Abstract
The invasion of inflammatory cells occurring after ischemic or traumatic brain injury (TBI) has a detrimental effect on neuronal survival and functional recovery after injury. We have recently demonstrated that not only the blood–brain barrier, but also the blood–cerebrospinal fluid (CSF) barrier (BCSFB), has a role in posttraumatic recruitment of neutrophils. Here, we show that TBI results in a rapid increase in synthesis and release into the CSF of a major chemoattractant for monocytes, CCL2, by the choroid plexus epithelium, a site of the BCSFB. Using an in vitro model of the BCSFB, we also show that CCL2 is released across the apical and basolateral membranes of the choroidal epithelium, a pattern of chemokine secretion that promotes leukocyte migration across epithelial barriers. Immunohistochemical and electron microscopic analyses of choroidal tissue provide evidence for the movement of monocytes, sometimes in tandem with neutrophils, along the paracellular pathways between adjacent epithelial cells. These data further support the pathophysiological role of BCSFB in promoting the recruitment of inflammatory cells to the injured brain.
Keywords: blood–cerebrospinal fluid barrier, choroid plexus, CC chemokines, monocytes, traumatic brain injury
Introduction
Brain injury, whether it is ischemic, hemorrhagic, or traumatic, is accompanied by pathological neuroinflammation, which is thought to significantly contribute to the formation of cerebral edema and loss of neural tissue, and to have an impact on functional recovery after injury. An important part of this brain inflammatory response to injury is the influx of neutrophils and monocytes. Neutrophils are highly toxic to vulnerable neurons (Neumann et al, 2008) and these inflammatory cells have been shown to exacerbate cerebral edema and brain tissue damage in rodent models of traumatic brain injury (TBI) (Schoettle et al, 1990; Semple et al, 2010b). Similarly, invading monocytes have been reported to substantially contribute to the formation of cerebral edema and loss of neural tissue, and to have an adverse effect on neurologic outcome in rodent models of stroke and TBI (Chen et al, 2003; Dimitrijevic et al, 2007; Semple et al, 2010a). Using the Brattleboro rats deficient in vasopressin, a neuropeptide found to substantially augment the posttraumatic synthesis of proinflammatory mediators, we have recently demonstrated that there is an association between the cortical production of neutrophil and monocyte chemoattractants, the magnitude of influx of inflammatory cells, and the extent of loss of neural tissue occurring after TBI (Szmydynger-Chodobska et al, 2010). These results are consistent with previous observations that treatments directed to counter the influx of inflammatory cells into the injured brain are therapeutically beneficial (Beech et al, 2001; Yamasaki et al, 1997).
Although the blood–brain barrier is the major route for inflammatory cells to invade the injured brain, increasing evidence suggests that the blood–cerebrospinal fluid (CSF) barrier (BCSFB) also has an important role in this pathophysiological process (Szmydynger-Chodobska et al, 2009). The BCSFB is formed by a single layer of epithelial cells enclosing blood microvessels in the highly vascularized choroid plexus (CP), a tissue located in all four cerebral ventricles (Strazielle and Ghersi-Egea, 2000). We have previously shown that the choroidal epithelium has the ability to produce neutrophil chemoattractants and provided electron microscopic evidence for neutrophil trafficking across the BCSFB (Szmydynger-Chodobska et al, 2009). However, investigations have not determined whether the BCSFB also has a role in the recruitment of monocytes to the injured brain.
The major chemoattractant for monocytes is monocyte chemotactic protein 1 (MCP-1) or CCL2, which belongs to the CC family of chemokines (Rollins, 1997). This chemokine has previously been shown to be synthesized by variety of epithelial cells in response to proinflammatory cytokines interleukin-1β (IL-1β) and tumor necrosis factor-α (Paine et al, 1993; Prodjosudjadi et al, 1995). Although other members of the MCP subfamily of CC chemokines, such as MCP-2/CCL8, MCP-3/CCL7, MCP-4/CCL13, and MCP-5/CCL12 (mouse only), have been identified, they seem to be weaker chemoattractants for monocytes compared with MCP-1/CCL2 (Sozzani et al, 1994; Takahashi et al, 2009). CCL2 binds to CCR2, a G-protein-coupled receptor (Rollins, 1997). This chemokine also binds to CCR10, a placental chemokine receptor (Bonini et al, 1997); however, CCR10 is not expressed on leukocytes.
The aim of this study was to define a role of the CP in monocyte recruitment to the injured brain. Accordingly, we characterized the ability of the CP to produce CCL2 in response to injury using a rat model of TBI and determined the direction of cytokine-induced release of this chemokine (across the apical versus basolateral membrane of choroidal epithelium) using an in vitro model of the BCSFB. Finally, we employed transmission electron microscopy to demonstrate monocyte trafficking across the BCSFB.
Materials and methods
Reagents and Antibodies
ThermoScript RNase H– reverse transcriptase and RNase inhibitor, RNaseOut, were obtained from Invitrogen (Carlsbad, CA, USA). HotStart Taq DNA polymerase was purchased from Qiagen (Valencia, CA, USA). Low-endotoxin bovine serum albumin (A2058) was obtained from Sigma (St Louis, MO, USA) and laminin was obtained from Becton Dickinson (Bedford, MA, USA). Recombinant rat IL-1β and CCL2, and human CCL2, were obtained from R&D Systems Europe (Lille, France) or PeproTech (Paris, France). 14C-sucrose was obtained from Amersham (Little Chalfont, UK).
Rabbit polyclonal antibody to rat CCL2 (1 μg/mL for Western blotting and 2 μg/mL for immunohistochemistry) was obtained from Antigenix America (Huntington Station, NY, USA). The following mouse monoclonal antibodies were used: anti-mouse β-catenin (clone 14; 2.5 μg/mL) from BD-Transduction Labs (Lexington, KY, USA), anti-rat trans-Golgi network-specific protein of 38 kDa (TGN38) (clone 2F7.1; diluted 1:2,000) from Novus Biologicals (Littleton, CO, USA), anti-rat aquaporin 1 (AQP1) (clone 1/A5F6; diluted 1:1,000) from Abnova (Taipei City, Taiwan), anti-rat CD68 (clone ED1; 1 μg/mL) from Serotec (Oxford, UK), and anti-chicken α-tubulin (clone DM1A; diluted 1:5,000) from Cell Signaling (Danvers, MA, USA). For detection on Western blots, horseradish peroxidase-conjugated anti-rabbit and anti-mouse antibodies from goat (Cell Signaling) were used diluted 1:5,000. Secondary antibodies for immunohistochemistry were obtained from Molecular Probes (Eugene, OR, USA). These were goat anti-rabbit and anti-mouse antibodies conjugated with Alexa Fluor 488 or 594. They were used at 2 μg/mL.
