolerance induction.
Figure 1. Increased response to self DNA in DCs from aged leads to loss of peripheral self tolerance and inflammation.
Defective clearance of apoptotic cells in aged leads to accumulation of late apoptotic, secondary necrotic cells which lyse to release DNA and other self antigens that are taken up by DCs along with other cell debris leading to their maturation and cytokine secretion. These DCs can prime T and B lymphocytes resulting in their activation and proliferation. The net outcome is loss of tolerance along with increased inflammation and autoantibody production.
More recently we have shown that the DNA from aged subjects is more immunogenic than DNA from young, thus DNA released by late apoptotic cells in aging induce increased amounts of cytokine IFN-a from DCs (Agrawal et al., 2010). This suggests that in addition to increased reactivity of DCs to self antigens, changes in self antigens may also aid in loss of self tolerance associated with aging.
2.3 Central Tolerance
In addition to oral and peripheral tolerance, DCs have a critical role in central tolerance in the thymus. Thymic DCs play an essential role in shaping T cell-mediated immune responses by deleting self-reactive thymocytes, and by inducing regulatory T-cell (Treg) development to establish central tolerance (Klein et al., 2009). Involution of thymus results in decreased output of naïve T cells during aging. However, the effect of thymic involution on the function of thymic DCs is not well known. Earlier studies (Nabarra and Andrianarison, 1996; Varas et al., 2003) reported an age-associated decline in medullary DC numbers. Furthermore, Varas et al observed decreased expression of antigen-presenting and costimulatory molecules HLA-DR, CD54, CD86 and CD40 in thymic DCs, which was associated with an impaired function of DCs from aged to induce proliferation of alloreactive T cells as compared to their young counterparts. Others have reported that the function of thymic DCs to negatively select autoreactive T cell is impaired only in very old mice (30–36 months). More recently van Dommelen et al (van Dommelen et al., 2010) also reported decreased numbers of thymic DCs in aged mice relative to young mice. They further showed that it was possible to restore the number of aged DCs to the level of that in young when the thymus was regenerated using sex steroid ablation therapy. Thus it seems that although the numbers of thymic DCs decrease with age, it does not have a major impact on central tolerance.
Recently, Tomohisha Baba (Baba et al., 2009) and colleague have identified a unique role of thymic Sirpα+ dendritic cell subpopulation in the establishment of central tolerance. It would be of interest to examine changes in Sirpα expression and function of Sirpα+ DCs in relation to aging.
3. Age-associated altered signaling pathways in DCs
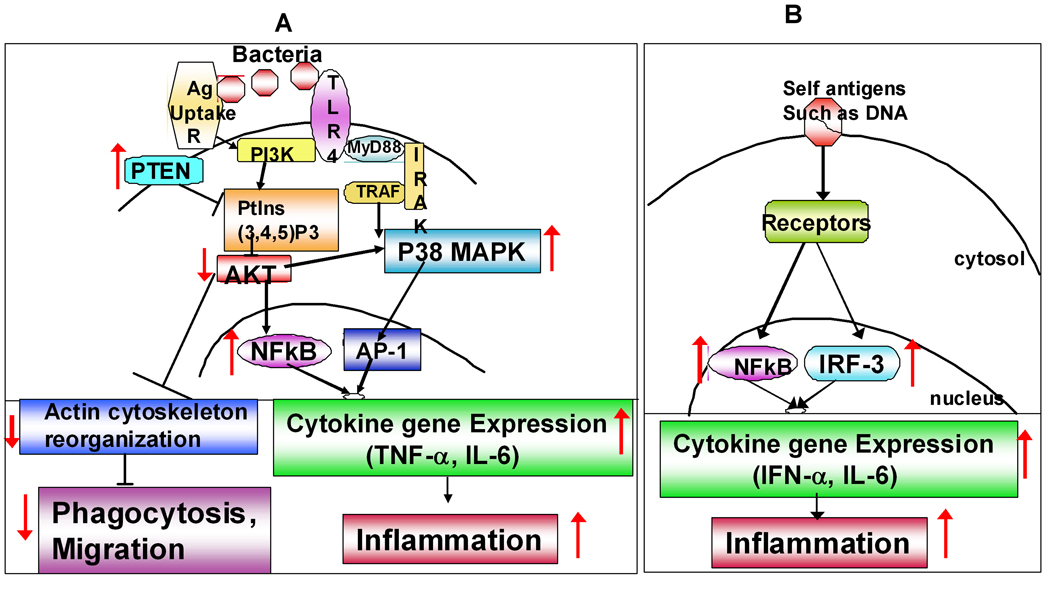
Though several studies document changes in functions of DCs with age, only a few studies have focused on dissecting the underlying signaling mechanisms responsible for the observed changes. This is particularly important in order to design interventions to restore the function of DCs in the aged. We have reported (Agrawal et al., 2007; Agrawal et al., 2009) several significant alterations in multiple signaling pathways in aged DCs, including PI3 kinase signaling pathway (Figure 2A). We observed (Agrawal et al., 2007) decreased activation of AKT kinase following activation of aged MODCs with LPS. Since PI3 kinase signaling pathway acts as a negative regulator of TLR signaling through LPS, impaired AKT activation in aging results in an increased activation and phosphorylation of p38 MAP kinase, and increased secretion of pro-inflammatory cytokines. In addition to regulating TLR signaling, PI3kinase plays an important role in cytoskeletal movements during phagocytosis and migration of DCs. Impaired PI3 kinase signaling thus results in decreased phagocytosis, and impaired migration of aged DCs. Furthermore, we observed that there is an increased PTEN expression in aged DCs, which negatively regulates the PI3kinase signaling pathway. In a subsequent study (Agrawal et al., 2009), we have demonstrated that DCs from aged exhibit increased activity of NFκB p65 unit at the basal level resulting in an increased activation of transcription factor IRF3, and consequently increased secretion of IFN-α in response to self-antigen, human DNA (Figure 2B). An Impaired phosphorylation of the transcription factor IRF-7 has also been reported in PDCs (Stout-Delgado et al., 2008). These studies suggest that aging has a significant effect on major signaling pathways in DCs. These effects appear to be more at the level of phosphorylation and activation of signaling molecules than at the gene expression levels. It is possible that reduced energy (ATP, GTP) production by the aging mitochondria results in altered phosphorylation of signaling molecules.
