Abstract
Introduction
Left atrial (LA) fibrosis and ablation related scarring are major predictors of success in rhythm control of atrial fibrillation (AF). We used delayed enhancement MRI (DE-MRI) to stratify AF patients based on pre-ablation fibrosis and also to evaluate ablation-induced scarring in order to identify predictors of a successful ablation.
Methods and Results
One hundred and forty-four patients were staged by percent of fibrosis quantified with DE-MRI, relative to the LA wall volume: minimal or Utah stage 1; <5%, mild or Utah stage 2; 5–20%, moderate or Utah stage 3; 20–35%, and extensive or Utah stage 4; >35%. All patients underwent pulmonary vein (PV) isolation and posterior wall and septal debulking. Overall, LA scarring was quantified and PV antra were evaluated for circumferential scarring 3 months post ablation. LA scarring post ablation was comparable across the 4 stages. Most patients had either no (36.8%) or 1 PV (32.6%) antrum circumferentially scarred. Forty-two patients (29%) had recurrent AF over 283 ± 167 days. No recurrences were noted in Utah stage 1. Recurrence was 28% in Utah stage 2, 35% in Utah stage 3, and 56% in Utah stage 4. Recurrence was predicted by circumferential PV scarring in Utah stage 2 and by overall LA wall scarring in Utah stage 3. No recurrence predictors were identified in Utah stage 4.
Conclusions
Circumferential PV antral scarring predicts ablation success in mild LA fibrosis, while posterior wall and septal scarring is needed for moderate fibrosis. This may help select the proper candidate and strategy in catheter ablation of AF.
Keywords: atrial fibrillation, atrial remodeling, catheter ablation, magnetic resonance imaging
Introduction
Atrial fibrillation (AF) is the most common sustained arrhythmia encountered in adult cardiology.1, 2 Several studies have demonstrated that AF is associated with electrical, contractile, and structural remodeling (SRM) in the left atrium (LA) that contributes to the persistence and sustainability of the arrhythmia.3–7 It has also been shown that the end result of this remodeling process is loss of atrial myocytes and increased collagen content and hence fibrosis of the LA wall.5 Delayed enhancement MRI (DE-MRI) using gadolinium contrast has been demonstrated to localize and quantify the degree of SRM or fibrosis associated with AF in the LA.8
DE-MRI has also been shown to be useful in localizing and quantifying scar formation in the LA following radiofrequency ablation (RFA).9, 10 The pulmonary vein (PV) antral region can be visualized to assess circumferential PV scarring that results from RFA lesions/ablation. In addition, the amount of scarring to the LA after catheter ablation can be quantified as a proportion of the total left atrial volume.
Rhythm control of AF using catheter ablation has yielded varying results in different patient populations.11 Identifying the ideal candidate for catheter ablation remains a significant challenge. In addition, a number of different approaches to catheter ablation have been reported and most experts agree that 1 ablation strategy does not fit all AF patients.11–15 Therefore, selecting the proper strategy for a particular patient is also an important determinant of procedure success.
We used DE-MRI to quantify both the degree of SRM/fibrosis pre-ablation and scar formation post ablation. Our aim was to identify predictors of successful ablation in a group of patients stratified according to pre-ablation fibrosis. This would help select the most appropriate ablation strategy for the individual AF ablation candidate.
Methods
This is a prospective observational study where 144 patients with AF presenting to the University of Utah for catheter ablation between November 2006 and November 2008 were included. Patient information gathered for the purposes of the study was de-identified and protected in compliance with HIPAA regulations. All patients underwent DE-MRI evaluation both pre-ablation to assess the extent of atrial tissue fibrosis and at 3 months following the procedure to quantify the degree of ablation related atrial tissue scarring.
MRI Image Acquisition
All studies were obtained on a 1.5 Tesla Avanto clinical scanner (Siemens Medical Solutions, Erlangen, Germany) using a TIM phased-array receiver coil. The scan was acquired 15 minutes following contrast agent injection (0.1 mmol/kg, Multihance [Bracco Diagnostic Inc., Princeton, NJ]) using a 3D inversion recovery, respiration navigated, ECG-gated, gradient echo pulse sequence. Typical acquisition parameters were: free-breathing using navigator gating, a transverse imaging volume with voxel size = 1.25 × 1.25 × 2.5 mm (reconstructed to 0.625 × 0.625 × 1.25 mm), TR/TE = 5.4/2.3 ms, flip angle = 20°, inversion time (TI) = 270–310 ms, and GRAPPA with R = 2 and 46 reference lines. ECG gating was used to acquire a small subset of phase encoding views during the diastolic phase of the LA cardiac cycle. The time interval between the R-peak of the ECG and the start of data acquisition was defined using the cine images of the LA. Fat saturation was used to suppress fat signal. The TE of the scan (2.3 ms) was chosen such that fat and water are out of phase and the signal intensity of partial volume fat-tissue voxels was reduced allowing improved delineation of the LA wall boundary. The TI value for the DE-MRI scan was identified using a scout scan. Typical scan time for the DE-MRI study was 5–10 minutes depending on subject respiratory and heart rate.