The Rat Model of Traumatic Brain Injury and Cerebrospinal Fluid Sampling
Adult male Long-Evans rats weighing 250 to 350 g (Harlan, Indianapolis, IN, USA) were used. They were kept at 22°C with a 12-hour light cycle and maintained on standard pelleted rat chow and water ad libitum. The surgical and animal care procedures were in accordance with the guidelines of the Institutional Animal Care and Use Committee of Rhode Island Hospital and conformed to international guidelines on the ethical use of animals (animal welfare assurance number A3922). The controlled cortical impact model of TBI was employed as previously described (Szmydynger-Chodobska et al, 2009, 2010). In brief, rats were anesthetized with intraperitoneal pentobarbital sodium (60 mg/kg) and a 4-mm craniotomy was performed on the right side of the skull to expose the dura, with the center of the opening located 3 mm posterior to the bregma and 2.5 mm lateral to the midline. The velocity of impact was 5 m/sec and the duration of impact was 50 milliseconds. The diameter of the impactor's tip was 2.5 mm and the depth of brain deformation was set at 3 mm. In sham-injured animals, the same surgical procedures were performed, but the injury was not produced.
At 6 hours after TBI or sham injury, rats (8 to 11 animals per group) were reanesthetized with pentobarbital sodium and the samples of CSF were collected from the cisterna magna. The concentration of CCL2 in these samples was measured by ELISA (enzyme-linked immunosorbent assay) as described below.
Real-Time Reverse-Transcriptase Polymerase Chain Reaction
At 2, 4, and 6 hours, and 1, 2, and 4 days post-TBI, rats (9 to 10 animals per time point) were reanesthetized with pentobarbital sodium and were perfused transcardially with ice-cold 0.9% NaCl. The lateral ventricle CPs, both ipsilateral and contralateral to injury, were collected separately and pooled into three subgroups (3 to 4 rats per subgroup) for each time point. The lateral ventricle CPs were also collected from sham-injured rats. Total RNA was isolated using NucleoSpin RNA II kit (Macherey-Nagel, Düren, Germany). First-strand cDNAs were synthesized using oligo(dT)20 primer (50 pmol) and 15 U of ThermoScript RNase H– reverse transcriptase. Forty units of RNase inhibitor, RNaseOut, were also added to the reverse transcription reactions. For each reaction, 0.5 μg of total RNA was used and the reactions were performed for 1 hour at 50°C.
Real-time polymerase chain reaction (PCR) was performed as previously described (Szmydynger-Chodobska et al, 2009, 2010). Cyclophilin A was used for the normalization of the data obtained. The following primers and TaqMan probes were used: 5′-TGTCTCAGCCAGATGCAGTTA-3′ (forward primer for CCL2), 5′-CATTCCTTATTGGGGTCAGC-3′ (reverse primer for CCL2), 5′-ATGCCCCACTCACCTGCTGCTA-3′ (probe for CCL2), 5′-GGTGAAAGAAGGCATGAGCA-3′ (forward primer for cyclophilin A), 5′-GCTACAGAAGGAATGGTTTGATG-3′ (reverse primer for cyclophilin A), and 5′-TTTGGGTCCAGGAATGGCAAGAC-3′ (probe for cyclophilin A). The predicted sizes of PCR products were 181 and 152 bp for CCL2 and cyclophilin A, respectively. The 50-μL PCR reaction mixtures contained 0.2 mmol/L mixed dNTPs, 0.2 μmol/L each primer, 0.1 μmol/L TaqMan probe, 5 mmol/L MgCl2, 1 U of HotStart Taq DNA polymerase, and 1/20 (CCL2) or 1/2,000 (cyclophilin A) of the reverse transcription reaction product. The reaction mixtures were heated to 95°C for 15 minutes and were then subjected to 45 cycles of denaturation (15 seconds) at 94°C and annealing/extension (60°C, 45 seconds). The results are presented as a number of copies of mRNA for CCL2 per 100 copies of mRNA for cyclophilin A.
Western Blotting
At 6 hours post-TBI, rats divided into two separate groups (four animals per group) were reanesthetized with pentobarbital sodium and were perfused transcardially with ice-cold 0.9% NaCl. The lateral ventricle CPs were collected and pooled separately for the ipsilateral and contralateral side. The lateral ventricle CPs were also collected from four sham-injured rats. Proteins were extracted using isotonic lysis buffer (150 mmol/L NaCl, 50 mmol/L Tris-HCl, pH 7.4, 2 mmol/L EDTA, 1% Triton X-100) containing protease inhibitors (1 mmol/L benzamidine, 100 U/mL aprotinin, 20 μg/mL antipain, 20 μg/mL leupeptin, 1 μg/mL pepstatin A, 1 mmol/L PMSF).
Immunoblotting procedures were performed as previously described (Szmydynger-Chodobska et al, 2010). In brief, proteins were resolved via sodium dodecyl sulfate-polyacrylamide gel electrophoresis (4% to 12%) under reducing conditions and proteins were transferred onto 0.2 μm nitrocellulose membranes (Invitrogen). After blocking with 5% ECL Advance blocking agent (GE Healthcare, Little Chalfont, UK) for 1 hour at room temperature, the membranes were incubated with primary antibodies overnight at 4°C. Membranes were subsequently incubated with horseradish peroxidase-conjugated anti-rabbit or anti-mouse antibody for 1 hour at room temperature. For detection, SuperSignal West Dura extended duration (Pierce; Thermo Fisher Scientific Inc., Rockford, IL, USA) or ECL Advance (GE Healthcare) chemiluminescence substrate and the Bio Imaging System Chemi Genius2 (Syngene, Frederick, MD, USA) were used.
Immunohistochemistry
Immunohistochemical procedures were performed as previously described (Szmydynger-Chodobska et al, 2009, 2010). Rats (2 to 4 animals per group) were reanesthetized with pentobarbital sodium and were perfused transcardially with ice-cold 0.9% NaCl, followed by ice-cold 4% paraformaldehyde in 0.05 mol/L PBS (phosphate-buffered saline), pH 7.4. Brains were removed and post-fixed for additional 4 hours in the paraformaldehyde/PBS solution at 4°C. They were then incubated overnight in 20% sucrose in PBS and embedded in Tissue-Tek OCT compound (Sakura Finetek, Torrance, CA, USA). Coronal brain sections were cut on a cryostat at 10 μm.
All procedures were performed at room temperature, except for the incubation with primary antibodies that was completed at 4°C. To minimize nonspecific staining, the brain sections were incubated for 30 minutes with 10% normal goat serum. Four percent of normal goat serum was also added when the sections were incubated with primary and secondary antibodies. After the initial blocking step, the sections were incubated overnight with primary antibodies and then were incubated for 1 hour with secondary antibodies. The sections were mounted with Vectashield mounting medium (Vector Labs, Burlingame, CA, USA).
Transmission Electron Microscopic Analysis
At 1 day after TBI or sham injury, rats (2 to 10 animals per group) were reanesthetized with pentobarbital sodium and the lateral ventricle CPs were removed and fixed overnight in 2.5% glutaraldehyde in 0.15 mol/L sodium cacodylate buffer, pH 7.4, at 4°C. Following fixation, the specimens were treated with 1% OsO4 in cacodylate buffer for 1 hour at 4°C. They were then dehydrated in a graded acetone series and embedded in Epox 812 resin (Fullam, Latham, NY, USA). Ultrathin sections (50 to 60 nm) were prepared, retrieved onto 300 mesh copper grids, and contrasted with uranyl acetate and lead citrate. Sections were examined using a Morgagni 268 transmission electron microscope (FEI Electron Optics.
