Abstract
BACKGROUND
Lone atrial fibrillation (AF) is thought to be a benign type or an early stage of the disease.
OBJECTIVE
This study sought to compare the left atrium (LA) substrate using delayed-enhanced magnetic resonance imaging (DE-MRI) in patients with lone AF versus those with comorbidities.
METHODS
Forty of 333 included patients met criteria for lone AF. All patients underwent DE-MRI to quantify atrial fibrosis as a marker for structural remodeling (SRM) and underwent catheter ablation. Based on the degree of SRM, patients were staged into 4 groups: Utah I (≤5% LA wall enhancement), Utah II (>5% to ≤20%), Utah III (>20% to ≤35%), or Utah IV (>35%).
RESULTS
Distribution in Utah I to IV was comparable in patients with lone AF and non–lone AF. In both groups, a number of patients showed extensive SRM. Mean enhancement (14.08 ± 8.94 vs. 16.94 ± 11.37) was not significantly different between the 2 groups (P = .0721). In the lone AF group, catheter ablation was successful in suppressing AF in all of Utah I, 81.82% of Utah II, 62.5% of Utah III, and none of Utah IV patients. Similar results were achieved in the non–lone AF group. Outcome after ablation was significantly dependent on the SRM of the LA (P < .001).
CONCLUSION
The degree of LA structural remodeling as detected using DE-MRI is independent of AF type and associated comorbidities. Selecting appropriate treatment candidates based on the quality and quantity of atrial fibrosis using DE-MRI would improve procedural outcome and avoid unnecessary intervention.
Keywords: Lone atrial fibrillation, Delayed-enhanced MRI, Structural remodeling, Pulmonary vein antrum isolation, Atrial fibrillation, Recurrence
In recent years, the designation of lone atrial fibrillation (AF) has been expressed in various ways and is thought to be a different or benign type of AF.1–4 Lone AF generally applies to young individuals (below 60 years of age) without clinical or echocardiographic evidence of cardiopulmonary disease.4 Currently, there is no uniform definition in use because intensive research has shown many conditions that may trigger AF. It is common that patients with lone AF have a better prognosis with respect to mortality and thromboembolism. However, as the disease progresses and the potential development of cardiac comorbidities rises, patients can move out of the so-called lone AF category over time and the risks of thromboembolism and mortality increase.5
Atrial remodeling is a well-described phenomenon in AF. Tachycardia-induced electrical remodeling reduces atrial refractoriness and conduction in the left atrium (LA).6 Over time this results in alterations of the structural components of the LA, creating a favorable substrate for sustaining AF.7–9 Although structural remodeling has been shown to induce an arrhythmogenic substrate, recent evidence indicates patients remote from the arrhythmia also have physiologically abnormal atria, signifying the importance of diseased atria on arrhythmia initiation and maintenance.10 However, noninvasive assessment of the underlying substrate in patients with lone AF is lacking.
Delayed-enhancement magnetic resonance imaging (DE-MRI) is an established method of characterizing cardiac tissue in a variety of disease processes.11–13 Recently, DE-MRI has emerged as an effective method to noninvasively assess and quantify the extent of LA structural remodeling (SRM). The extent of LA fibrosis assessed by high-resolution DE-MRI has been introduced as an independent predictor of radiofrequency (RF) ablation failure.14 These findings imply that patients with less atrial remodeling might have a better benefit from therapeutic intervention. Although patients with AF often have acceptable overall outcomes after ablation, there remains a significant portion of this cohort who experience AF recurrence. Therefore, in our study we examine the LA SRM with DE-MRI in patients with lone AF and compare them with non–lone AF patients to better understand the underlying substrate in these patients. Moreover, we examine how lone AF and non–lone AF patients with varying degrees of fibrosis respond to RF ablation.
Methods
Study population
This study is comprised of 376 consecutive patients who underwent DE-MRI at the electrophysiology-MRI lab at the University of Utah between December 2006 and November 2009. Of these patients, 11 patients had prior catheter ablation and were excluded. Thirty-two patients were excluded due to poor-quality MRI characterized as blurring from patient motion, incorrect inversion time, and/or significant gating artifacts. Thus, 333 patients were eligible for the final analysis.
The study patients were a part of the AF database approved by our institutional review board and were Health Insurance Portability and Accountability Act compliant. Lone AF was defined in patients who were age 60 years and younger; who had an absence of structural heart disease based on patient history, physical examination, and imaging tests including chest x-ray and echocardiography; and did not have history of coronary artery disease, diabetes mellitus, or hypertension. The baseline AF type was categorized as paroxysmal, persistent, or long-lasting persistent AF as defined by the American College of Cardiology/American Heart Association/European Society of Cardiology guidelines.5 Forty of the total patient cohort of 333 complied with lone-AF defining criteria and were included in the final analysis (see Table 1 for patient demographics). Two hundred and ninety-three patients with cardiac comorbidities, i.e., hypertension, coronary artery disease, mitral valve regurgitation, diabetes mellitus, etc., were categorized as non–lone AF patients in this study. The demographics of the non–lone AF patients are listed in Table 2.
Table 1.