DE-MRI Quantification of Pre-Ablation Fibrosis/Structural Remodeling and Postablation Scarring
In all images, the epicardial and endocardial LA borders were manually contoured with image display and analysis software in Seg3D (segmentation and processing tool developed by the NIH Center for Integrative Biomedical Computing at the University of Utah Scientific Computing and Imaging [SCI] Institute). The relative extent of pre-ablation enhancement and postablation scar were quantified within the LA wall with a threshold-based algorithm utilizing pixel intensities from normal based on a bimodal distribution with analysis software written in MATLAB (The Mathworks Inc, Natick, MA, USA). Qualitative confirmation of the percent enhancement was performed for all scans using 3D visualization of the MRI performed using a custom made image-processing software. Initial visualization used a maximum intensity projection to assess contrast consistency, followed by volume rendering with a ray-cast engine with linear table opacity for pre-ablation images and smooth table opacity for postablation images. A color lookup table mask was applied to better differentiate enhanced and nonenhanced tissue.
Ablation Procedure
The details of the PV isolation in addition to posterior wall and septal debulking has been described elsewhere.16 In brief, the LA was accessed through 2 transseptal punctures under intracardiac echo guidance using a phased array catheter (Acunav, Siemens Medical Solutions USA, Inc, Mountain View, CA, USA). A 10-pole circular mapping catheter (Lasso, Biosense Webster, Diamond Bar, CA, USA) and a 3.5 mm Thermocool ablation catheter (Biosense Webster) were advanced into the LA for mapping and ablation. A 14-pole catheter (TZ Medical, Portland, OR, USA) was used to record right atrial and coronary sinus electrograms and was used as the reference catheter for 3D electroanatomical mapping with CARTO (Biosense Webster). Radiofrequency energy was delivered with 50 Watts at a catheter tip temperature of 50 °C for no longer than 10 seconds seconds, guided by electrograms abolition recorded on the Lasso catheter. Ablation lesions were placed in a circular fashion in the PV antral region until electrical isolation of the PVs was achieved. Additional lesions were placed along the left atrial posterior wall and septum.
Assessment of postablation scarring
The overall scarring was quantified 3 months following the ablation procedure as described above. The scar burden was reported as a proportion of the left atrial wall volume. In addition, the PV antral regions were analyzed to determine the presence of contiguous lesions encircling the PV ostia. The PV antral regions were included in the overall scar quantification. A particular vein was considered encircled if 100% of its antral area was circumferentially scarred (Fig. 3).
Figure 3.
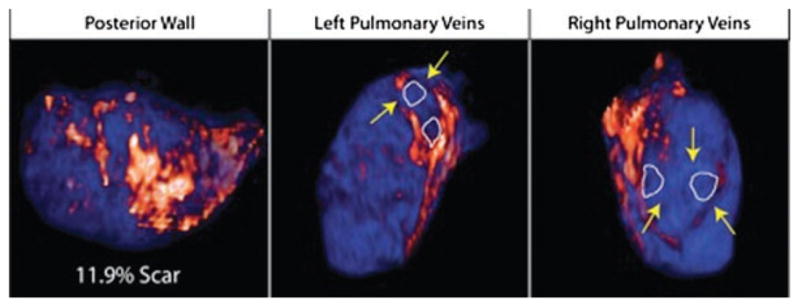
Evaluation of the overall left atrial scarring as well as pulmonary vein encirclement with ablation scar. The left panel shows a patient with 11.9% overall LA wall scar 3 months postablation with only one vein circumferentially encircled. The middle and right panels show another patient with 32.4% overall scarring of the LA wall with all 4 pulmonary veins circumferentially encircled.
Pre-ablation fibrosis vs postablation scarring
Compared to pre-ablation DE-MRI, imaging of the LA 3 months post ablation reveals an enhancement pattern characterized by a brighter signal and distribution of pixel intensity shifted towards higher intensity (Figs. 1 and 3). The high intensity signals due to ablation-related scarring effectively mask the fibrosis pattern noted on the pre-ablation scans. To quantify scarring, we therefore utilized a different algorithm with higher thresholds to distinguish scarred from unscarred tissue. Pre-ablation fibrosis signals fall below the threshold used for postablation scarring. Therefore, it is very unlikely that the quantification of scarring includes in part pre-ablation fibrosis.
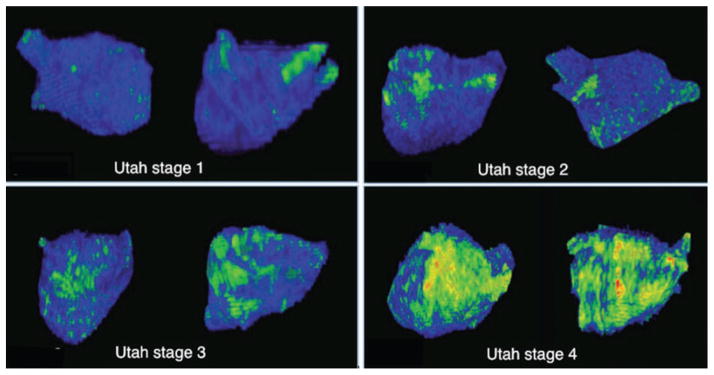
Figure 1.