Figure 2. Age-associated alterations in signaling pathways in DCs impacting their functions.
A. Pathogens: Figure depicts age-associated changes in signaling mechanisms leading to increased cytokine secretion in response to pathogens and decreased phagocytosis and migration of DCs. Increased basal expression of PTEN results in inhibition of activation of AKT kinase of PI3kinase signaling pathway. Reduced AKT phosphorylation functions as a positive regulator for TLR signaling leading to increased activation of p38 MAP kinase and NFκB which in turn result in increased proinflammatory cytokine secretion and inflammation. Reduced activation of AKT however inhibits phagocytosis and migration of DCs. B. Self Antigens: Figure depicts age-associated changes in signaling mechanisms leading to increased reactivity of DCs to self- antigens such as DNA. Increased basal level activation of NFκB leads to semi-maturation of DCs which results in enhanced response to self antigens and subsequent inflammation.
Conclusions
In summary, advancing age significantly impairs various DC functions in both humans and mice (Summarized in Table 2). The response of DCs to infections is compromised either due to a reduction in their frequency or due to reduced expression of PRRs on CDC and PDC subsets. The effect seems to be primarily on the cytokine secretion by DCs. The antigen capture and migratory capacity of CDCs is also severely affected with age, suggesting that the functions related to motility of DCs may be affected. The capacity of DCs to prime T cells is also impaired.
It would be of interest to determine the effect of aging on non-TLR induced DC functions as well as in the context of infections, as the response will be physiologically more relevant, and sum total of all receptors engaged and not just TLRs. Furthermore, studies may be focused on the antigen-specific T cell stimulatory capacity of DCs particularly in humans where the majority of the studies have used an allostimulatory model to study DC-induced T cell proliferation. Another area of particular relevance would be the study of DCs at the mucosal surfaces since infections such as influenza and pneumonia are the major cause of morbidity and mortality associated with aging. The age-associated alterations in DC functions at neuronal-immunological interface is another area of significant interest since most neurodegenerative disorders display altered reactivity to a self antigen.
ACKNOWLEDEMENT
This study is supported in part by grant AG027512 from NIH and by New Scholar grant from the Ellison Medical Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat. Immuno. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 2.Agrawal S, Agrawal A, Doughty B, Gerwitz A, Van Dyke T, Pulendran B. Cutting edge, Different TLR Agonist Instruct Dendritic Cells to Induce Diverse T helper Responses, via Differential Activation of MAP Kinases. J Immunol. 2003;171:4984–4989. doi: 10.4049/jimmunol.171.10.4984. [DOI] [PubMed] [Google Scholar]
- 3.Agrawal S, Dillon S, Banerjee K, Letterio J, O-Ritcher K, Kasprowicz DJ, Keller K, Pare J, Van Dyke T, Ziegler S, Unutmaz D, Pulendran B. Yeast Zymosan, Ligand for TLR-2 and Dectin-1, induce regulatory antigen-presenting cells and Immunological tolerance. J Clin Invest. 2006;116:916–928. doi: 10.1172/JCI27203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agrawal A, Agrawal S, Cao JN, Su H, Osann K, Gupta S. Altered innate immune functioning of dendritic cells in elderly humans, a role of phosphoinositide 3-kinase-signaling pathway. J Immunol. 2007;178:6912–6922. doi: 10.4049/jimmunol.178.11.6912. [DOI] [PubMed] [Google Scholar]
- 5.Agrawal A, Tay J, Ton S, Agrawal S, Gupta S. Increased Reactivity of Dendritic Cells from Aged Subjects to Self Antigen, the Human DNA. J Immunol. 2009;182:1138–1145. doi: 10.4049/jimmunol.182.2.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agrawal A, Tay J, Yang GE, Agrawal S, Gupta S. Age-associated epigenetic modifications in human DNA increase its immunogenicity. Aging Jan 10. 2010 doi: 10.18632/aging.100121. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amigorena S, Savina A. Intracellular mechanisms of antigen cross presentation in dendritic cells. Curr Opin Immunol. 2010;22:109–117. doi: 10.1016/j.coi.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 8.Ank N, West H, Bartholdy C, Eriksson K, Thomsen AR, Paludan SR. Lambda Interferon. INF, a type III IFN, is induced by viruses and IFNs and displays potent antiviral activity against select virus infections in vivo. J Virol. 2006;80:4501–4509. doi: 10.1128/JVI.80.9.4501-4509.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baba T, Nakamoto Y, Mukaida N. Crucial Contribution of Thymic Sirpα+ Conventional Dendritic Cells to Central Tolerance against Blood-Borne Antigens in a CCR2-Dependent Manner. J. Immunol. 2009;183:3053–3063. doi: 10.4049/jimmunol.0900438. [DOI] [PubMed] [Google Scholar]