Patient population characteristics for patients with lone atrial fibrillation
| Total (n = 40) | Utah I (n = 4) | Utah II (n = 26) | Utah III (n = 9) | Utah IV (n = 1) | |
|---|---|---|---|---|---|
| Age, y | 48.1 ± 9.7 | 44.25 ± 5.1 | 49 ± 10.1 | 45.8 ± 10.1 | 58 |
| Gender, n (%) | |||||
| Female | 10 (25) | 0 (0) | 8 (30.77) | 2 (22.22) | 0 (0) |
| Male | 30 (75) | 4 (100) | 18 (69.23) | 7 (77.78) | 1 (100) |
| Hypertension, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Diabetes mellitus, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| CAD, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| History of smoking, n (%) | 7 (17.5) | 2 (50) | 3 (11.54) | 2 (22.22) | 0 (0) |
| Valve surgery, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| CABG, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| MI, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Implanted device, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| CHF, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| CMP, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| MVR, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Stroke, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Duration (months) | 68.8 ± 119.8 | ||||
CABG = coronary artery bypass graft; CAD = coronary artery disease; CHF = congestive heart failure; CMP = cardiomyopathy; MI = myocardial infarction; MVR = mitral valve regurgitation.
Continuous measurements are presented as mean ± SD. Categorical measurements are presented as number positive for the condition and percentage of the total.
Table 2.
Patient population characteristics for patients with non–lone atrial fibrillation
| Total (n = 293) | Utah I (n = 17) | Utah II (n = 187) | Utah III (n = 67) | Utah IV (n = 22) | |
|---|---|---|---|---|---|
| Age, y | 68.8 ± 6.9 | 67.7 ± 8.8 | 66.1 ± 9.2 | 67.6 ± 11.8 | 70.2 ± 7.7 |
| Gender, n (%) | |||||
| Female | 110 (37.54) | 5 (29.41) | 59 (31.56) | 33 (49.25) | 11 (50) |
| Male | 183 (62.46) | 12 (70.59) | 128 (68.44) | 34 (50.75) | 11 (50) |
| Hypertension, n (%) | 193 (65.87) | 13 (76.47) | 125 (66.84) | 41 (61.19) | 15 (68.18) |
| Diabetes mellitus, n (%) | 46 (15.7) | 3 (17.65) | 25 (13.37) | 14 (20.90) | 5 (22.73) |
| CAD, n (%) | 48 (16.38) | 5 (29.41) | 24 (12.83) | 16 (23.88) | 3 (13.64) |
| CABG, n (%) | 24 (8.19) | 4 (23.53) | 14 (7.49) | 4 (5.97) | 2 (9.09) |
| MI, n (%) | 16 (5.46) | 2 (11.76) | 9 (4.81) | 4 (5.97) | 1 (4.55) |
| History of smoking, n (%) | 48 (16.38) | 3 (17.65) | 31 (16.58) | 10 (14.93) | 4 (18.18) |
| Implanted device, n (%) | 23 (7.85) | 0 (0) | 9 (4.81) | 10 (14.93) | 4 (18.18) |
| CHF, n (%) | 30 (10.24) | 3 (17.65) | 17 (9.09) | 8 (11.94) | 3 (13.64) |
| CMP, n (%) | 30 (10.24) | 0 (0) | 19 (10.16) | 7 (10.45) | 5 (22.73) |
| MVR, n (%) | 15 (5.12) | 0 (0) | 8 (4.28) | 6 (8.96) | 1 (4.55) |
| Valve surgery, n (%) | 8 (2.73) | 0 (0) | 4 (4.14) | 2 (2.99) | 2 (9.09) |
| Stroke, n (%) | 26 (8.87) | 2 (11.76) | 12 (6.41) | 9 (13.43) | 3 (13.64) |
| Duration (months) | 68.4 ± 93.6 | ||||
Abbreviations as in Table 1.
Continuous measurements are presented as mean ± SD. Categorical measurements are presented as number positive for the condition and percentage of the total.
DE-MRI
DE-MRI was obtained to assess for the extent of LA fibrosis or nonviable tissue using methods previously described.14 Briefly, studies were performed on a 1.5-T Avanto clinical scanner 15 ± 35 days prior to ablation (Siemens Medical Solutions, Erlangen, Germany) using a TIM (Total Imaging Matrix) phased-array receiver coil. In all patients, there was only 1 DE-MRI examination performed at our institute prior to ablation. The scan was acquired 15 minutes after contrast agent injection (0.1 mmol/kg, Mulithance [Bracco Diagnostic Inc., Princeton, NJ]) using a 3-dimensional inversion recovery, respiration navigated, electrocardiogram (ECG)-gated, gradient echo pulse sequence. Typical acquisition parameters were: free breathing using navigator gating, a transverse imaging volume with voxel size 1.25 × 1.25 × 2.5 mm (reconstructed to 0.625 × 0.625 × 1.25 mm), repetition time/TE = 5.4/2.3 ms, inversion time (TI) = 270 to 310 ms; GRAPPA (Gene-Ralized Autocalibrating Partially Parallel Acquisition) with R = 2 and 46 reference lines. ECG gating was used to acquire a small subset of phase-encoding views during the diastolic phase of the LA cardiac cycle. The time interval between the R-peak of the ECG and the start of data acquisition was defined using the cine images of the LA. Fat saturation was used to suppress fat signal. The TE of the scan (2.3 ms) was chosen such that fat and water are out of phase and the signal intensity of partial volume fat-tissue voxels was reduced, allowing improved delineation of the LA wall boundary. The TI value for the DE-MRI scan was identified using a scout scan. Typical scan time for the DE-MRI study was 5 to 10 minutes depending on subject respiration and heart rate.