A series of left atrial MRI 3D reconstructions displayed in the RAO and PA projections illustrating areas of fibrosis (bright green) across the 4 stages of fibrosis. Utah stage 1: <5% fibrosis, Utah stage 2: 5–20% fibrosis, Utah stage 3: 20–25% fibrosis, Utah stage 4: >35% fibrosis.
Follow-Up and Definition of Recurrence
A postablation blanking period was observed for 3 months during which all patients received an 8-week automatic trigger cardiac event monitor for assessment of early AF recurrence. Early recurrences were treated with direct current cardioversion, antiarrhythmic drugs (AADs) or both. AADs were discontinued at the end of the blanking period. All patients were seen in follow-up at 3 months following ablation and at 3-month intervals thereafter. Each patient received a 12 lead ECG and an 8-day Holter monitor for detection of arrhythmia recurrence post blanking. Additional ECG recordings were obtained as suggested by the patients’ reported symptoms through weekly telephone calls. Recurrence was defined as any atrial arrhythmia sustained for longer than 30 seconds without AAD treatment following the 3-month blanking period, as suggested by the HRS consensus statement.11 All ablation procedures were performed on therapeutic anticoagulation with warfarin. Warfarin was continued post procedure as well to maintain an international therapeutic ratio of 2.0–3.0.
Data Analysis
Statistical analysis was performed using STATA 11 (Stata-Corp, College Station, TX, USA). Continuous variables are reported as means and standard deviations and categorical variables are reported as percentages of the cohort. Student’s t-test was used to compare continuous variables and Chi-square test to compare proportions. A Cox proportional hazard multivariate regression model was used to determine significant predictors of AF recurrence following ablation. To avoid overfitting, nonsignificant predictor variables were removed from the regression model in a stepwise fashion. Two-sided P-values <0.05 were considered significant.
Results
Pre-Ablation Fibrosis/Structural Remodeling Based Staging
DE-MRI scans were of adequate quality to obtain quantification of pre-ablation SRM in 120 of the 144 total patient cohort (85%). Motion artifact often due to AF at the time of MRI acquisition was the main contributing factor for poor scans quality.
Of the 120 patients successfully quantified, the average pre-ablation fibrosis was 18.06 ± 13.49% of the LA wall volume. These patients were then divided into 4 categories as follows: Utah stage 1 or minimal fibrosis (at least 1 standard deviation below the cohort mean, i.e., <5% enhancement), Utah stage 2 or mild fibrosis (5–20% enhancement), Utah stage 3 or moderate fibrosis (20–35% enhancement) and Utah stage 4 or extensive fibrosis (greater than 35% enhancement). Figure 1 shows examples of patients in each of these stages. Of the patients with successful quantification, 10 (7%) were in Utah stage 1, 71 (49%) in Utah stage 2, 23 (16%) in Utah stage 3 and 16 (11%) in Utah stage 4. Age at the time of initial MRI acquisition, prevalence of hypertension, coronary artery disease, congestive heart failure, diabetes and left ventricular ejection fraction were comparable across the 4 groups. The patients’ characteristics are detailed in Table 1.
TABLE 1.
Characteristics of 120 Patients with Preablation Quantification of Left Atrial Fibrosis
| Utah Stage 1 (<5%) (N = 10) | Utah Stage 2 (5–20%) (N = 71) | Utah Stage 3 (20–35%) (N = 23) | Utah Stage 4 (>35%) (N = 16) | P-value | |
|---|---|---|---|---|---|
| Age (years) | 58 ± 14 | 62 ± 13 | 67 ± 13 | 68 ± 8 | ns |
| HTN (%) | 50.0 | 53.5 | 56.5 | 43.8 | ns |
| Diabetes (%) | 10 | 7.0 | 21.7 | 6.3 | ns |
| Coronary disease (%) | 30 | 12.7 | 13.0 | 18.8 | ns |
| CHF (%) | 10 | 5.6 | 4.3 | 12.5 | ns |
| LV EF (%) | 57.2 ± 3.5 | 51.8 ± 9.5 | 49.7 ± 11.4 | 44.8 ± 13.2 | ns |
| Paroxysmal/persistent AF (%) | 60/40 | 45/55 | 35/65 | 25/75 | ns |
ns = nonsignificant.
AF Phenotype and Degree of Fibrosis
Paroxysmal AF was more prevalent in Utah stage 1 and persistent AF was more prevalent in Utah stage 4; however, all groups were a heterogeneous mix of both AF clinical phenotypes as illustrated in Figure 2.
Figure 2.