- 10.Banchereau J, Steinman RM. Dendritic cells and the control of immunity [review] Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 11.Batista FD, Harwood NE. The who, how and where of antigen presentation to B cells. Nat Rev Immunol. 2009;9:15–27. doi: 10.1038/nri2454. [DOI] [PubMed] [Google Scholar]
- 12.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu. Rev. Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 13.Benito MJ, Lopez-Hoyos M, Fernandez-Fresnedo G, Ruiz JC, Benito A, San Segundo D, Gomez-Alamillo C, Arias M. Changes in the expression of the immunoglobulin-like transcript 3. ILT3. and ILT4 receptors in renal allograft recipients, effect of donor and recipient aging. Transplant Proc. 2008;40:2894–2896. doi: 10.1016/j.transproceed.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 14.Bergtold A, Desai DD, Gavhane A, Clynes R. Cell surface recycling of internalized antigen permits dendritic cell priming of B cells. Immunity. 2005;23:503–514. doi: 10.1016/j.immuni.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 15.Bhushan M, Cumberbatch M, Dearman RJ, Andrew SM, Kimber I, Griffiths CE. Tumour necrosis factor-alpha-induced migration of human Langerhans cells, the influence of ageing. Br J Dermatol. 2002;146:32–40. doi: 10.1046/j.1365-2133.2002.04549.x. [DOI] [PubMed] [Google Scholar]
- 16.Blachere NE, Darnell RB, Albert ML. Apoptotic cells deliver processed antigen to dendritic cells for cross-presentation. PLoS Biol. 2005;3:e185. doi: 10.1371/journal.pbio.0030185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bodineau A, Coulomb B, Folliguet M, Igondjo-Tchen S, Godeau G, Brousse N, Séguier S. Do Langerhans cells behave similarly in elderly and younger patients with chronic periodontitis? Arch Oral Biol. 2007;52:189–194. doi: 10.1016/j.archoralbio.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 18.Bodineau A, Coulomb B, Tedesco AC, Séguier S. Increase of gingival matured dendritic cells number in elderly patients with chronic periodontitis. Arch Oral Biol. 2009;54:12–16. doi: 10.1016/j.archoralbio.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 19.Boren E, Gershwin ME. Inflamm-aging, autoimmunity, and the immune-risk phenotype. Autoimmun Rev. 2004;3:401–406. doi: 10.1016/j.autrev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Brown GD, Gordon S. Immune recognition, a new receptor for_-glucans. Nature. 2001;413:36–37. doi: 10.1038/35092620. [DOI] [PubMed] [Google Scholar]
- 21.Burgdorf S, Schölz C, Kautz A, Tampé R, Kurts C. Spatial and mechanistic separation of cross-presentation and endogenous antigen presentation. Nature Immunol. 2008;9:558–566. doi: 10.1038/ni.1601. [DOI] [PubMed] [Google Scholar]
- 22.Canaday DH, Amponsah NA, Jones L, Tisch DJ, Hornick TR, Ramachandra L. Influenza-Induced Production of Interferon-Alpha is Defective in Geriatric Individuals. J Clin Immunol. 2010;30:373–383. doi: 10.1007/s10875-010-9374-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cella M, Sallusto F, Lanzavecchia A. Origin, maturation and antigen presenting function of dendritic cells. Curr Opin Immunol. 1997;9:10–16. doi: 10.1016/s0952-7915(97)80153-7. [DOI] [PubMed] [Google Scholar]
- 24.Chiu BC, Stolberg VR, Zhang H, Chensue SW. Increased Foxp3.+. Treg cell activity reduces dendritic cell co-stimulatory molecule expression in aged mice. Mech Ageing Dev. 2007;128:618–627. doi: 10.1016/j.mad.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 25.Choi KL, Sauder DN. Epidermal Langerhans cell density and contact sensitivity in young and aged BALB/c mice. Mech Age Dev. 1987;39:69–79. doi: 10.1016/0047-6374(87)90087-x. [DOI] [PubMed] [Google Scholar]
- 26.Cohen PL, Caricchio R, Abraham V, Camenisch TD, Jennette JC. Delayed apoptotic cell clearance and lupus-like autoimmunity in mice lacking the c-mer membrane tyrosine kinase. J Exp Med. 2002;196:135–140. doi: 10.1084/jem.20012094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coquerelle C, Moser M. DC subsets in positive and negative regulation of immunity. Immunol Rev. 2010;234:317–334. doi: 10.1111/j.0105-2896.2009.00887.x. [DOI] [PubMed] [Google Scholar]