Primary Cell Cultures of Rat Choroidal Epithelial Cells
The procedures were conducted according to the guidelines approved by the French Ethical Committee (decree 87-848) and by the European Community directive 86-609-EEC. Animals were obtained from Harlan (Gannat, France). Choroidal epithelial cells were isolated as previously described (Strazielle and Ghersi-Egea, 1999) and were seeded on Transwell Clear inserts with the pore size of 0.4 μm (Costar, Cambridge, MA, USA) precoated with laminin. The cells were kept at 37°C in a humidified atmosphere of 5% CO2/95% air and were fed every other day with medium containing 10% fetal bovine serum. The experiments were performed 5 days after the cells reached confluence.
Secretion of CCL2
The cells were serum starved in SFM (serum-free medium) supplemented with 0.1% bovine serum albumin for 24 hours before experimentation. On the day of experiment, bovine serum albumin-containing SFM was renewed in both chambers and the apical surface of epithelial monolayers was exposed for 6 hours to recombinant rat IL-1β at concentrations of 2 to 1,000 pg/mL (dose-response studies). In time-course studies, the cells were incubated with 10 pg/mL of IL-1β for 1, 3, 6, and 24 hours. Control monolayers (incubated in SFM without IL-1β) were processed simultaneously. At the end of incubation, media were collected from the apical and basolateral chambers and stored at −80°C until further processing for CCL2 quantification. The epithelial monolayers were subsequently analyzed for possible changes in paracellular permeability (see below). The concentration of CCL2 in the culture media and CSF was determined using the Rat MCP-1 ELISA Development kit from PeproTech. The samples were assayed in duplicates after appropriate dilution.
Permeability of Epithelial Monolayers to CCL2
The epithelial cells were serum starved as described above and were then exposed for 6 hours to recombinant rat CCL2, which was added to either the apical (100 ng/mL) or basolateral (50 ng/mL) chamber. After this incubation, media were sampled from both compartments to determine the concentration of CCL2. Recombinant human CCL2 was used to measure the epithelial permeability to this chemokine in IL-1β-treated cells. In these experiments, the cells were pretreated for 3 hours by adding 100 pg/mL of IL-1β to the apical chamber. Control monolayers (incubated in SFM without IL-1β) were processed simultaneously. The concentrations of human CCL2 in the apical and basolateral compartments were determined using the Human MCP-1 ELISA Development kit (PeproTech). This assay showed no crossreactivity with rat CCL2 at a concentration as high as 50 ng/mL. No degradation of the recombinant chemokines during the incubation periods was observed.
Measurement of Paracellular Permeability of Epithelial Monolayers
The integrity of epithelial monolayers was examined by measuring the permeability of each filter to the paracellular permeability marker 14C-sucrose. The permeability × surface area product, PSt, was calculated from the 14C-sucrose clearance rates as previously described (Strazielle and Preston, 2003). The permeability coefficient, Pt, was calculated by dividing PSt by the surface area of the cell monolayer.
Statistical Analysis
For statistical evaluation of data, analysis of variance was used, followed by the tests for multiple comparisons among means, as described in figure 1 and 3 legends. P<0.05 was considered statistically significant.
Results
Posttraumatic Increase in Choroidal CCL2 Synthesis
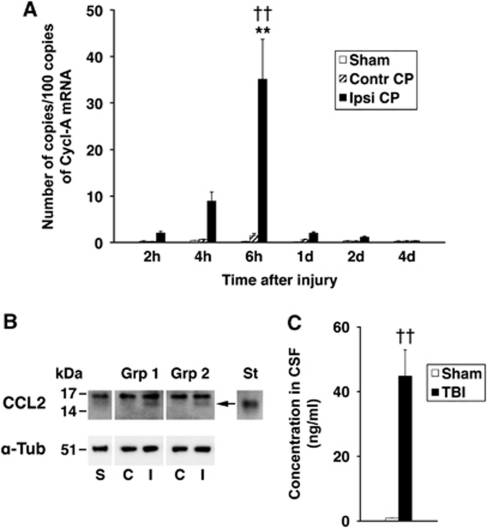
Posttraumatic changes in CCL2 expression in the lateral ventricle CP were initially analyzed using real-time reverse transcriptase (RT) polymerase chain reaction. This analysis showed a gradual increase in CCL2 mRNA, which peaked at 6 hours after injury (Figure 1A). This increase in choroidal synthesis of CCL2 was transient and at 1 day after TBI, CCL2 mRNA in the ipsilateral CP dropped abruptly to a level, which was not significantly different from that found in the contralateral CP or in the CP from sham-injured rats. No changes in CCL2 mRNA in the contralateral CP or the CP from sham-injured animals were observed.
Figure 1.
Posttraumatic production of CCL2 by the lateral ventricle choroid plexus (CP). (A) Temporal changes in CCL2 mRNA in the ipsilateral CP (Ipsi CP) compared with the contralateral CP (Contr CP) and the CP from sham-injured rats (Sham) (n=9 to 10 rats per time point). These data were obtained by real-time reverse transcriptase polymerase chain reaction (RT-PCR). The number of copies of transcripts for CCL2 relative to the message for cyclophilin A (Cycl-A) is shown. Data represent mean values±s.e.m. **P<0.01 for the ipsilateral versus contralateral CP (Newman–Keuls test). ††P<0.01 for the ipsilateral CP versus the CP from sham-injured rats (Newman–Keuls test). (B) Western blot analysis of choroidal synthesis of CCL2. The CPs were collected at 6 hours after sham injury or traumatic brain injury (TBI) (n=4 rats per group). Data from two independent groups of injured rats, group 1 and group 2 (Grp 1 and Grp 2, respectively), are shown. Consistent with RT-PCR analysis, a band (arrow) with the same apparent molecular weight as recombinant rat CCL2 (St; 1 ng) was detected in the ipsilateral CP. This band was not present in protein extracts from the contralateral CP or the CP collected from sham-injured animals (30 μg per lane). The recombinant rat CCL2 was run on the same gel, but was detected with five times lower concentration of anti-CCL2 antibody. α-Tub is α-tubulin (10 μg of total protein was loaded per lane to be probed with anti-α-tubulin antibody). C, I, and S are contralateral and ipsilateral CPs, and the CPs collected from sham-injured rats, respectively. (C) The levels of CCL2 in the cerebrospinal fluid (CSF) from injured (TBI) versus sham-injured rats (Sham) (n=8 to 11 per group). The samples of CSF were collected from the cisterna magna at 6 hours after TBI. Data represent mean values±s.e.m. ††P<0.01 for injured versus sham-injured rats (Student's t-test).
We next used Western blotting to determine whether the posttraumatic increase in choroidal CCL2 synthesis observed at the message level is also associated with increased choroidal production of CCL2 protein. The choroidal tissue was harvested at 6 hours post-TBI, a time point at which a peak in posttraumatic increase in CCL2 mRNA was observed. Consistent with RT-PCR analysis, an increase in CCL2 protein expression was observed in the ipsilateral CP in two independent groups of animals (Figure 1B). This increase in choroidal CCL2 synthesis was followed by a 50-fold elevation of CCL2 concentration in the CSF collected from the cisterna magna at 6 hours after injury when compared with sham-injured animals (Figure 1C).