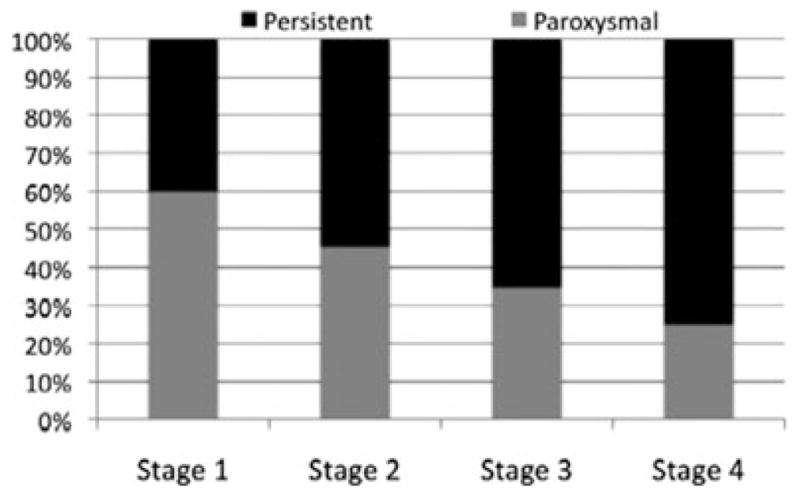
Distribution of paroxysmal and persistent atrial fibrillation across the 4 stages of fibrosis. Note that each stage is a heterogeneous mix of both AF phenotypes with more predominant persistent AF in advanced stages.
Postablation Scarring Analysis
All patient were in sinus rhythm at the time of MRI acquisition at 3 months following RFA. Therefore, quantification of LA scarring was successful in all 144 patients. The scar was analyzed to quantify the overall scarring due to the ablation and to determine whether the scarring encircled the entire PV antral circumference.
Overall Left Atrial Scarring
All patients received a similar ablation lesion set that covered the PV antral regions as well as the posterior and septal left atrial walls. The overall scarring at 3 months following ablation averaged 14.94 ± 9.58% of the left atrial wall volume. The average scar burden was found to be similar across the pre-ablation Utah stages (Table 2). Notice that the quantification obtained for the average scar quantified relative to the left atrial wall volume is different than the average pre-ablation fibrosis. This is due to the fact that ablation induced injury and scarring yields a much stronger signal on DE-MRI compared to pre-ablation fibrosis. Therefore, a different quantification algorithm and threshold was used for postablation quantification than the one used for pre-ablation fibrosis.
TABLE 2.
MRI Quantification of Structural Remodeling Pre-Ablation and Assessment of Scarring and Pulmonary Vein Encirclement Postablation
| Utah Stage 1 (<5%) (N = 10) | Utah Stage 2 (5–20%) (N = 71) | Utah Stage 3 (20–35%) (N = 23) | Utah Stage 4 (>35%) (N = 16) | P-value | |
|---|---|---|---|---|---|
| Pre-ablation delayed | 2.8 ± 1 | 11.8 ± 4.5 | 24.7 ± 7 | 45.8 ± 9.0 | <0.01 |
| Enhancement (%) | |||||
| Pulmonary veins encircled | 1.8 ± 1.0 | 1.1 ± 1.2 | 0.8 ± 1.2 | 1.0 ± 1.15 | 0.16 |
| Overall posterior wall scarring (%) | 15.6 ± 7.2 | 15.4 ± 10.1 | 12.2 ± 8.2 | 14.5 ± 12.5 | 0.41 |
Pulmonary Vein Antral Scarring
The distribution of scar around the PV antral region was analyzed in the entire patient cohort to determine the success of encirclement of the PV antra by ablation lesions. We found that the majority of patients had zero (53 of 144 or 36.8%) or 1 vein antrum (47 of 144 or 32.6%) circumferentially encircled by scar. In only a minority of patients, all 4 veins were encircled by scar (10 of 144 pts or 7%) (Fig. 4). PV encirclement by ablation scar was correlated with the overall left atrial scarring (r2 = 0.36, P < 0.01).
Figure 4.
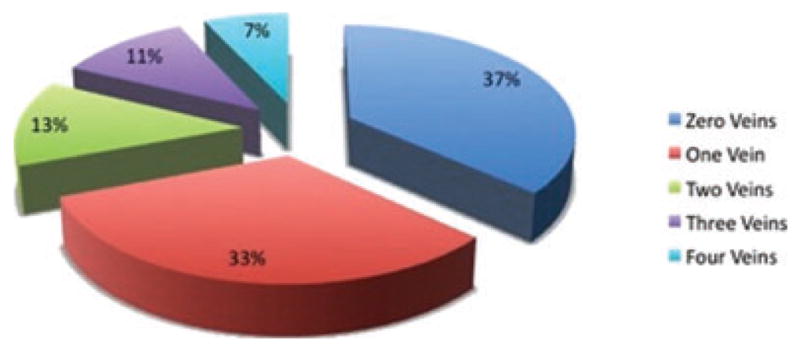
Pie chart depicting the success of ablation in creating circumferential scarring around the pulmonary vein antra. Only a small proportion of patients (7%) had complete encirclement of all 4 pulmonary veins.
Recurrence Predictors
Of 120 patients staged based on pre-ablation fibrosis, 37 (31%) experienced recurrent atrial arrhythmia over an average follow-up period of 283 ± 167 days. We analyzed the recurrence predictors in each of the 4 SRM groups. The follow-up period was comparable across the pre-ablation staging groups.