Utah classification of atrial remodeling and detection of atrial fibrosis
Quantification of LA remodeling was obtained using methods previously described.14 In all DE-MRI images, the epicardial and endocardial LA borders were manually contoured with image display and analysis software in Seg3D (Scientific Computing and Imaging Institute, Salt Lake City, UT). The relative extent of contrast enhancement was quantified within the LA wall using a threshold-based algorithm based on pixel intensity distribution of healthy myocardium and nonviable myocardium, utilizing pixel intensities from normal based on a bimodal distribution with analysis using Marrek, Inc. segmentation and quantification software (Marrek, Inc., Salt Lake City, UT). Qualitative confirmation of the percent enhancement was performed for all scans using 3-dimensional visualization of the MRI performed with OsiriX 3.7.1 (Rosset, Los Angeles, CA) using a maximum intensity projection to assess contrast consistency followed by ray cast volume rendering with an opacity-weighted linear table for the preablation images. Patients were assigned to one of 4 groups (Utah I to Utah IV) based on the percentage of LA wall enhancement. Utah I was defined as ≤5% LA wall enhancement, Utah II as >5% and ≤20%, Utah III as >20% and ≤35%, and Utah IV as >35%.
Pulmonary vein antrum isolation
RF ablation was performed under intracardiac echocardiography in all study patients as described previously.15–17 A 10-F, 64-element, phased-array ultrasound catheter (Acu-Nav, Siemens Medical Solutions USA, Malvern, PA) was used to visualize the interatrial septum and to guide the transseptal puncture. After the transseptal punctures and for the remainder of the procedure, an infusion of heparin was maintained to achieve an activated clotting time (ACT) >350 seconds. ACT measurements were performed every 30 minutes thereafter. A circular mapping catheter (Lasso, Biosense Webster, Diamond Bar, CA) and an ablation catheter were inserted into the LA. Intracardiac echocardiography was used to define the pulmonary vein ostia, their antra, and the posterior wall, and was also used to position the circular mapping catheter and ablation catheter. All study patients underwent pulmonary vein antrum isolation (PVAI), defined as electric disconnection of the pulmonary vein antrum from the LA, together with posterior wall and septal debulking.17
Postablation care and follow-up
A postablation blanking period was observed for 3 months, during which all patients received an 8-week automatic trigger cardiac event monitor for assessment of early AF recurrence. Early recurrences were treated with direct current cardioversion, antiarrhythmic drugs, or both. Antiar-rhythmic drugs were discontinued at the end of the blanking period, whereas other medications such as angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin-receptor blockers (ARBs) for comorbidities, e.g., hypertension or heart failure, were continued; 297 patients were seen at 3 months after ablation and at 3-month intervals thereafter. Each patient received a 12-lead ECG and an 8-day Holter monitor for detection of arrhythmia recurrence postblanking at each of these follow-up visits. Additional ECG recordings were obtained as suggested by the patients’ reported symptoms through weekly telephone calls and by evaluation of ECGs sent by primary care providers. Recurrence was defined as any atrial arrhythmia sustained for longer than 30 seconds without antiarrhythmic drug treatment after the 3-month blanking period, as suggested by the Heart Rhythm Society consensus statement.18 All ablation procedures were performed on therapeutic anticoagulation with warfarin. Warfarin was continued postprocedure as well to maintain an international therapeutic ratio of 2.0 to 3.0.
Statistical analysis
Normal continuous variables are presented as mean ± standard deviations. A 2-sample t-test and one-way analysis of variance (ANOVA) was used to test for statistical significance and was further addressed using the Tukey-Kramer method to correct for multiple comparisons. Categorical variables are presented as number and percentage of total. Pearson chi-square or Fisher exact tests were used to assess for statistical significance. Differences were considered significant at a P value of <.05.
Results
The population reported here included 333 patients (213 male; mean age 60.2 ± 13.1 years). Forty patients (30 male; 48 ± 9.7 years) met criteria for lone AF, whereas 293 patients (183 male; 68.8 ± 6.9 years) showed AF with comorbidities.
Noninvasive assessment of preablation structural remodeling
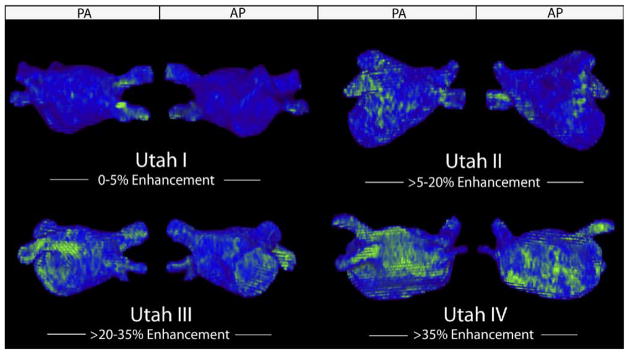
Four patients (10%) in the lone AF group were staged in Utah I and 26 patients (65%) in Utah II. A number of patients with lone AF showed distinctive and extensive structural remodeling, as 9 patients (22.5%) with lone AF were staged in Utah III and 1 patient (2.5%) in Utah IV. Seventeen patients (5.8%) with non–lone AF were staged in Utah I, 187 patients (63.82%) in Utah II, 67 patients (22.87%) in Utah III, and 22 patients (7.51%) in Utah IV (Table 3). Figure 1 shows patient examples of lone AF with less (Utah I), mild (Utah II), distinctive (Utah III), and extensive (Utah IV) SRM.
Table 3.