- 28.Crotzer VL, Blum JS. Autophagy and its role in MHC-mediated antigen presentation. J Immunol. 2009;182:3335–3341. doi: 10.4049/jimmunol.0803458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Della Bella S, Bierti L, Presicce P, Arienti R, Valenti M, Saresella M, Vergani C, Villa ML. Peripheral blood dendritic cells and monocytes are differently regulated in the elderly. Clin Immunol. 2007;122:220–228. doi: 10.1016/j.clim.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 30.Donnini A, Argentati K, Mancini R, Smorlesi A, Bartozzi B, Bernardini G, Provinciali M. Phenotype, antigen-presenting capacity, and migration of antigen-presenting cells in young and old age. Exp Gerontol. 2002;37:1097–1112. doi: 10.1016/s0531-5565(02)00087-6. [DOI] [PubMed] [Google Scholar]
- 31.Dorshkind K, Montecino-Rodriguez E, Signer RA. The ageing immune system, is it ever too old to become young again? Nat Rev Immunol. 2009;9:57–62. doi: 10.1038/nri2471. [DOI] [PubMed] [Google Scholar]
- 32.Dumoutier L, Tounsi A, Michiels T, Sommereyns C, Kotenko SV, Renauld JC. Role of the interleukin. IL.-28 receptor tyrosine residues for antiviral and antiproliferative activity of IL-29/interferon-λ1, similarities with type I interferon signaling. J. Biol. Chem. 2004;279:32269–32274. doi: 10.1074/jbc.M404789200. [DOI] [PubMed] [Google Scholar]
- 33.Faria AMC, Garcia G, Rios MJC, Michalaros CL, Vaz NM. Decrease in susceptibility to oral tolerance induction and occurrence of oral immunization to ovalbumin in 20–38-week-old mice. The effect of interval between oral exposures and rate of antigen intake in the oral immunization. Immunology. 1993;78:147–151. [PMC free article] [PubMed] [Google Scholar]
- 34.de Faria AM, Ficker SM, Speziali E, Menezes JS, Stransky B, Silva Rodrigues V, Vaz NM. Aging affects oral tolerance induction but not its maintenance in mice. Mech Ageing Dev. 1998;102:67–80. doi: 10.1016/s0047-6374(98)00024-4. [DOI] [PubMed] [Google Scholar]
- 35.Ferwerda G, Netea MG, Joosten LA, van der Meer JW, Romani L, Kullberg BJ. The role of Toll-like receptors and C-type lectins for vaccination against Candida albicans. Vaccine. 2010;28:614–622. doi: 10.1016/j.vaccine.2009.10.082. [DOI] [PubMed] [Google Scholar]
- 36.Forster R, Schubel A, Brietfeld D, Kremmer E, Renner-Muller I, Wolf E, Lipp M. CCR-7 coordinates primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23–33. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- 37.Franceschi C, Monti D, Sansoni P, Cossarizza A. The immunology of exceptional individuals, the lesson of Centenarians. Immunol. Today. 1995;16:12–16. doi: 10.1016/0167-5699(95)80064-6. [DOI] [PubMed] [Google Scholar]
- 38.Fujihashi K, Kiyono H. Mucosal immunosenescence, new developments and vaccines to control infectious diseases. Trends Immunol. 2009;30:334–343. doi: 10.1016/j.it.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 39.Gaipl US, Brunner J, Beyer TD, Voll RE, Kalden JR, Herrmann M. Disposal of dying cells, a balancing act between infection and autoimmunity. Arthritis Rheum. 2003;48:6–11. doi: 10.1002/art.10744. [DOI] [PubMed] [Google Scholar]
- 40.Gebe JA, Unrath KA, Falk BA, Ito K, Wen L, Daniels TL, Lernmark A, Nepom GT. Age-dependent loss of tolerance to an immunodominant epitope of glutamic acid decarboxylase in diabetic-prone RIP-B7/DR4 mice. Clin Immunol. 2006;121:294–304. doi: 10.1016/j.clim.2006.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Geijtenbeek TB, Gringhuis SI. Signalling through C-type lectin receptors, shaping immune responses. Nat Rev Immunol. 2009;9:465–479. doi: 10.1038/nri2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ginaldi L, De Martinis M, Monti D, Franceschi C. The immune system in the elderly, activation-induced and damage-induced apoptosis. Immunol Res. 2004;30:81–94. doi: 10.1385/IR:30:1:081. [DOI] [PubMed] [Google Scholar]
- 43.Goodridge HS, Wolf AJ, Underhil DM. b-glucan recognition by the innate immune system. Immunological Reviews. 2009;230:38–50. doi: 10.1111/j.1600-065X.2009.00793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grainger JR, Hall JA, Bouladoux N, Oldenhove G, Belkaid Y. Microbe-dendritic cell dialog controls regulatory T-cell fate. Immunol Rev. 2010;234:305–316. doi: 10.1111/j.0105-2896.2009.00880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grewe M. Chronological ageing and photoageing of dendritic cells. Clin Exp Dermatol. 2001;26:608–612. doi: 10.1046/j.1365-2230.2001.00898.x. [DOI] [PubMed] [Google Scholar]