Using a Cox multivariate regression model, the patient’s age, AF type, left atrial volume, number of PVs encircled by scarring, and overall left atrial scarring at 3 months were included as predictor variables for arrhythmia recurrence. Since ablation scar and PV encirclement were statistically correlated, an interaction term (scar × PV encirclement) was included in the model and it showed that the observed correlations of PV encirclement and overall scarring were independent. Recurrence was analyzed across the 4 groups as shown in the Kaplan–Meier curve in Figure 5.
Figure 5.
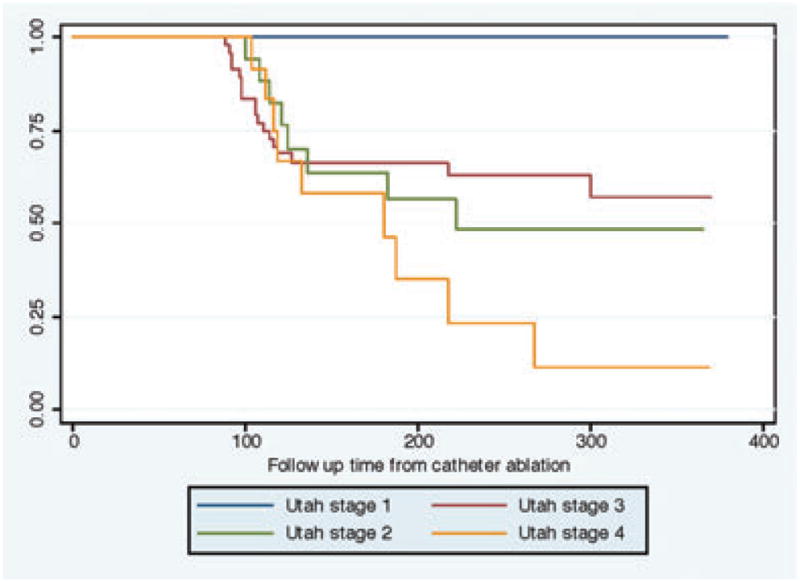
Kaplan–Meier depicting AF recurrence stratified over the different stages of structural remodeling. Utah stage 1: <5% fibrosis, Utah stage 2: 5–20% fibrosis, Utah stage 3: 20–35% fibrosis, Utah stage 4: >35% fibrosis.
No recurrences were observed in the Utah stage 1 group over the follow-up period.
In the Utah stage 2 group, 20 of 71 patients experienced recurrence (28.1%). The number of PVs encircled by scar was found to be the strongest predictor of arrhythmia recurrence with a hazard ratio of 0.43 (95% confidence interval 0.20–0.92; P = 0.03). Left atrial volume pre-ablation was a minor predictor with a hazard ratio of 1.01 (95% confidence interval 1.00–1.02; P = 0.01).
In the Utah stage 3 group, 8 of 23 patients experienced recurrence (34.7%). The overall left atrial scar at 3 months postablation was found to be the strongest predictor of recurrence with a hazard ratio of 0.63 (95% confidence interval 0.42–0.94; P = 0.03). The patient’s age was also found to be an important predictor of recurrence in this group with a hazard ratio of 0.80 (95% confidence interval 0.65–0.98; P = 0.03).
In the Utah stage 4 group, 9 of 16 patients had recurrence atrial arrhythmia (56.3%). None of the parameters included in the regression model successfully predicted recurrence in this group.
The results of the multivariate Cox regression are summarized in Table 3.
TABLE 3.
Recurrence Predictors Following Ablation for 120 Patients with Pre- and Postablation Assessment of Left Atrial Fibrosis and Scarring
| Utah Stage 1 (<5%) (N = 10) No Recurrence | Utah Stage 2 (5–20%) (N = 71)
|
Utah Stage 3 (20–35%) (N = 23)
|
Utah Stage 4 (>35%) (N = 16)
|
||||
|---|---|---|---|---|---|---|---|
| Hazard Ratio | P-value | Hazard Ratio | P-value | Hazard Ratio | P-value | ||
| Age | 1.01 | 0.74 | 0.80 | 0.03 | 0.97 | 0.60 | |
| AF type | 0.55 | 0.22 | 1.3 | 0.76 | 1.13 | 0.90 | |
| LA volume | 1.01 | 0.01 | 1.0 | 0.86 | 0.99 | 0.49 | |
| Pulmonary veins encircled | 0.43 | 0.03 | 5.33 | 0.10 | 0.54 | 0.43 | |
| Posterior wall scarring | 1.0 | 0.84 | 0.63 | 0.03 | 0.99 | 0.92 | |
Discussion
Pre-ablation staging identified 2 subgroups of AF patients with either an excellent (minimal fibrosis) or a poor (extensive fibrosis) prognosis following ablation. In addition, postablation assessment of the PV antral region scarring demonstrated that catheter ablation targeting acute electrical isolation does not correlate with PV antral circumferential scarring at 3 months, as seen using DE-MRI. We also demonstrate circumferential PV scarring to be an important predictor of arrhythmia recurrence in the subgroup of patients with mild fibrosis (Utah stage 2), as opposed to the moderate fibrosis group (Utah stage 3), where overall LA scarring, specifically LA posterior wall and septum, is shown to be the most important determinant of procedural failure.