Injury-Dependent Changes in CCL2-Immunopositive Staining of the Choroidal Tissue
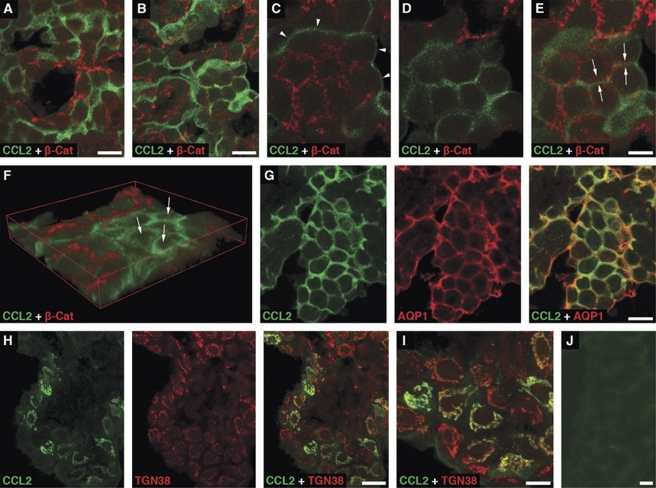
The immunohistochemical analysis of choroidal tissue harvested at 6 hours after sham injury showed a distinct pattern of CCL2-positive staining of choroidal epithelium associated with the apical domain of epithelial cells (Figure 2A). Similar pattern of immunostaining for CCL2 was observed in both the contralateral (Figures 2B–2G) and ipsilateral (data not shown) CPs harvested at 6 hours post-TBI. Double immunostaining of choroidal tissue with anti-CCL2 antibody and an antibody to β-catenin, a component of the adherens junction complex, demonstrated a clear separation of the CCL2-immunoreactive product from this basolateral marker (Figures 2C–2E). The apical localization of the CCL2-immunoreactive product was further confirmed by double immunostaining of the choroidal tissue with anti-CCL2 antibody and an antibody to AQP1, a water channel expressed apically in choroidal epithelial cells (Praetorius and Nielsen, 2006; Figure 2G). Intense cytoplasmic CCL2-positive staining of epithelial cells in the ipsilateral, but not contralateral, CP was associated with the Golgi complex (Figures 2H and 2I). The localization of CCL2 to the Golgi complex was consistent with increased synthesis of this secreted protein occurring after TBI. Unlike apical CCL2 staining that was rather evenly distributed throughout the CP, the Golgi-associated CCL2 immunostaining was found in clusters of epithelial cells scattered through the choroidal tissue. Such clusters of cells were observed as early as 4 hours after TBI. The number of epithelial cells with the Golgi-associated CCL2 immunostaining peaked at 6 hours post-TBI, which was in line with the results from the real-time RT-PCR analysis and Western blotting. The distribution of CCL2-immunopositive staining in the contralateral or ipsilateral CP was confined to the epithelial cells. The CCL2-immunoreactive product was not associated with other types of cells normally present in the choroidal tissue, such as endothelial and epiplexus cells or stromal macrophages.
Figure 2.
Immunohistochemical localization of CCL2 in the lateral ventricle choroid plexus (CP). Confocal microscopy images of choroidal tissue harvested at 6 hours after sham injury (A) or traumatic brain injury (TBI) (B–J) are shown. (A) Double immunostaining of the CP from a sham-injured rat performed with anti-CCL2 antibody and an antibody to β-catenin, a component of the adherens junction complex. (B) Similar pattern of staining for CCL2 observed in the contralateral CP of an injured rat. The same pattern of CCL2 expression was also found in the ipsilateral CP. (C) A higher magnification view of a fragment of the image shown in (B). The choroidal epithelial cells were scanned at the level of adherens junctions. Note that the CCL2-immunoreactive product is associated with the apical surface of epithelial cells (arrowheads). (D) A scan performed 5.5 μm above the level shown in (C) close to the apical surface of choroidal cells. (E) The projection of consecutive optical sections scanned at the level shown in (C) through the level shown in (D). Note that the CCL2-immunoreactive product does not colocalize with that found for β-catenin (arrows). (F) A 3D reconstruction of images scanned through the whole 10 μm volume of choroidal tissue. This reconstruction clearly demonstrates the apical localization of CCL2-immunoreactive product (arrows). (G) The apical localization of the CCL2-immunoreactive product further confirmed by double immunostaining of the contralateral CP with anti-CCL2 antibody and an antibody to aquaporin 1 (AQP1), a water channel expressed apically in choroidal epithelial cells. (H) Double immunostaining of the ipsilateral CP with anti-CCL2 antibody and an antibody to the Golgi marker, TGN38. The localization of CCL2 to the Golgi complex in the ipsilateral CP is consistent with increased synthesis of this secreted protein occurring after TBI. This pattern of staining for CCL2 was observed in neither the contralateral CP nor the CP from sham-injured rats. (I) A higher magnification view of a fragment of the image shown in (H). (J) Negative control staining for CCL2. In these experiments, the choroidal tissue was incubated with anti-CCL2 antibody that had been preabsorbed overnight with recombinant rat CCL2 (PeproTech) at 50 μg/mL and low-molecular weight heparin from porcine intestinal mucosa (Sigma) at 10 mg/mL. The ipsilateral CP is shown. Bars: panels (A, B, G, H, J), 20 μm; panels (C–E, I), 10 μm.
Polarity of CCL2 Secretion by Choroidal Epithelium
To test whether IL-1β induces CCL2 synthesis in the choroidal epithelium and to determine the direction of CCL2 release (across the apical versus basolateral membrane), we used an in vitro polarized model of the BCSFB (Strazielle and Ghersi-Egea, 1999), which reproduces the in vivo properties of the choroidal epithelium. The type I IL-1 receptor has previously been identified by in situ hybridization to be expressed in the CP (Ericsson et al, 1995), and we have confirmed its expression on epithelial monolayers (Szmydynger-Chodobska et al, 2009).
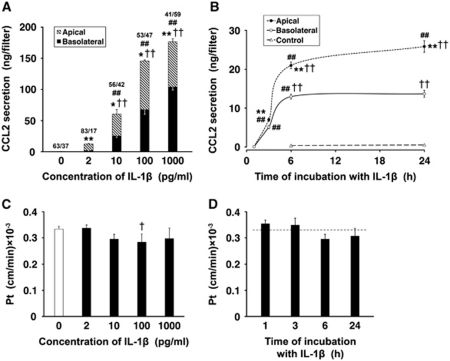
Under control conditions, the low-level production of CCL2 was observed. This constitutive secretion of CCL2 was polarized, with the chemokine being largely released across the apical (CSF-facing) membrane of choroidal epithelium. In dose-response studies, the epithelial monolayers were exposed to 2, 10, 100, and 1,000 pg/mL of IL-1β for 6 hours (Figure 3A). CCL2 secretion was increased 25 and 120 times over its constitutive production when the monolayers were exposed to IL-1β at concentrations as low as 2 and 10 pg/mL, respectively. The exposure of epithelial cells to IL-1β at 100 pg/mL resulted in a 290-fold increase in CCL2 synthesis relative to control. The choroidal production of CCL2 appeared to reach its maximum capacity when the monolayers were incubated with IL-1β at a concentration of 1 ng/mL. With all IL-1β concentrations tested, except 1 ng/mL, the larger amounts of CCL2 were secreted across the apical versus basolateral membrane of choroidal epithelium. In time-course studies, the cells were incubated with 10 pg/mL of IL-1β for 1, 3, 6, and 24 hours (Figure 3B). At this concentration of IL-1β, CCL2 secretion was predominantly apical throughout the 24-hour observation period. The CCL2 levels in the apical and basolateral chambers increased rapidly between 1 and 6 hours of exposure to IL-1β; however, later on, the CCL2 levels in the apical chamber increased at much slower rate, whereas no further increase in CCL2 concentration was observed in the basolateral compartment.