Distribution in Utah I to IV
| Total (n = 333) | Lone AF (n = 40) | Non–lone AF (n = 293) | P value | |
|---|---|---|---|---|
| Utah I, n (%) | 21 | 4 (10) | 17 (5.8) | .298 |
| Utah II, n (%) | 141 | 26 (65) | 187 (63.82) | .884 |
| Utah III, n (%) | 148 | 9 (22.5) | 67 (22.87) | .959 |
| Utah IV, n (%) | 23 | 1 (2.5) | 22 (7.51) | .334 |
Categorical measurements are presented as number positive for the condition and percentage of the total. Pearson chi-square or Fisher exact test were used to assess for statistical significance.
Figure 1.
Utah I to IV in patients with lone AF. Posterior–anterior and anterior–posterior view of enhancement (green pattern) versus normal healthy tissue (blue) before ablation in patients with lone AF. AF = atrial fibrillation.
Clinical outcome after ablation
At a mean follow-up period of 324 ± 234 days after ablation, 27 patients (77.14%) with lone AF remained free of AF recurrence, whereas 170 patients (64.89%) with non–lone AF stayed in stable sinus rhythm (P = .150) (Table 4). In detail, the success rate in patients with lone AF and Utah I was 100%, in Utah II 81.82%, in Utah III 62.5%, and in Utah IV 0%, whereas the success rate in patients with non–lone AF and Utah I was 100%, in Utah II 71.26%, in Utah III 63.49%, and in Utah IV 4.55%. Patients who suffered from recurrence showed a higher amount of fibrosis prior to ablation (13.72 ± 7.39 vs. 23.30 ± 14.92; P < .001), independent of AF type.
Table 4.
Recurrence rate for Utah I to IV
| Lone AF | Non–lone AF | P value | |
|---|---|---|---|
| Patients | 35 | 262 | |
| Total n (%) | 8 (22.86) | 92 (35.11) | .150 |
| Utah I, n (%) | 0 (0) | 0 (0) | 1.000 |
| Utah II, n (%) | 4 (18.18) | 48 (28.74) | .297 |
| Utah III, n (%) | 3 (37.5) | 40 (36.51) | .956 |
| Utah IV, n (%) | 1 (100) | 21 (95.45) | .827 |
Categorical measurements are presented as number positive for the condition and percentage of the total. Pearson chi-square or Fisher exact test were used to assess for statistical significance.
Comparison of lone AF and non–lone AF
Persistent AF was significantly higher in patients with non–lone AF (P < .005), whereas paroxysmal AF was significantly higher in patients with lone AF (P < .001). Gender was not a predictor for the type of AF (P = .131). No significant difference was observed between the mean enhancements in the 2 study populations (14.08 ± 8.94 vs. 16.94 ± 11.37; P = .0721). Table 5 summarizes these results. The distribution of groups Utah I to IV shows no significant differences between patients with lone AF and non–lone AF (Figure 2). In all patients, the amount of enhancement in the LA was independent from the AF duration (R2 = .05; P = NS). The mean duration of AF was 68.8 ± 119.8 months in patients with lone AF, ranging from 1 month to 720 months. In patients with non–lone AF, the mean duration of AF was 68.42 ± 93.63 months within the range from 1 to 528 months. There was no significant difference between the burden in patients with lone AF and non–lone AF (P = .985). One hundred and thirty-two patients (45.05%) in the non–lone AF group were taking ARBs or ACEIs on presentation. The degree of LA fibrosis was similar in patients taking ARBs or ACEIs when compared with the patients not taking the drug (17.14 ± 11.38 vs. 16.78 ± 11.39; P = .78) in the non–lone AF group.
Table 5.
LA wall enhancement prior to ablation
| Lone AF | Non–lone AF | P value | |
|---|---|---|---|
| Total | 14.08 ± 8.94 | 16.94 ± 11.37 | 0.0721 |
| Utah I | 2.84 ± 1.33 | 3.34 ± 1.17 | |
| Utah II | 11.15 ± 4.93 | 11.96 ± 4.49 | |
| Utah III | 24.99 ± 4.8 | 24.68 ± 4.08 | |
| Utah IV | 36.89 | 46.22 ± 10.24 |
Continuous measurements are presented as mean ± SD. Pearson chi-square or Fisher exact test were used to assess for statistical significance.
Figure 2.
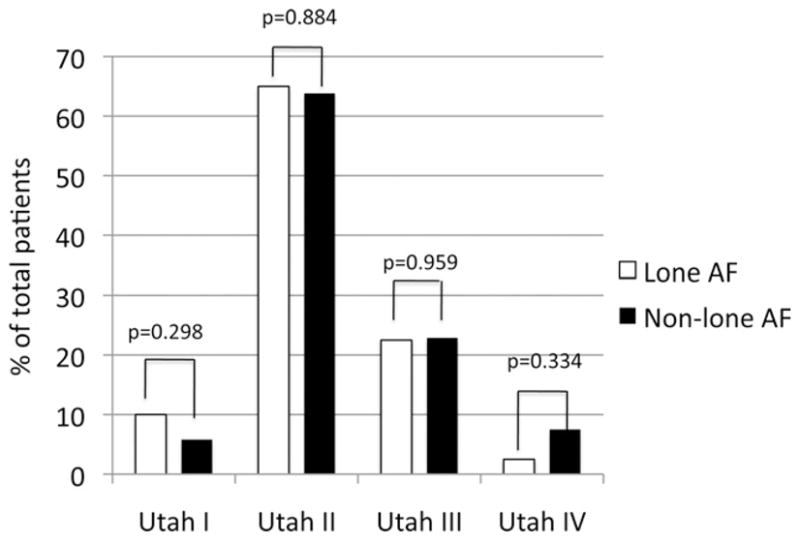
Distribution in groups Utah I to IV.