Most approaches to ablation for AF aim at isolation of PV triggers.11, 12 This is accomplished by encircling the PV antral region with ablation lesions and achieving electrical isolation, which is an accepted acute endpoint.12, 17 Some operators advocate for additional ablation aimed at “substrate modification” for patients with longstanding or persistent arrhythmia.11, 13–15 This study examines the anatomical correlate of electrical isolation by examining scarring in the PV antral region as well as the overall scarring in the LA. We show that even though electrical isolation is achieved acutely, this does not translate to persistent circumferential scarring around the PVs at 3 months. This indicates that either (1) electrical isolation does not require complete encirclement of the PVs or (2) that initial ablation is ineffective at creating irreversible scarring, i.e., there is recovery from the initial injury. The first scenario is less likely as operators currently ablate away from the true ostia of the PVs to avoid PV stenosis. Lesions therefore target continuous left atrial tissue rather than muscle sleeves inside the PVs. The second scenario is more likely especially when it is suspected that electrical reconnection of the PVs to the main left atrial musculature has occurred in the majority of recurrent cases.18–21 Our study also shows that PV encirclement with scar correlates with the overall scarring in the LA 3 months postablation. Patients with fewer veins encircled with scarring have a lower overall left atrial scar burden at 3 months. This is seen despite the fact that all patients were ablated using the same approach and achieved the same endpoints at the end of the procedure. This indicates that the mechanism leading to ineffective lesion formation or tissue recovery following ablation is the same in the antral region and throughout the LA.
PV antrum encirclement with ablation scar and overall ablation scar in the LA predicted recurrence independently in patients with mild and moderate fibrosis/SRM subgroups, respectively. This is in line with other publications suggesting that isolation of PV triggers may be acceptable in paroxysmal AF, whereas patients with more clinically advanced AF are likely to have a better long-term outcome with more extensive ablation. Variable success rates have been reported with adding linear lesions to PV circumferential isolation,14, 22 targeting areas of complex fractionated electrograms (CFAEs)13 and dominant frequency,23 as well as disrupting ganglionated plexi connections to the atrium.24 The common denominator to all these approaches helping improve outcomes following ablation is that, especially in patients with more advanced clinical AF, there is more left atrial tissue involved in creating the AF substrate than the triggers in the PVs and that going outside the PV antral region to modify this substrate leads to improved ablation outcomes. Our study offers an insight onto this AF substrate by quantifying the extent of LA remodeling and fibrosis associated with it, which we demonstrate to require more extensive ablation in order to overcome and achieve better long-term success.
Clinical Implication
The results of this study have an important impact on clinical decision making both for the AF patient and the physician managing the arrhythmia. For the patient, expectations for the outcomes of the ablation procedure can be satisfactorily estimated and the patient can then weigh the risks of undergoing the ablation procedure against the benefits of maintaining sinus rhythm. For the ablationist, quantification of fibrosis/SRM can be used to counsel the patients better about the expected outcomes of catheter ablation. Moreover, the operator can plan the procedure better with the knowledge that patients with advanced fibrosis/SRM will have a better outcome with a more extensive ablation rather than a PV isolation.
Limitations
This is an observational study that included a selected population of patients with AF presenting for catheter ablation. Prospective randomized studies are needed to validate that patients with early stages of atrial remodeling derive a good outcome from PV isolation, whereas patients with advanced stages require additional atrial substrate modification.
The distribution of fibrosis in this ablation cohort was centered in the mild and moderate categories with a small number of patients in the minimal and extensive SRM groups. The inclusion of a larger and more representative group of patients, including AF patients treated with AADs and permanent AF patients treated with a rate control strategy would also provide a more comprehensive picture of the distribution of SRM at the various stages of the disease process. A more balanced sample size across the different stages would potentially allow for a better comparison between these groups. This distribution, however, may be representative of AF patients seeking ablation, where LA fibrosis has progressed passed the initial stages.
While this study sheds an important light on the scarring that follows AF ablation, it is limited by the fact that DE-MRI scans obtained post catheter ablation demonstrate a different degree and distribution of enhancement. This makes it technically very challenging to distinguish ablation related scarring from pre-existing fibrosis.
Transmural lesion formation at the time of ablation is a function of tissue temperature, contact force and tissue thickness. While acute edema may result in electrical isolation, long term scarring may not have been achieved. A real-time assessment of lesion formation remains the ultimate goal to ensure adequate lesion delivery and better long-term outcome.
Acknowledgments
E. Kholmovski and R. MacLeod report research support from Surgivision, Inc. N. Marrouche reports research support from Surgivision, Inc and Biosense Webster. Other authors: No disclosures.