Figure 3.
Secretion of CCL2 by primary cultures of choroidal epithelial cells. The cells were seeded on Transwell Clear inserts as described in Materials and methods. The experiments were performed 5 days after the cells reached confluence (n=3 to 6 per group). The apical surface of epithelial monolayers was exposed to interleukin-1β (IL-1β). (A) Dose-response studies. The cells were exposed to IL-1β for 6 hours. The fractions above the columns represent the proportion (%) of apical versus basolateral release of CCL2 into the culture media. Under control conditions, CCL2 was constitutively produced, and the total amount of CCL2 secreted into the apical and basolateral chambers during the 6-hour observation period was 0.5 ng/filter. This secretion was polarized, with CCL2 being predominantly released across the apical (cerebrospinal fluid (CSF)-facing) membrane. Note that, with all IL-1β concentrations tested, except 1 ng/mL, the larger amounts of CCL2 were secreted into the apical chamber when compared with those released into the basolateral compartment. (B) Time-course studies. The cells were incubated with 10 pg/mL of IL-1β for up to 24 hours. Note that during the initial 6-hour period of exposure to IL-1β, the CCL2 levels in the apical and basolateral chambers increased rapidly; however, later on, the CCL2 levels either did not change (basolateral) or increased at a much slower rate (apical). (C) The permeability of epithelial monolayers to 14C-sucrose after the 6-hour incubation with varying concentrations of IL-1β. The permeability coefficient, Pt, was calculated from the 14C-sucrose clearance rates as described in Materials and methods. (D) The permeability of epithelial monolayers to 14C-sucrose after the exposure to 10 pg/mL of IL-1β for up to 24 hours. The dashed line represents the Pt value observed in untreated cells. All data represent mean values±s.d. *P<0.05, **P<0.01 for apical versus basolateral CCL2 secretion (Student's t-test with Bonferroni correction). †P<0.05, ††P<0.01 for CCL2 secretion in response to IL-1β versus control (Dunnett test). ##P<0.01 for a difference in CCL2 secretion in response to the next higher concentration of IL-1β or between two consecutive time points during the exposure to the cytokine (Tukey–Kramer test).
The possible paracellular diffusion and/or transcellular transfer of CCL2, which might have had an effect on our estimates of polarity of chemokine secretion by epithelial monolayers, was also evaluated. To this end, recombinant rat CCL2 was added to either the apical or basolateral chamber, and the media were sampled from both compartments 6 hours later. The levels of CCL2 measured in the respective opposite chambers at the end of the incubation period were higher than those resulting from constitutive secretion, which was assessed in parallel in separate monolayers. However, the amounts of CCL2 that actually permeated the monolayers represented only 0.6% to 1.6% of the amount of the chemokine added to each chamber. Similar low permeability to recombinant human CCL2 was observed for both control monolayers and the epithelial cells pretreated with IL-1β. This suggested that IL-1β did not enhance a leak and/or transcellular transfer of CCL2 across the epithelial monolayer. The exposure of epithelial cells to IL-1β or CCL2 also did not cause any apparent changes in the integrity of the monolayers, as shown by the lack of increase in permeability to sucrose (Figures 3C and 3D, and data not shown).
Monocyte Trafficking Across the Blood–Cerebrospinal Fluid Barrier
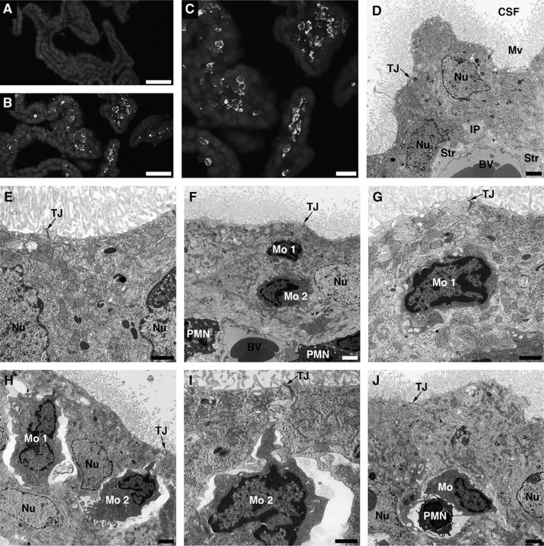
Posttraumatic increase in choroidal synthesis of CCL2 was followed by monocyte recruitment to the ipsilateral CP; however, there was a delay between an increase in the production of this chemokine and the accumulation of monocytes in choroidal tissue. Monocytes infiltrating the ipsilateral CP were found at 1 day after injury (Figures 4B and 4C), whereas at 6 hours post-TBI, these inflammatory cells were only sporadically observed to accumulate in the ipsilateral CP. Monocytes did not infiltrate the whole ipsilateral CP, but only accumulated in certain areas of the choroidal tissue. Monocytes did not accumulate in the contralateral CP (Figure 4A), which was consistent with the lack of changes in CCL2 synthesis in the contralateral CP (see Figures 1A and 1B).
Figure 4.
Posttraumatic accumulation of monocytes in the lateral ventricle choroid plexus (CP). (A, B) Low-power microphotographs of the contralateral and ipsilateral CPs, respectively, at 1 day after traumatic brain injury (TBI). Monocytes were stained with anti-CD68 antibody. These inflammatory cells were only sporadically found in the ipsilateral CP at 6 hours post-TBI. (C) A higher magnification microphotograph of the ipsilateral CP shown in (B). (D) Transmission electron microscopic analysis of the CP. The choroidal tissue was harvested at 1 day after TBI. The contralateral CP, which has normal morphology, similar to the morphology of choroidal tissue from sham-injured rats, is shown. Note that the choroidal epithelial cells have well-defined microvilli and the lateral membrane is folded into elaborate interdigitating processes near the base of the cells. The space between adjacent epithelial cells is narrow and the tight junctions between the cells are well discernible. (E) A higher magnification microphotograph of the contralateral CP. (F) The ipsilateral CP. The microphotograph shows two monocytes (Mo 1 and Mo 2) that reached the intercellular space between the choroidal epithelial cells. Note that the space between invading monocytes and the epithelial cells is narrow. The microphotograph also shows two neutrophils or polymorphonuclear leukocytes (PMN) that extravasated into the choroidal stroma. (G) A higher magnification view of monocyte 1 from (F). (H) The ipsilateral CP. Two monocytes (Mo 1 and Mo 2) that reached the intercellular space between the epithelial cells are shown. A significant expansion of space between invading inflammatory cells and the choroidal epithelial cells is well visible on this microphotograph. Such enlarged, rather than tight, space between migrating monocytes and the epithelial cells was predominantly seen in the ipsilateral CP. (I) A higher magnification view of monocyte 2 from (H). Note that the movement of monocytes between the epithelial cells toward their apical domain does not appear to affect the integrity of tight junctions. (J) The ipsilateral CP. A monocyte and neutrophil migrating in tandem between adjacent epithelial cells. Bars: panels (A, B), 50 μm; panel (C), 20 μm; panels (D, F, H, J), 2 μm; panels (E, G, I), 1 μm. BV, blood microvessel; CSF, cerebrospinal fluid; IP, interdigitating processes; Mo, monocyte; Mv, microvilli; Nu, nucleus of the choroidal epithelial cell; PMN, neutrophil; Str, choroidal stroma; TJ, tight junction.