The success rate in Utah I to IV was comparable in patients with lone AF and non–lone AF (P = NS). The recurrence rate was significantly correlated to the Utah staging groups for SRM (P < .001) (Figure 3) and showed no difference between patients with lone AF and patients with non–lone AF (Figure 4).
Figure 3.
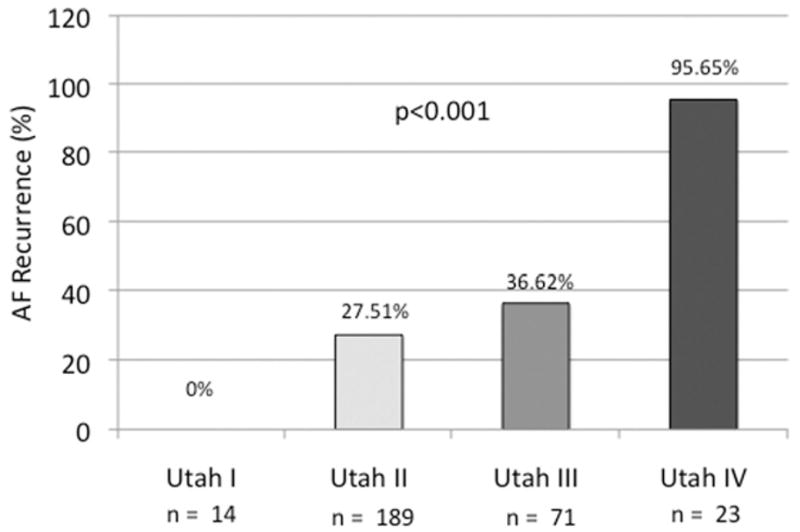
Recurrence in groups Utah I to IV.
Figure 4.
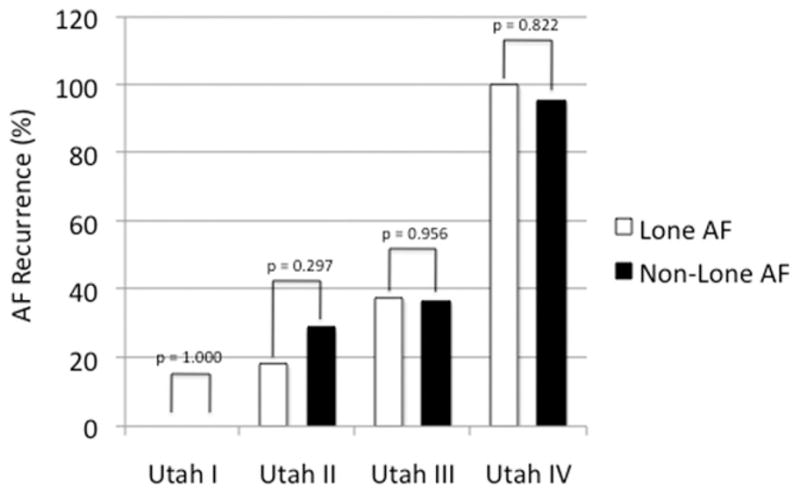
Comparison of recurrence rate in patients with lone AF and non–lone AF. AF = atrial fibrillation.
Discussion
In the present study, we show that the degree of atrial SRM detected using DE-MRI does not differ in patients with lone AF and non–lone AF. Our results indicate that an increased amount of enhancement within the LA wall is strongly associated with AF recurrence after PVAI, independent of AF type and associated comorbidities.
Structural remodeling of the LA
A recent study by Stiles et al10 demonstrated that patients with paroxysmal lone AF, without any preceding arrhythmic event, demonstrated biatrial abnormalities, including loss of myocardial voltage and prolonged atrial refractoriness. These authors concluded that the rate of atrial remodeling could not account for all structural abnormalities found in AF patients. Similarly, prior histological studies found that patients with lone AF had increased levels of patchy LA fibrosis as compared with control subjects.19 Our study is consistent with these prior findings, as a number of patients with lone AF displayed increased regions of LA enhancement primarily in the posterior wall of the LA. Compared with a previous study by our group that looked at healthy volunteers,14 there was substantially more remodeling in the LA wall in patients with lone AF and non–lone AF in comparison to healthy volunteers. In our study, SRM of the LA showed no correlation to the time that AF was first diagnosed. It is improbable to reconstruct exactly how many episodes of AF, especially asymptomatic AF, that each patient has had since AF was initially diagnosed or to precisely identify how long these episodes were sustained since the onset of AF. Thus, DE-MRI seems to be the ideal noninvasive tool for classifying patients with AF. In addition, it appears that DE-MRI may be beneficial for assessing the optimal treatment in any patient with AF, regardless of AF type.
It is known that AF itself produces changes in atrial structure and function.20–22 Allessie et al20 concluded that short durations of AF result in less structural atrial remodeling. A recent study23 is consistent with these findings, as patients with persistent AF compared with patients with paroxysmal AF showed more fibrosis in the LA. In our study, more patients with lone AF suffered from paroxysmal AF (episodes that generally last 7 days or less), whereas the majority of patients with non–lone AF suffered from persistent AF (episodes that last longer than 7 days).5
The rennin-angiotensin system (RAS) has been noted to play a role in AF and has been suggested by various human studies to demonstrate a beneficial effect of angiotensin-converting enzyme inhibition on atrial remodeling and fibrillation.24–27 Moreover, Boldt et al28 reported in a study that angiotensin-converting enzyme inhibitors reduce fibrosis in patients with lone AF. In our study ARB/ACEI did not seem to have an effect on the degree of LA fibrosis. Nevertheless, further larger prospective studies are needed to better understanding of the effect of these drugs on atrial fibrosis.