References
- 1.Fuster VRL, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Le Heuzey JY, Kay GN, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL, Wann S, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Halperin JL, Hunt SA, Nishimura R, Ornato JP, Page RL, Riegel B, Priori SG, Blanc JJ, Budaj A, Camm AJ, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo JL, Zamorano JL. American College of Cardiology; American Heart Association Task Force; European Society of Cardiology Committee for Practice Guidelines; European Heart Rhythm Association; Heart Rhythm Society: ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): Developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2007;114:e257–354. doi: 10.1161/CIRCULATIONAHA.106.177292. [DOI] [PubMed] [Google Scholar]
- 2.Go ASHE, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: National implications for rhythm management and stroke prevention: The AnTicoagulation and Risk Factors in Atrial Fribrillation (ATRIA) Study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 3.Ausma J, Wijffels M, Thone F, Wouters L, Allessie M, Borgers M. Structural changes of atrial myocardium due to sustained atrial fibrillation in the goat. Circulation. 1997;96:3157–3163. doi: 10.1161/01.cir.96.9.3157. [DOI] [PubMed] [Google Scholar]
- 4.Bosch RF, Zeng X, Grammer JB, Popovic K, Mewis C, Kühlkamp V. Ionic mechanisms of electrical remodeling in human atrial fibrillation. Cardiovascular Research. 1999;44:121–131. doi: 10.1016/s0008-6363(99)00178-9. [DOI] [PubMed] [Google Scholar]
- 5.Allessie MAJ, Schotten U. Electrical, contractile and structural remodeling during atrial fibrillation. Cardiovascular Research. 2002;54:230–236. doi: 10.1016/s0008-6363(02)00258-4. [DOI] [PubMed] [Google Scholar]
- 6.Casaclang-Verzosa GGB, Tsang TS. Structural and functional remodeling of the left atrium: Clinical and therapeutic implications for atrial fibrillation. Journal of the American College of Cardiology. 2008;51:1–11. doi: 10.1016/j.jacc.2007.09.026. [DOI] [PubMed] [Google Scholar]
- 7.Schotten UDM, Ausma J, Eijbouts S, Neuberger HR, Allessie M. Electrical and contractile remodeling during the first days of atrial fibrillation go hand in hand. Circulation. 2003;107:1433–1439. doi: 10.1161/01.cir.0000055314.10801.4f. [DOI] [PubMed] [Google Scholar]
- 8.Oakes RS, Badger TJ, Kholmovski EG, Akoum N, Burgon NS, Fish EN, Blauer JJE, Rao SN, DiBella EVR, Segerson NM, Daccarett M, Windfelder J, McGann CJ, Parker D, MacLeod RS, Marrouche NF. Detection and quantification of left atrial structural remodeling with delayed-enhancement magnetic resonance imaging in patients with atrial fibrillation. Circulation. 2009;119:1758–1767. doi: 10.1161/CIRCULATIONAHA.108.811877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McGann CJ, Kholmovski EG, Oakes RS, Blauer JJE, Daccarett M, Segerson N, Airey KJ, Akoum N, Fish E, Badger TJ, DiBella EVR, Parker D, MacLeod RS, Marrouche NF. New magnetic resonance imaging-based method for defining the extent of left atrial wall injury after the ablation of atrial fibrillation. J Am Coll Cardiol. 2008;52:1263–1271. doi: 10.1016/j.jacc.2008.05.062. [DOI] [PubMed] [Google Scholar]
- 10.Peters DC, Wylie JV, Hauser TH, Kissinger KV, Botnar RM, Essebag V, Josephson ME, Manning WJ. Detection of pulmonary vein and left atrial scar after catheter ablation with three-dimensional navigator-gated delayed enhancement MR imaging: Initial experience. Radiology. 2007;243:690–695. doi: 10.1148/radiol.2433060417. [DOI] [PubMed] [Google Scholar]
- 11.Calkins HBJ, Packer DL, Cappato R, Chen SA, Crijns HJ, Damiano RJ, Jr, Davies DW, Haines DE, Haisaguerre M, Iesaka Y, Jackman W, Jais P, Kottkamp H, Kuck KH, Lindsay BD, Marchlinski FE, McCarthy PM, Mont JL, Morady F, Nademanee K, Natale A, Pappone C, Prystowsky E, Raviele A, Ruskin JN, Shemin RJ. HRS/EHRA/ECAS expert Consensus Statement on catheter and surgical ablation of atrial fibrillation: Recommendations for personnel, policy, procedures and follow-up. A report of the Heart Rhythm Society (HRS) Task Force on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2007;4:816–861. doi: 10.1016/j.hrthm.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 12.Haissaguerre M, Jais P, Shah DC, Garrigue S, Takahashi A, Lavergne T, Hocini M, Peng JT, Roudaut R, Clementy J. Electrophysiological end point for catheter ablation of atrial fibrillation initiated from multiple pulmonary venous foci. Circulation. 2000;101:1409–1417. doi: 10.1161/01.cir.101.12.1409. [DOI] [PubMed] [Google Scholar]