- 46.Grolleau-Julius A, Garg MR, Mo R, Stoolman LL, Yung RL. Effect of Aging on Bone Marrow-Derived Murine CD11c+CD4-CD8 {alpha}- Dendritic Cell Function. J Gerontol A Biol Sci Med Sci. 2006;61:1039–1047. doi: 10.1093/gerona/61.10.1039. [DOI] [PubMed] [Google Scholar]
- 47.Grolleau-Julius A, Harning EK, Abernathy LM, Yung RL. Impaired dendritic cell function in aging leads to defective antitumor immunity. Cancer Res. 2008;68:6341–6349. doi: 10.1158/0008-5472.CAN-07-5769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grubeck-Loebenstein B, Della Bella S, Iorio AM, Michel JP, Pawelec G, Solana R. Immunosenescence and vaccine failure in the elderly. Aging Clinical and Experimental Research. 2009;21:201–209. doi: 10.1007/BF03324904. [DOI] [PubMed] [Google Scholar]
- 49.Gunn MD. Chemokine mediated control of dendritic cell migration and function. Semin. Immunol. 2003;15:271–276. doi: 10.1016/j.smim.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 50.Hainz U, Jenewein B, Asch E, Pfeiffer KP, Berger P, Grubeck-Loebenstein B. Insufficient protection for healthy elderly adults by tetanus and TBE vaccines. Vaccine. 2005;23:3232–3235. doi: 10.1016/j.vaccine.2005.01.085. [DOI] [PubMed] [Google Scholar]
- 51.Hardin JA. Directing autoimmunity to nucleoprotein particles, the impact of dendritic cells and interferon αin lupus. J Exp Med. 2003;197:681–685. doi: 10.1084/jem.20030130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haruna H, Inaba M, Inaba K, Taketani S, Sugiura K, Fukuba Y, Doi H, Toki J, Tokunaga R, Ikehara S. Abnormalities of B cells and dendritic cells in SAMP1 mice. Eur J Immunol. 1995;25:1319–1325. doi: 10.1002/eji.1830250528. [DOI] [PubMed] [Google Scholar]
- 53.Hawiger D, Inaba K, Dorsett Y, Guo M, Mahnke K, Rivera M, Ravetch JV, Steinman RM, Nussenzweig MC. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J. Exp. Med. 2001;194:769–779. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Herrmann M, Voll RE, Zoller OM, Hagenhofer M, Ponner BB, Kalden JR. Impaired phagocytosis of apoptotic cell material by monocyte-derived macrophages from patients with systemic lupus erythematosus. Arthritis Rheum. 1998;41:1241–1250. doi: 10.1002/1529-0131(199807)41:7<1241::AID-ART15>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 55.Inaba K, Metlay JP, Crowley MT, Steinman RM. Dendritic cells pulsed with protein antigens in vitro can prime antigen-specific, MHC-restricted T cells in situ. J Exp Med. 1990;172:631–640. doi: 10.1084/jem.172.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ishii KJ, Akira S. Toll or toll-free adjuvant path toward the optimal vaccine development. J Clin Immunol. 2007;27:363–371. doi: 10.1007/s10875-007-9087-x. [DOI] [PubMed] [Google Scholar]
- 57.Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science. 2010;327:291–295. doi: 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jego G, Pascual V, Palucka AK, Banchereau J. Dendritic cells control B cell growth and differentiation. Curr Dir Autoimmun. 2005;8:124–139. doi: 10.1159/000082101. [DOI] [PubMed] [Google Scholar]
- 59.Jing Y, Shaheen E, Drake RR, Chen N, Gravenstein S, Deng Y. Aging is associated with a numerical and functional decline in plasmacytoid dendritic cells, whereas myeloid dendritic cells are relatively unaltered in human peripheral blood. Human Immunology. 2009;70:777–784. doi: 10.1016/j.humimm.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kaml M, Weiskirchner I, Keller M, Luft T, Hoster E, Hasford J, Young L, Bartlett B, Neuner C, Fischer KH, Neuman B, Würzner R, Grubeck-Loebenstein B. Booster vaccination in the elderly, their success depends on the vaccine type applied earlier in life as well as on pre-vaccination antibody titers. Vaccine. 2006;24:6808–6811. doi: 10.1016/j.vaccine.2006.06.037. [DOI] [PubMed] [Google Scholar]
- 61.Kapsenberg ML. Dendritic-cell control of pathogen-driven T-cell polarization. Nat Rev Immunol. 2003;3:984–993. doi: 10.1038/nri1246. [DOI] [PubMed] [Google Scholar]
- 62.Kato H, Fujihashi K, Kato R, Dohi T, Fujihashi K, Hagiwara Y, Kataoka K, McGhee JR. Lack of oral tolerance in aging is due to sequential loss of Peyer's patch cell interactions. International Immunology. 2003;15:145–158. doi: 10.1093/intimm/dxg011. [DOI] [PubMed] [Google Scholar]
- 63.Kellermann SA, Hudak S, Oldham ER, Liu YJ, McEvoy LM. The CCchemokine receptor-7 ligands 6Ckine and macrophage inflammatory protein 3 are potent chemoattractants for in vitro- and in vivo-derived dendritic cells. J. Immunol. 1999;162:3859–3864. [PubMed] [Google Scholar]