Rationale for staging patients in Utah I to IV
In recent studies from our group,14,29 patients were classified into 3 groups: mild, moderate, and extensive enhancement. In the present study, we classified the patients into 4 groups (Utah I to IV), based on the percentage of LA wall enhancement. The hypothesis of this new classification was to find a more precise distribution for SRM staging for patients with AF. Furthermore, these recent studies demonstrated that preablation DE-MRI is an independent predictor of response to PVAI in patients with AF.14 Our current study is consistent with these prior findings, as the recurrence rate was strongly dependent on the amount of enhancement in the LA. Furthermore, as no patients in Utah I had a recurrence, independent of the underlying type of AF, this new classification holds promise to be a more exact predictor for responders to AF ablation and may help to avoid less promising interventions.
Clinical outcome after ablation procedure
Recent studies reported a long-term success rate of 29% to 89% after RF ablation in patients with AF.30–32 In our study, the mean success rate for patients with lone AF and non–lone AF was consistent with these findings. Certainly, the amount of patients with recurrence increased from an early stage of remodeling (Utah I) to the more progressed stage of remodeling (Utah IV) in patients with lone AF and non–lone AF. This finding indicates that SRM prior to ablative therapy can be used as a predictor for the outcome after PVAI, independent of the underlying type of AF and comorbidities.
Duration of AF
We would hypothesize that the number of years that the patient has been symptomatic for the arrhythmia might not be the crucial factor. Rather the number and length of episodes might be the more important factors, as it is known that short durations of AF result in less structural atrial remodeling20 and agree that it would be very interesting to compare the frequency and duration of AF episodes with the structural remodeling in the LA. Understandably, it is clinically difficult to detect and record all episodes, especially the silent ones since AF was first diagnosed; however, this correlation should be considered for future studies.
Extensive structural remodeling of the LA
The phenomenon of electrical and structural remodeling is well described in patients with AF and comorbidities.2,33,34 In the present study, extensive structural remodeling was proven in patients with lone AF. Our results pointed out that the amount of enhancement in the LA was independent from the AF duration, comorbidities, and underlying baseline type of AF.
Recent studies are engaged with the genetics of AF35,36 and have identified several genetic loci associated with typical AF. A recent study has identified an association on chromosome 1q21 to lone AF.37 Therefore, a genetic background could be a potential cause of extensive structural remodeling in patients with lone AF. Hence, more genetic studies are necessary to understand all mechanisms in patients with lone AF.
It is known that AF is a multifactorial disease, and the pathogenesis of AF remains incompletely understood. Over the last decade, more and more factors have been shown to be related to the appearance of atrial fibrosis. One of these factors might be an inflammatory process. To our knowledge only 1 study investigated the structural changes associated with lone AF.19 These authors reported from abnormal atrial histology in all included patients with lone AF. However, the cause of the pathological changes in that recent study, which were found only in atrial septal biopsies but not in biatrial biopsies, remained unknown in 75% of patients. This histological study found patients with lone AF had increased levels of patchy LA fibrosis as compared with control subjects.19 Therefore, this might yet be another reason for structural remodeling in patients with lone AF, aside from those of a genetic background.
Study limitations
Only 1 lone AF patient presented with Utah IV SRM in this study. Therefore, it may be premature to appraise the overall recurrence rate in this group, although this patient did suffer from recurrence. However, recurrence rate in the Utah I, II, and III groups shows no significant difference comparing patients with lone AF and non–lone AF. Larger studies are needed to improve the outcome for lone AF patients with an extensive amount of SRM (i.e., 1 lone AF patient was classified as Utah IV) of the LA, as well as to increase the patient populations between non–lone AF and lone AF patients. In addition, the MRI operator selection of a wrong inversion time, the presence of respiratory navigator artifacts, and other MRI noise may lead to the inappropriate detection and quantification of fibrosis; in spite of this, such effects seemed to be minimal in this study because all included DE-MRIs were analyzable for segmentation and quantification.
Conclusion
The degree of LA structural remodeling and fibrosis as detected using DE-MRI is independent of the AF type and associated comorbidities. Selecting appropriate treatment candidates based on the quality and quantity of atrial fibrosis using DE-MRI would improve procedural outcome and avoid unnecessary intervention.
Acknowledgments
Drs. Kholmovski and Marrouche are partially supported by grants from the SurgiVision Corporation.