To demonstrate monocyte trafficking across the BCSFB, transmission electron microscopy was used. Ten rats were analyzed at 1 day post-TBI, and in 8 out of these 10 animals (80%), the infiltration of the ipsilateral CP by monocytes was observed. The contralateral CP had normal morphology (Figures 4D and 4E), similar to the morphology of choroidal tissue from sham-injured rats (data not shown). The choroidal epithelial cells had well-defined microvilli and the lateral membrane was folded into elaborate interdigitating processes near the base of the cells. The space between adjacent epithelial cells was narrow and the tight junctions between the cells were well discernible. In the ipsilateral CP, monocytes were found to reach the intercellular space between the epithelial cells and were apparently moving toward the apical domain of choroidal epithelium (Figures 4F–4I). The space between invading monocytes and the choroidal epithelial cells was occasionally found to be narrow (Figures 4F and 4G), but predominantly an enlarged, rather than tight, space between migrating monocytes and the epithelial cells was seen in the ipsilateral CP (Figures 4H and 4I). We were also able to find monocytes invading the ipsilateral CP in tandem with neutrophils (Figure 4J). Usually, one to four such events were observed in the areas of the ipsilateral CP where the accumulation of inflammatory cells occurred. The movement of monocytes between the epithelial cells toward the apical domain of choroidal epithelium did not appear to affect the integrity of tight junctions.
Discussion
Monocytes invading the brain parenchyma in response to ischemia or TBI have detrimental effect on neuronal survival and functional recovery after injury (Chen et al, 2003; Dimitrijevic et al, 2007; Semple et al, 2010a). The influx of these inflammatory cells is driven by monocyte chemoattractants, such as CCL2, whose synthesis is rapidly increased in the injured cortex (Semple et al, 2010a; Szmydynger-Chodobska et al, 2010). In the present study, we demonstrated that neurotrauma also results in a rapid increase in production of CCL2 by the lateral ventricle CP located ipsilaterally to injury. This increase in choroidal CCL2 synthesis was not associated with posttraumatic accumulation of monocytes in the choroidal tissue, but resulted from production of CCL2 by the choroidal epithelium. Indeed, although monocytes can produce CCL2 in response to proinflammatory mediators (Colotta et al, 1992), these inflammatory cells were rarely found in the ipsilateral CP at 6 hours post-TBI, a time point at which a maximum increase in choroidal CCL2 synthesis was observed. An increase in choroidal production of CCL2 was associated with a significant elevation of CCL2 concentration in the CSF, which was comparable to the levels of this chemokine found in the CSF of patients with severe TBI (Semple et al, 2010a).
These observations raise the question about the importance of the CP as a source of CCL2 in the injured brain. Using the primary cultures of choroidal epithelial cells, we found that the rate of apical secretion of CCL2 in response to IL-1β is relatively stable during the first 6 hours of incubation with the cytokine (see Figure 3B) and amounts to 15 ng/h per cm2 of surface area of epithelial monolayer. In these experiments, the epithelial monolayers were exposed to 10 pg/mL of IL-1β, the average concentration of IL-1β found in ventricular CSF in patients with severe TBI at 6 hours after injury (Shiozaki et al, 2005). To extrapolate these in vitro data to the in vivo situation, we assumed that the apical surface of choroidal epithelium of one lateral ventricle CP is 0.7 cm2 (without taking into account the surface area of apical microvilli; unpublished data). Accordingly, the rate of CCL2 secretion by one lateral ventricle CP would amount to 10.5 ng/h. In our previous study, we have determined that with a substance continually secreted by the CP, the time needed for this substance to reach half of its maximal concentration in the CSF is ∼70 minutes in rats (Batisson et al, 2006). Given this time and assuming that the rate of CSF production in Long-Evans rats is 177 μL/h (DePasquale et al, 1989), the concentration of CCL2 in the CSF at 6 hours post-TBI would be 59 ng/mL. This estimated concentration of CCL2 is comparable to the average level of CCL2 (44 ng/mL) found in the CSF collected at 6 hours after injury, which clearly indicates that the CP represents a significant source of CCL2 in the injured brain. These calculations are based on the simplified compartmental model and do not take into consideration the loss of CCL2 from the CSF due to its diffusion into the brain parenchyma.
The immunohistochemical analysis of choroidal tissue demonstrated that CCL2 is produced by the epithelial cells. This chemokine did not appear to be synthesized by other types of cells normally present in the choroidal tissue, such as endothelial and epiplexus cells or stromal macrophages, in both sham-injured and traumatized rats. These results are in line with the previous studies, in which in situ hybridization histochemistry was used to demonstrate the upregulation of CCL2 expression in the CP in response to peripheral administration of proinflammatory mediators, such as lipopolysaccharide, IL-1β, and tumor necrosis factor-α (Thibeault et al, 2001). The hybridization signal shown by these authors was consistent with the expression of CCL2 by the choroidal epithelial cells. On the other hand, Mitchell et al (2009), who also used an in situ hybridization technique, reported the increased expression of CCL2 in choroidal stromal cells after the carrageenan-induced peripheral inflammation, but the types of cells producing this chemokine were not identified. The reason for these discrepant results is not immediately clear, but may be associated with the different animal models used. Unlike the cerebrovascular endothelium that is an important source of CCL2 in the injured brain (Szmydynger-Chodobska et al, 2010), the endothelial cells of choroidal microvessels did not appear to produce this chemokine. The fenestrated phenotype of choroidal endothelial cells (Strazielle and Ghersi-Egea, 2000) may be a factor differentiating the choroidal and cerebrovascular endothelia in their ability to synthesize CCL2.