- 64.Khaitov MR, Laza-Stanca V, Edwards MR, Walton RP, Rohde G, Contoli M, Papi A, Stanciu LA, Kotenko SV, Johnston SL. Respiratory virus induction of alpha-, beta- and lambda-interferons in bronchial epithelial cells and peripheral blood mononuclear cells. Allergy. 2009;64:375–386. doi: 10.1111/j.1398-9995.2008.01826.x. [DOI] [PubMed] [Google Scholar]
- 65.Klein L, Hinterberger M, Wirnsberger G, Kyewski B. Antigen presentation in the thymus for positive selection and central tolerance induction. Nat Rev Immunol. 2009;9:833–844. doi: 10.1038/nri2669. [DOI] [PubMed] [Google Scholar]
- 66.Kotenko SV, Gallagher G, Baurin VV, Lewis-Antes A, Shen M, Shah NK, Langer JA, Sheikh F, Dickensheets H, Donnelly RP. IFN-λs mediate antiviral protection through a distinct class II cytokine receptor complex. Nat. Immunol. 2003;4:69–77. doi: 10.1038/ni875. [DOI] [PubMed] [Google Scholar]
- 67.Lambrecht BN, Hammad H. Biology of lung dendritic cells at the origin of asthma. Immunity. 2009;31:412–424. doi: 10.1016/j.immuni.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 68.Lee W, Jin F, Weksler ME. The nature and significance of age-associated autoimmunity chap 63. In: Bona C, Theophilopoulos A, editors. Molecular Pathology of Autoimmune Disease. Langhorne, PA: Harwood Academic Publishers; 2001. [Google Scholar]
- 69.Linton PJ, Li SP, Zhang Y, Bautista B, Huynh Q, Trinh T. Intrinsic versus environmental influences on T-cell responses in aging. Immunol Rev. 2005;205:207–219. doi: 10.1111/j.0105-2896.2005.00266.x. [DOI] [PubMed] [Google Scholar]
- 70.Looney RJ, Falsey AR, Walsh E, Campbell D. Effect of aging on cytokine production in response to respiratory syncytial virus infection. J Infect Dis. 2002;185:682–685. doi: 10.1086/339008. [DOI] [PubMed] [Google Scholar]
- 71.Luckashenak N, Schroeder S, Endt K, Schmidt D, Mahnke K, Bachmann MF, Marconi P, Deeg CA, Brocker T. Constitutive crosspresentation of tissue antigens by dendritic cells controls CD8+ T cell tolerance in vivo. Immunity. 2008;28:521–532. doi: 10.1016/j.immuni.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 72.Lung TL, Saurwein-Teissl M, Parson W, Schonitzer D, Grubeck-Loebenstein B. Unimpaired dendritic cells can be derived from monocytes in old age and can mobilize residual function in senescent T cells. Vaccine. 2000;18:1606–1612. doi: 10.1016/s0264-410x(99)00494-6. [DOI] [PubMed] [Google Scholar]
- 73.Manicassamy S, Pulendran B. Modulation of adaptive immunity with Toll-like receptors. Semin Immunol. 2009;21:185–193. doi: 10.1016/j.smim.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McElhaney JE, Effros RB. Immunosenescence, what does it mean to health outcomes in older adults? Curr Opin Immunol. 2009;21:418–424. doi: 10.1016/j.coi.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Meager A, Visvalingam K, Dilger P, Bryan D, Wadhwa M. Biological activity of interleukins-28 and −29, comparison with type I interferons. Cytokine. 2005;31:109–118. doi: 10.1016/j.cyto.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 76.Mevorach D, Zhou JL, Song X, Elkon KB. Systemic exposure to irradiated apoptotic cells induces autoantibody production. J Exp Med. 1998;188:387–392. doi: 10.1084/jem.188.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mezayen RE, Gazzar ME, Myer R. High, Aging-dependent upregulation of IL-23p19 gene expression in dendritic cells is associated with differential transcription factor binding and histone modifications. Aging Cell. 2009;8:553–565. doi: 10.1111/j.1474-9726.2009.00502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mueller DL. Mechanisms maintaining peripheral tolerance. Nat Immunol. 2010;11:21–27. doi: 10.1038/ni.1817. [DOI] [PubMed] [Google Scholar]
- 79.Nabarra B, Andrianarison I. Ultrastructural study of thymic microenvironment involution in aging mice. Exp Gerontol. 1996;31:489–506. doi: 10.1016/0531-5565(95)02038-1. [DOI] [PubMed] [Google Scholar]
- 80.Okabe Y, Kawane K, Akira S, Taniguchi T, Nagata S. Toll-like receptor-independent gene induction program activated by mammalian DNA escaped from apoptotic DNA degradation. J. Exp. Med. 2005;202:1333–1339. doi: 10.1084/jem.20051654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ohnmacht C, Pullner A, King SB, Drexler I, Meier S, Brocker T, Voehringer D. Constitutive ablation of dendritic cells breaks self-tolerance of CD4 T cells and results in spontaneous fatal autoimmunity. J Exp Med. 2009;206:549–559. doi: 10.1084/jem.20082394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Onoguchi K, Yoneyama M, Takemura A, Akira S, Taniguchi T, Namiki H, Fujita T. Viral infections activate types I and III interferon genes through a common mechanism. J. Biol. Chem. 2007;282:7576–7581. doi: 10.1074/jbc.M608618200. [DOI] [PubMed] [Google Scholar]