ABBREVIATIONS
- ACEI
angiotensin-converting enzyme inhibitor
- ACT
activated clotting time
- AF
atrial fibrillation
- ARB
angiotensin-receptor blocker
- DE-MRI
delayed-enhanced magnetic resonance imaging
- ECG
electrocardiogram
- LA
left atrium
- PVAI
pulmonary vein antrum isolation
- RAS
rennin-angiotensin system
- RF
radiofrequency
- SRM
structural remodeling
- TE
echo time
- TI
inversion time
References
- 1.Davidson E, Rotenberg Z, Weinberger I, Fuchs J, Agmon J. Diagnosis and characteristics of lone atrial fibrillation. Chest. 1989;95:1048–1050. doi: 10.1378/chest.95.5.1048. [DOI] [PubMed] [Google Scholar]
- 2.Dunn M, Alexander J, de Silva R, Hildner F. Antithrombotic therapy in atrial fibrillation. Chest. 1986;89:685–645. doi: 10.1378/chest.89.2_supplement.68s. [DOI] [PubMed] [Google Scholar]
- 3.Potpara T, Grujic M, Marinkovic J, Vujisic-Tesic B, Ostojic M, Polovina M. Mortality of patients with lone and idiopathic atrial fibrillation is similar to mortality in general population of Serbia. Vojnosanit Pregl. 2010;67:132–135. doi: 10.2298/vsp1002132p. [DOI] [PubMed] [Google Scholar]
- 4.Kopecky SL, Gersh BJ, McGoon MD, et al. The natural history of lone atrial fibrillation: a population-based study over three decades. N Engl J Med. 1987;317:669–674. doi: 10.1056/NEJM198709103171104. [DOI] [PubMed] [Google Scholar]
- 5.Fuster V, Ryde’n LE, Cannom DS, et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines; European Society of Cardiology Committee for Practice Guidelines; European Heart Rhythm Association; Heart Rhythm Society ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients with Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114:700–752. doi: 10.1161/CIRCULATIONAHA.106.177292. [DOI] [PubMed] [Google Scholar]
- 6.Ehrlich J, Nattel S, Hohnloser S. Atrial fibrillation and congestive heart failure: specific considerations at the intersection of two common and important cardiac disease sets. J Cardiovasc Electrophysiol. 2002;12:399–405. doi: 10.1046/j.1540-8167.2002.00399.x. [DOI] [PubMed] [Google Scholar]
- 7.Parkash R, Green MS, Kerr CR, et al. Canadian Registry of Atrial Fibrillation: the association of left atrial size and occurrence of atrial fibrillation: a prospective cohort study from the Canadian Registry of Atrial Fibrillation. Am Heart J. 2004;148:649–654. doi: 10.1016/j.ahj.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 8.Li D, Fareh S, Leung T, Nattel S. Promotion of atrial fibrillation by heart failure in dogs: atrial remodeling of a different sort. Circulation. 1999;100:87–95. doi: 10.1161/01.cir.100.1.87. [DOI] [PubMed] [Google Scholar]
- 9.Corradi D, Callegari S, Benussi S, et al. Myocyte changes and their left atrial distribution in patients with chronic atrial fibrillation related to mitral valve disease. Hum Pathol. 2005;36:1080–1089. doi: 10.1016/j.humpath.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 10.Stiles MK, John B, Wong CX, et al. Paroxysmal lone atrial fibrillation is associated with an abnormal atrial substrate: characterizing the “second factor. J Am Coll Cardiol. 2009;53:1182–1191. doi: 10.1016/j.jacc.2008.11.054. [DOI] [PubMed] [Google Scholar]
- 11.Kim RJ, Fieno DS, Parrish TB, et al. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation. 1999;100:1992–2002. doi: 10.1161/01.cir.100.19.1992. [DOI] [PubMed] [Google Scholar]
- 12.Klein C, Nekolla SG, Bengel FM, et al. Assessment of myocardial viability with contrast-enhanced magnetic resonance imaging: comparison with positron emission tomography. Circulation. 2002;105:162–167. doi: 10.1161/hc0202.102123. [DOI] [PubMed] [Google Scholar]
- 13.Kim RJ, Wu E, Rafael A, et al. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000;343:1445–1453. doi: 10.1056/NEJM200011163432003. [DOI] [PubMed] [Google Scholar]
- 14.Oakes RS, Badger TJ, Kholmovski EG, et al. Detection and quantification of left atrial structural remodeling with delayed-enhancement magnetic resonance imaging in patients with atrial fibrillation. Circulation. 2009;119:1758–1767. doi: 10.1161/CIRCULATIONAHA.108.811877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marrouche NF, Guenther J, Segerson NM, et al. Randomized comparison between open irrigation technology and intracardiac-echo-guided energy delivery for pulmonary vein antrum isolation: procedural parameters, outcomes, and the effect on esophageal injury. J Cardiovasc Electrophysiol. 2007;18:583–588. doi: 10.1111/j.1540-8167.2007.00879.x. [DOI] [PubMed] [Google Scholar]
- 16.Verma A, Marrouche NF, Natale A. Pulmonary vein antrum isolation: intracardiac echocardiography-guided technique. J Cardiovasc Electrophysiol. 2004;15:1335–1340. doi: 10.1046/j.1540-8167.2004.04428.x. [DOI] [PubMed] [Google Scholar]
- 17.Segerson NM, Daccarett M, Badger TJ, et al. Magnetic resonance imaging-confirmed ablative debulking of the left atrial posterior wall and septum for treatment of persistent atrial fibrillation: rationale and initial experience. J Cardiovasc Electrophysiol. 2009;21:126–132. doi: 10.1111/j.1540-8167.2009.01611.x. [DOI] [PubMed] [Google Scholar]