Interestingly, both in sham-injured rats and in animals subjected to TBI, a distinct CCL2-positive staining of the apical surface of choroidal epithelium was observed. This finding was consistent with the results from in vitro experiments, in which a constitutive, predominantly apical, secretion of CCL2 was observed in epithelial monolayers. Since CCL2 has the ability to bind to glycosaminoglycans (Kuschert et al, 1999), it is possible that the apical localization of CCL2 was associated with the binding of this chemokine to glycosaminoglycans expressed on the apical surface of choroidal epithelium. In the ipsilateral CP, the intense Golgi complex-associated staining for CCL2 was observed. This pattern of CCL2-positive staining was found in clusters of epithelial cells scattered through the choroidal tissue, suggesting an uneven response to proinflammatory mediators and/or varying ability to produce the chemokine by individual epithelial cells. This likely resulted in uneven distribution of the chemokine gradients across the CP, which may explain our electron microscopic observations that monocytes accumulated only in certain areas of the ipsilateral CP.
The experiments involving the epithelial monolayers showed that CCL2 is secreted across both the apical and basolateral membranes of choroidal epithelium. This finding is in line with the previous studies, in which bidirectional secretion of chemokines by intestinal epithelia has been demonstrated and found to be necessary for leukocyte migration across this epithelial barrier (McCormick et al, 1995, 1998). In our in vitro studies, the possible leak and/or transcellular transfer of CCL2, which could affect our estimates of polarity of chemokine secretion by epithelial monolayers, was also evaluated. The results showed low paracellular permeability of control and stimulated monolayers, and suggested that neither IL-1β nor CCL2 enhances a leak and/or transcellular transfer of the chemokine. These results validate our estimates of polarity of chemokine secretion by the choroidal epithelium.
The lack of changes in paracellular permeability of epithelial monolayers after exposure to CCL2 contrasts with increased permeability of the blood–brain barrier observed in response to this chemokine under both in vivo and in vitro conditions (Stamatovic et al, 2005). These discrepant responses of the two barriers to CCL2 may be related to different levels of expression of CCR2 at the blood–brain barrier versus BCSFB, differences in the concentrations of CCL2 used, and/or differences in signal transduction associated with CCL2 binding to its cognate receptor.
The above-discussed features of choroidal epithelium, such as the ability to synthesize CCL2 in response to injury and bidirectional secretion of this chemokine across the apical and basolateral membranes of choroidal epithelial cells, strongly suggest that the BCSFB has a role in posttraumatic invasion of monocytes. This idea is further supported by our electron microscopic analysis of choroidal tissue. Similar to the movement of neutrophils (Szmydynger-Chodobska et al, 2009), the migration of monocytes across the BCSFB appeared to involve the paracellular pathway. The movement of monocytes along the paracellular pathway was frequently associated with the widening of space between invading inflammatory cells and the adjacent epithelial cells (see Figures 4H and 4I), a phenomenon not observed for migrating neutrophils (Szmydynger-Chodobska et al, 2009). Interestingly, monocytes were sometimes found to invade the ipsilateral CP in tandem with neutrophils (see Figure 4J). The synergistic interactions between monocyte and neutrophil chemoattractants (Gouwy et al, 2004) could have a role in the movement of these two types of inflammatory cells together across the BCSFB.
Presently, it is not well defined how monocytes, or other inflammatory cells, invade the brain parenchyma after crossing the BCSFB. Also, little is known about the possible expression of cell adhesion molecules at ependymal and pial/glial linings bordering the CSF space. Monocytes have the ability to produce a variety of matrix metalloproteinases (Newby, 2008), and the activation of these metalloproteinases have been shown to be a critical factor in the recruitment of inflammatory cells to the brain tissue (Toft-Hansen et al, 2006). Carried by the bulk flow of CSF, monocytes may passage from the lateral cerebral ventricle to the cistern of velum interpositum located above the third cerebral ventricle, and may also enter the subarachnoid CSF space near the injury site. Our previous studies (Chodobski et al, 2003) suggest that these parts of the brain CSF space have an important role in influx of peripheral inflammatory cells into the brain parenchyma. The investigations of experimental autoimmune encephalomyelitis in rodents, an animal model of multiple sclerosis, have also shown that peripheral inflammatory cells, such as T cells, may enter the neural tissue from the CSF by moving along the perivascular, Virchow-Robin, space (Bartholomäus et al, 2009). Our preliminary observations (unpublished data) suggest that after injury, monocytes may use the same route to invade the brain parenchyma from the CSF.
Acknowledgments
The authors thank Ms Julie Sarri for her technical assistance, Ms Virginia Hovanesian for her help in acquiring and processing confocal microscopy images, and Ms Carol Ayala for her help with electron microscopy.
The authors declare no conflict of interest.
Footnotes
This work was supported by Grant NS49479 from the NIH (to AC), HEALTH-F2-2009-241778 from the European Union (to NS), and by funds from the Department of Emergency Medicine at the Alpert Medical School of Brown University (to JSC and AC).
References
- Bartholomäus I, Kawakami N, Odoardi F, Schläger C, Miljkovic D, Ellwart JW, Klinkert WE, Flügel-Koch C, Issekutz TB, Wekerle H, Flügel A. Effector T cell interactions with meningeal vascular structures in nascent autoimmune CNS lesions. Nature. 2009;462:94–98. doi: 10.1038/nature08478. [DOI] [PubMed] [Google Scholar]
- Batisson M, Strazielle N, Hejmadi M, Thomas D, Ghersi-Egea JF, Etienne J, Vandenesch F, Lina G. Toxic shock syndrome toxin-1 challenges the neuroprotective functions of the choroidal epithelium and induces neurotoxicity. J Infect Dis. 2006;194:341–349. doi: 10.1086/505428. [DOI] [PubMed] [Google Scholar]
- Beech JS, Reckless J, Mosedale DE, Grainger DJ, Williams SC, Menon DK. Neuroprotection in ischemia-reperfusion injury: an antiinflammatory approach using a novel broad-spectrum chemokine inhibitor. J Cereb Blood Flow Metab. 2001;21:683–689. doi: 10.1097/00004647-200106000-00006. [DOI] [PubMed] [Google Scholar]