- 83.Opitz B, Rejaibi A, Dauber B, Eckhard J, Vinzing M, Schmeck B, Hippenstiel S, Suttorp N, Wolff T. IFNβ induction by influenza A virus is mediated by RIG-I which is regulated by the viral NS1 protein. Cellular Microbiology. 2007;9:930–938. doi: 10.1111/j.1462-5822.2006.00841.x. [DOI] [PubMed] [Google Scholar]
- 84.Pagliaccetti NE, Eduardo R, Kleinstein SH, Mu XJ, Bandi P, Robek MD. Interleukin-29 functions cooperatively with interferon to induce antiviral gene expression and inhibit hepatitis C virus replication. J Biol Chem. 2008;283:30079–30089. doi: 10.1074/jbc.M804296200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Panda A, Qian F, Mohanty S, van Duin D, Newman FK, Zhang L, Chen S, Towle V, Belshe RB, Fikrig E, Allore HG, Montgomery RR, Shaw AC. Age-associated decrease in TLR function in primary human dendritic cells predicts influenza vaccine response. J Immunol. 2010;184:2518–2527. doi: 10.4049/jimmunol.0901022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pietschmann P, Hahn P, Kudlacek S, Thomas R, Peterlik M. Surface markers and transendothelial migration of dendritic cells from elderly subjects. Exp Gerontol. 2000;35:213–222. doi: 10.1016/s0531-5565(99)00089-3. [DOI] [PubMed] [Google Scholar]
- 87.Pérez-Cabezas B, Naranjo-Gómez M, Fernández MA, Grífols JR, Pujol-Borrell R, Borràs FE. Reduced numbers of plasmacytoid dendritic cells in aged blood donors. Exp Gerontol. 2007;42:1033–1038. doi: 10.1016/j.exger.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 88.Qi H, Egen JG, Huang AY, Germain RN. Extrafollicular activation of lymph node B cells by antigen-bearing dendritic cells. Science. 2006;312:1672–1676. doi: 10.1126/science.1125703. [DOI] [PubMed] [Google Scholar]
- 89.Ramos-Casals M, Garcia-Carrasco M, Brito MP, Lopez-Soto A, Font J. Autoimmunity and geriatrics, clinical significance of autoimmune manifestations in the elderly. Lupus. 2003;12:341–355. doi: 10.1191/0961203303lu383ed. [DOI] [PubMed] [Google Scholar]
- 90.Reid DM, Gow NA, Brown GD. Pattern recognition, recent insights from dectin-1. Curr. Opin. Immunol. 2009;21:30–37. doi: 10.1016/j.coi.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past, clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2002;2:965–975. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- 92.Sauter B, Albert ML, Francisco L, Larsson M, Somersan S, Bhardwaj N. Consequences of cell death, exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med. 2000;191:423–434. doi: 10.1084/jem.191.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schmitt N, Morita R, Bourdery L, Bentebibel SE, Zurawski SM, Banchereau J, Ueno H. Human dendritic cells induce the differentiation of interleukin-21-producing T follicular helper-like cells through interleukin-12. Immunity. 2009;31:158–169. doi: 10.1016/j.immuni.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shodell M, Siegal FP. Circulating, interferon-producing plasmacytoid dendritic cells decline during human ageing. Scand J Immunol. 2002;56:518–521. doi: 10.1046/j.1365-3083.2002.01148.x. [DOI] [PubMed] [Google Scholar]
- 95.Shoshan Y, Mevorach D. Accelerated autoimmune disease in MRL/MpJ-Fas.lpr but not in MRL/MpJ following immunization with high load of syngeneic late apoptotic cells. Autoimmunity. 2004:37103–37109. doi: 10.1080/08916930410001666622. [DOI] [PubMed] [Google Scholar]
- 96.Sprecher E, Becker Y, Kraal G, Hall E, Harrison D, Shultz LD. Effect of aging on epidermal dendritic cell populations in C57BL/6J mice. J Invest Dermatol. 1990;94:247–253. doi: 10.1111/1523-1747.ep12874586. [DOI] [PubMed] [Google Scholar]