- 13.Nademanee K, McKenzie J, Kosar E, Schwab M, Sunsaneewitayakul B, Vasavakul T, Khunnawat C, Ngarmukos T. A new approach for catheter ablation of atrial fibrillation: Mapping of the electrophysiologic substrate. J Am Coll Cardiol. 2004;43:2044–2053. doi: 10.1016/j.jacc.2003.12.054. [DOI] [PubMed] [Google Scholar]
- 14.O’Neill M, Jaïs P, Takahashi Y, Jonsson A, Sacher F, Hocini M, Sanders P, Rostock T, Rotter M, Pernat A, Clementy J, Haïssaguerre M. The stepwise ablation approach for chornic atrial fibrillation-evidence for a cumulative effect. J Interv Card Electrophysiol. 2006;16:153–167. doi: 10.1007/s10840-006-9045-1. [DOI] [PubMed] [Google Scholar]
- 15.Oral H, Chugh A, Good E, Wimmer A, Dey S, Gadeela N, Sankaran S, Crawford T, Sarrazin J, Kuhne M, Chalfoun N, Wells D, Frederick M, Fortino J, Benloucif-Moore S, Jongnarangsin K, Pelosi FJ, Bogun F, Morady F. Radiofrequency catheter ablation of chronic atrial fib-rillation guided by complex electrograms. Circulation. 2007;115:2606–2612. doi: 10.1161/CIRCULATIONAHA.107.691386. [DOI] [PubMed] [Google Scholar]
- 16.Segerson N, Daccarett M, Badger T, Shabaan A, Akoum N, Fish E, Rao S, Burgon N, Adjei-Poku Y, Kholmovski E, Vijayakumar S, Dibella E, MacLeod R, Marrouche N. Magnetic resonance imaging-confirmed ablative debulking of the left atrial posterior wall and septum for treatment of persistent atrial fibrillation: Rationale and initial experience. J Cardiovasc Electrophysio. 2009;21:126–132. doi: 10.1111/j.1540-8167.2009.01611.x. [DOI] [PubMed] [Google Scholar]
- 17.Stevenson WG, Epstein LM. Endpoints for ablation of atrial fibrillation. Heart Rhythm. 2006;3:146–147. doi: 10.1016/j.hrthm.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 18.Kim R, Peter MK, Mark JE, Glyn T, Maite I, Simon CS, Richard JS. Acute and chronic pulmonary vein reconnection after atrial fibrillation ablation: A prospective characterization of anatomical sites. Pacing and Clinical Electrophysiology. 2008;31:1598–1605. doi: 10.1111/j.1540-8159.2008.01232.x. [DOI] [PubMed] [Google Scholar]
- 19.Ouyang F, Antz M, Ernst S, Hachiya H, Mavrakis H, Deger FT, Schaumann A, Chun J, Falk P, Hennig D, Liu X, Bansch D, Kuck KH. Recovered pulmonary vein conduction as a dominant factor for recurrent atrial tachyarrhythmias after complete circular isolation of the pulmonary veins: Lessons from double Lasso technique. Circulation. 2005;111:127–135. doi: 10.1161/01.CIR.0000151289.73085.36. [DOI] [PubMed] [Google Scholar]
- 20.Verma A, Kilicaslan F, Pisano E, Marrouche NF, Fanelli R, Brachmann J, Geunther J, Potenza D, Martin DO, Cummings J, Burkhardt JD, Saliba W, Schweikert RA, Natale A. Response of atrial fibrillation to pulmonary vein antrum isolation is directly related to resumption and delay of pulmonary vein conduction. Circulation. 2005;112:627–635. doi: 10.1161/CIRCULATIONAHA.104.533190. [DOI] [PubMed] [Google Scholar]
- 21.Lemola K, Hall B, Cheung P, Good E, Han J, Tamirisa K, Chugh A, Bogun F, Pelosi F, Jr, Morady F, Oral H. Mechanisms of recurrent atrial fibrillation after pulmonary vein isolation by segmental ostial ablation. Heart Rhythm. 2004;1:197–202. doi: 10.1016/j.hrthm.2004.03.071. [DOI] [PubMed] [Google Scholar]
- 22.Oral H, Scharf C, Chugh A, Hall B, Cheung P, Good E, Veerareddy S, Pelosi F, Jr, Morady F. Catheter ablation for paroxysmal atrial fibrillation: Segmental pulmonary vein ostial ablation versus left atrial ablation. Circulation. 2003;108:2355–2360. doi: 10.1161/01.CIR.0000095796.45180.88. [DOI] [PubMed] [Google Scholar]
- 23.Sanders P, Berenfeld O, Hocini M, Jaïs P, Vaidyanathan R, Hsu L, Garrigue S, Takahashi Y, Rotter M, Sacher F, Scavee C, Ploutz-Snyder R, Jalife J, Haïssaguerre M. Spectral analysis identifies sites of high-frequency activity maintaining atrial fibrillation in humans. Circulation. 2005;112:789–797. doi: 10.1161/CIRCULATIONAHA.104.517011. [DOI] [PubMed] [Google Scholar]
- 24.Lu Z, Scherlag BJ, Lin J, Yu L, Guo J-H, Niu G, Jackman WM, Lazzara R, Jiang H, Po SS. Autonomic mechanism for initiation of rapid firing from atria and pulmonary veins: Evidence by ablation of ganglionated plexi. Cardiovasc Res. 2009;84:245–252. doi: 10.1093/cvr/cvp194. [DOI] [PMC free article] [PubMed] [Google Scholar]