- 18.Calkins H, Brugada J, Packer DL, et al. HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow-up: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation developed in partnership with the European Heart Rhythm Association (EHRA) and the European Cardiac Arrhythmia Society (ECAS); in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), and the Society of Thoracic Surgeons (STS): endorsed and approved by the governing bodies of the American College of Cardiology, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, and the Heart Rhythm Society. Europace. 2007;9:335–379. doi: 10.1093/europace/eum120. [DOI] [PubMed] [Google Scholar]
- 19.Frustaci A, Chimenti C, Bellocci F, Morgante E, Russo M, Maseri A. Histological substrate of atrial biopsies in patients with lone atrial fibrillation. Circulation. 1997;96:1180–1184. doi: 10.1161/01.cir.96.4.1180. [DOI] [PubMed] [Google Scholar]
- 20.Allessie M, Ausma J, Schotten U. Electrical, contractile and structural remodeling during atrial fibrillation. Cardiovasc Res. 2002;54:230–246. doi: 10.1016/s0008-6363(02)00258-4. [DOI] [PubMed] [Google Scholar]
- 21.Morillo CA, Klein GJ, Jones DL, Guiraudon CM. Chronic rapid atrial pacing. Structural, functional, and electrophysiological characteristics of a new model of sustained atrial fibrillation. Circulation. 1995;91:1588–1595. doi: 10.1161/01.cir.91.5.1588. [DOI] [PubMed] [Google Scholar]
- 22.Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation. 1995;92:1954–1968. doi: 10.1161/01.cir.92.7.1954. [DOI] [PubMed] [Google Scholar]
- 23.Kuppahally SS, Akoum N, Burgon NS, et al. Left atrial strain and strain rate in patients with paroxysmal and persistent atrial fibrillation: relationship to left atrial structural remodeling detected by delayed enhancement-MRI. Circ Cardiovasc Imgaging. 2010 May;3(3):231–9. doi: 10.1161/CIRCIMAGING.109.865683. [DOI] [PubMed] [Google Scholar]
- 24.Burstein B, Nattel S. Atrial fibrosis: mechanisms and clinical relevance in atrial fibrillation. J Am Coll Cardiol. 2008;51:802–809. doi: 10.1016/j.jacc.2007.09.064. [DOI] [PubMed] [Google Scholar]
- 25.L’Allier PL, Ducharme A, Keller PF, Yu H, Guertin MC, Tardif JC. Angiotensin-converting enzyme inhibition in hypertensive patients is associated with a reduction in the occurrence of atrial fibrillation. J Am Coll Cardiol. 2004;44:159–164. doi: 10.1016/j.jacc.2004.03.056. [DOI] [PubMed] [Google Scholar]
- 26.Murray KT, Rottman JN, et al. Inhibition of angiotensin II signaling and recurrence of atrial fibrillation in AFFIRM. Heart Rhythm. 2004;1:669–675. doi: 10.1016/j.hrthm.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 27.Vermes E, Tardif JC, Bourassa MG, et al. Enalapril decreases the incidence of atrial fibrillation in patients with left ventricular dysfunction: insight from the Studies Of Left Ventricular Dysfunction (SOLVD) trials. Circulation. 2003;107:2926–2931. doi: 10.1161/01.CIR.0000072793.81076.D4. [DOI] [PubMed] [Google Scholar]
- 28.Boldt A, Scholl A, Garbade J, Resetar ME, Mohr FW, Gummert JF, Dhein S. ACE-inhibitor treatment attenuates atrial structural remodeling in patients with lone chronic atrial fibrillation. Basic Res Cardiol. 2006;101:261–267. doi: 10.1007/s00395-005-0571-2. [DOI] [PubMed] [Google Scholar]
- 29.Akoum N, Badger T, Adjei-Poku, et al. Abstract pre-ablation assessment of structural remodeling in atrial fibrillation helps select successful ablation strategy. Circulation. 2009;120:S691. [Google Scholar]
- 30.Jais P, Cauchemez B, Macle L, et al. Catheter ablation versus antiarrhythmic drugs for atrial fibrillation: the A4 study. Circulation. 2008;118:2488–2490. doi: 10.1161/CIRCULATIONAHA.108.772582. [DOI] [PubMed] [Google Scholar]
- 31.Shah AN, Mittal S, Sichrovsky TC, et al. Long-term outcome following successful pulmonary vein isolation: pattern and prediction of very late recurrence. J Cardiovasc Electrophysiol. 2008;19:661–667. doi: 10.1111/j.1540-8167.2008.01101.x. [DOI] [PubMed] [Google Scholar]
- 32.Oral H, Knight B, Tada H, et al. Pulmonary vein isolation for paroxysmal and persistent atrial fibrillation. Circulation. 2002;105:1077–1081. doi: 10.1161/hc0902.104712. [DOI] [PubMed] [Google Scholar]
- 33.Bharti S, Lev M. Histology of the normal and diseases atrium. In: Falk RH, Podrid PJ, editors. Atrial Fibrillation: Mechanism and Management. 3. New York: Raven Press; 1992. pp. 15–39. [Google Scholar]
- 34.Dittrich HC, Pearce LA, Asinger RW, et al. Left atrial diameter in nonvalvular atrial fibrillation: an echocardiographic study. Stroke Prevention in Atrial Fibrillation Investigators. Am Heart J. 1999;137:494–499. doi: 10.1016/s0002-8703(99)70498-9. [DOI] [PubMed] [Google Scholar]
- 35.Lubitz SA, Yi BA, MacRae CA, Ellinor PT. Genetics of atrial fibrillation. Cardiol Clin. 2009;27:25–vii. doi: 10.1016/j.ccl.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sabeh MK, MacRae CA. The genetics of atrial fibrillation. Curr Opin Cardio. 2010 May;25(3):186–191. doi: 10.1097/HCO.0b013e3283385734. [DOI] [PubMed] [Google Scholar]
- 37.Ellinor PT, Lunetta KL, Glazer NL, et al. Common variants in KCNN3 are associated with lone atrial fibrillation. Nat Genet. 2010;42:240–244. doi: 10.1038/ng.537. [DOI] [PMC free article] [PubMed] [Google Scholar]