- 97.Steger MM, Maczek C, Grubeck-Loebenstein B. Morphologically and functionally intact dendritic cells can be derived from the peripheral blood of aged individuals. Clin Exp Immunol. 1996;105:544–550. doi: 10.1046/j.1365-2249.1996.d01-790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Steinman RM, Turley S, Mellman I, Inaba K. The induction of tolerance by dendritic cells that have captured apoptotic cells. J. Exp. Med. 2000;191:411–416. doi: 10.1084/jem.191.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Steinman RM, Nussenzweig MC. Avoiding horror autotoxicus, the importance of dendritic cells in peripheral T cell tolerance. Proc. Natl. Acad. Sci. USA. 2002;99:351–358. doi: 10.1073/pnas.231606698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells [review] Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 101.Stout-Delgado HW, Yang X, Walker WE, Tesar BM, Goldstein DR. Aging impairs IFN regulatory factor 7 up-regulation in plasmacytoid dendritic cells during TLR9 activation. J Immunol. 2008;181:6747–6756. doi: 10.4049/jimmunol.181.10.6747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Strober W. The multifaceted influence of the mucosal microflora on mucosal dendritic cell responses. Immunity. 2009;31:377–388. doi: 10.1016/j.immuni.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 103.Takeuchi O, Akira S. Pattern Recognition Receptors and Inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 104.Tesar BM, Walker WE, Unternaehrer J, Joshi NS, Chandele A, Haynes L, Kaech S, Goldstein DR. Murine [corrected] myeloid dendritic cell-dependent toll-like receptor immunity is preserved with aging. Aging Cell. 2007;5:473–486. doi: 10.1111/j.1474-9726.2006.00245.x. [DOI] [PubMed] [Google Scholar]
- 105.Tisch R, Wang B. Dysrulation of T cell peripheral tolerance in type 1 diabetes. Adv Immunol. 2008;100:125–149. doi: 10.1016/S0065-2776(08)00805-5. [DOI] [PubMed] [Google Scholar]
- 106.Toapanta FR, Ross TM. Impaired immune responses in the lungs of aged mice following influenza infection. Respir Res. 2009;10:112. doi: 10.1186/1465-9921-10-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ueno H, Schmitt N, Klechevsky E, Pedroza-Gonzalez A, Matsui T, Zurawski G, Oh S, Fay J, Pascual V, Banchereau J, Palucka K. Harnessing human dendritic cell subsets for medicine. Immunol Rev. 2010;234:199–212. doi: 10.1111/j.0105-2896.2009.00884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.van Dommelen SL, Rizzitelli A, Chidgey A, Boyd R, Shortman K, Wu L. Regeneration of dendritic cells in aged mice. Cell Mol Immunol. 2010;7:108–115. doi: 10.1038/cmi.2009.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Varas A, Sacedón R, Hernandez-López C, Jiménez E, García-Ceca J, Arias-Díaz J, Zapata AG, Vicente A. Age-dependent changes in thymic macrophages and dendritic cells. Microsc. Res. Tech. 2003;62:501–507. doi: 10.1002/jemt.10411. [DOI] [PubMed] [Google Scholar]
- 110.Wakabayashi A, Utsuyama M, Hosoda T, Sato K, Hirokawa K. Differential age effect of oral administration of an antigen on antibody response, an induction of tolerance in young mice but enhancement of immune response in old mice. Mech. Ageing Dev. 1999;109:191–201. doi: 10.1016/s0047-6374(99)00036-6. [DOI] [PubMed] [Google Scholar]
- 111.Warshakoon HJ, Hood JD, Kimbrell MR, Malladi S, Wu WY, Shukla NM, Agnihotri G, Sil D, David SA. Potential adjuvantic properties of innate immune stimuli. Hum Vaccin. 2009;5:381–394. doi: 10.4161/hv.5.6.8175. [DOI] [PubMed] [Google Scholar]
- 112.Weksler ME, Goodhardt M. Do age-associated changes in "physiologic" autoantibodies contribute to infection, atherosclerosis, and Alzheimer’s disease? Exp Gerontol. 2002;37:971–979. doi: 10.1016/s0531-5565(02)00091-8. [DOI] [PubMed] [Google Scholar]
- 113.Weyand CM, Fulbright JW, Goronzy JJ. Immunosenescence, autoimmunity, and rheumatoid arthritis. Exp Gerontol. 2003;38:833–841. doi: 10.1016/s0531-5565(03)00090-1. [DOI] [PubMed] [Google Scholar]
- 114.Williams A, Flavell RA, Eisenbarth SC. The role of NOD-like Receptors in shaping adaptive immunity. Curr Opin Immunol. 2010;22:34–40. doi: 10.1016/j.coi.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, Basham B, Smith K, Chen T, Morel F, Lecron JC, Kastelein RA, Cua DJ, McClanahan TK, Bowman EP, de Waal Malefyt R. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat. Immunol. 2007;8:950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 116.Wong CP, Magnusson KR, Ho E. Aging is associated with altered dendritic cells subset distribution and impaired proinflammatory cytokine production. Experimental Gerontology. 2010;45:163–169. doi: 10.1016/j.exger.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 117.Zhao L, Sun L, Wang H, Ma H, Liu G, Zhao Y. Changes of CD4+CD25+Foxp3+ regulatory T cells in aged Balb/c mice. J Leukoc Biol. 2007;81:1386–1394. doi: 10.1189/jlb.0506364. [DOI] [PubMed] [Google Scholar]
- 118.Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30:646–655. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]