- Bonini JA, Martin SK, Dralyuk F, Roe MW, Philipson LH, Steiner DF. Cloning, expression, and chromosomal mapping of a novel human CC-chemokine receptor (CCR10) that displays high-affinity binding for MCP-1 and MCP-3. DNA Cell Biol. 1997;16:1249–1256. doi: 10.1089/dna.1997.16.1249. [DOI] [PubMed] [Google Scholar]
- Chen Y, Hallenbeck JM, Ruetzler C, Bol D, Thomas K, Berman NE, Vogel SN. Overexpression of monocyte chemoattractant protein 1 in the brain exacerbates ischemic brain injury and is associated with recruitment of inflammatory cells. J Cereb Blood Flow Metab. 2003;23:748–755. doi: 10.1097/01.WCB.0000071885.63724.20. [DOI] [PubMed] [Google Scholar]
- Chodobski A, Chung I, Koźniewska E, Ivanenko T, Chang W, Harrington JF, Duncan JA, Szmydynger-Chodobska J. Early neutrophilic expression of vascular endothelial growth factor after traumatic brain injury. Neuroscience. 2003;122:853–867. doi: 10.1016/j.neuroscience.2003.08.055. [DOI] [PubMed] [Google Scholar]
- Colotta F, Borré A, Wang JM, Tattanelli M, Maddalena F, Polentarutti N, Peri G, Mantovani A. Expression of a monocyte chemotactic cytokine by human mononuclear phagocytes. J Immunol. 1992;148:760–765. [PubMed] [Google Scholar]
- DePasquale M, Patlak CS, Cserr HF. Brain ion and volume regulation during acute hypernatremia in Brattleboro rats. Am J Physiol. 1989;256:F1059–F1066. doi: 10.1152/ajprenal.1989.256.6.F1059. [DOI] [PubMed] [Google Scholar]
- Dimitrijevic OB, Stamatovic SM, Keep RF, Andjelkovic AV. Absence of the chemokine receptor CCR2 protects against cerebral ischemia/reperfusion injury in mice. Stroke. 2007;38:1345–1353. doi: 10.1161/01.STR.0000259709.16654.8f. [DOI] [PubMed] [Google Scholar]
- Ericsson A, Liu C, Hart RP, Sawchenko PE. Type 1 interleukin-1 receptor in the rat brain: distribution, regulation, and relationship to sites of IL-1-induced cellular activation. J Comp Neurol. 1995;361:681–698. doi: 10.1002/cne.903610410. [DOI] [PubMed] [Google Scholar]
- Gouwy M, Struyf S, Catusse J, Proost P, Van Damme J. Synergy between proinflammatory ligands of G protein-coupled receptors in neutrophil activation and migration. J Leukoc Biol. 2004;76:185–194. doi: 10.1189/jlb.1003479. [DOI] [PubMed] [Google Scholar]
- Kuschert GS, Coulin F, Power CA, Proudfoot AE, Hubbard RE, Hoogewerf AJ, Wells TN. Glycosaminoglycans interact selectively with chemokines and modulate receptor binding and cellular responses. Biochemistry. 1999;38:12959–12968. doi: 10.1021/bi990711d. [DOI] [PubMed] [Google Scholar]
- McCormick BA, Hofman PM, Kim J, Carnes DK, Miller SI, Madara JL. Surface attachment of Salmonella typhimurium to intestinal epithelia imprints the subepithelial matrix with gradients chemotactic for neutrophils. J Cell Biol. 1995;131:1599–1608. doi: 10.1083/jcb.131.6.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick BA, Parkos CA, Colgan SP, Carnes DK, Madara JL. Apical secretion of a pathogen-elicited epithelial chemoattractant activity in response to surface colonization of intestinal epithelia by Salmonella typhimurium. J Immunol. 1998;160:455–466. [PubMed] [Google Scholar]
- Mitchell K, Yang HY, Berk JD, Tran JH, Iadarola MJ. Monocyte chemoattractant protein-1 in the choroid plexus: a potential link between vascular pro-inflammatory mediators and the CNS during peripheral tissue inflammation. Neuroscience. 2009;158:885–895. doi: 10.1016/j.neuroscience.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann J, Sauerzweig S, Rönicke R, Gunzer F, Dinkel K, Ullrich O, Gunzer M, Reymann KG. Microglia cells protect neurons by direct engulfment of invading neutrophil granulocytes: a new mechanism of CNS immune privilege. J Neurosci. 2008;28:5965–5975. doi: 10.1523/JNEUROSCI.0060-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newby AC. Metalloproteinase expression in monocytes and macrophages and its relationship to atherosclerotic plaque instability. Arterioscler Thromb Vasc Biol. 2008;28:2108–2114. doi: 10.1161/ATVBAHA.108.173898. [DOI] [PubMed] [Google Scholar]
- Paine R, III, Rolfe MW, Standiford TJ, Burdick MD, Rollins BJ, Strieter RM. MCP-1 expression by rat type II alveolar epithelial cells in primary culture. J Immunol. 1993;150:4561–4570. [PubMed] [Google Scholar]
- Praetorius J, Nielsen S. Distribution of sodium transporters and aquaporin-1 in the human choroid plexus. Am J Physiol. 2006;291:C59–C67. doi: 10.1152/ajpcell.00433.2005. [DOI] [PubMed] [Google Scholar]
- Prodjosudjadi W, Gerritsma JS, Klar-Mohamad N, Gerritsen AF, Bruijn JA, Daha MR, van Es LA. Production and cytokine-mediated regulation of monocyte chemoattractant protein-1 by human proximal tubular epithelial cells. Kidney Int. 1995;48:1477–1486. doi: 10.1038/ki.1995.437. [DOI] [PubMed] [Google Scholar]
- Rollins BJ. Chemokines. Blood. 1997;90:909–928. [PubMed] [Google Scholar]
- Schoettle RJ, Kochanek PM, Magargee MJ, Uhl MW, Nemoto EM. Early polymorphonuclear leukocyte accumulation correlates with the development of posttraumatic cerebral edema in rats. J Neurotrauma. 1990;7:207–217. doi: 10.1089/neu.1990.7.207. [DOI] [PubMed] [Google Scholar]
- Semple BD, Bye N, Rancan M, Ziebell JM, Morganti-Kossmann MC. Role of CCL2 (MCP-1) in traumatic brain injury (TBI): evidence from severe TBI patients and CCL2–/– mice. J Cereb Blood Flow Metab. 2010a;30:769–782. doi: 10.1038/jcbfm.2009.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple BD, Bye N, Ziebell JM, Morganti-Kossmann MC. Deficiency of the chemokine receptor CXCR2 attenuates neutrophil infiltration and cortical damage following closed head injury. Neurobiol Dis. 2010b;40:394–403. doi: 10.1016/j.nbd.2010.06.015. [DOI] [PubMed] [Google Scholar]
- Shiozaki T, Hayakata T, Tasaki O, Hosotubo H, Fuijita K, Mouri T, Tajima G, Kajino K, Nakae H, Tanaka H, Shimazu T, Sugimoto H. Cerebrospinal fluid concentrations of anti-inflammatory mediators in early-phase severe traumatic brain injury. Shock. 2005;23:406–410. doi: 10.1097/01.shk.0000161385.62758.24. [DOI] [PubMed] [Google Scholar]
- Sozzani S, Zhou D, Locati M, Rieppi M, Proost P, Magazin M, Vita N, van Damme J, Mantovani A. Receptors and transduction pathways for monocyte chemotactic protein-2 and monocyte chemotactic protein-3. Similarities and differences with MCP-1. J Immunol. 1994;152:3615–3622. [PubMed] [Google Scholar]
- Stamatovic SM, Shakui P, Keep RF, Moore BB, Kunkel SL, Van Rooijen N, Andjelkovic AV. Monocyte chemoattractant protein-1 regulation of blood-brain barrier permeability. J Cereb Blood Flow Metab. 2005;25:593–606. doi: 10.1038/sj.jcbfm.9600055. [DOI] [PubMed] [Google Scholar]
- Strazielle N, Ghersi-Egea JF. Demonstration of a coupled metabolism-efflux process at the choroid plexus as a mechanism of brain protection toward xenobiotics. J Neurosci. 1999;19:6275–6289. doi: 10.1523/JNEUROSCI.19-15-06275.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strazielle N, Ghersi-Egea JF. Choroid plexus in the central nervous system: biology and physiopathology. J Neuropathol Exp Neurol. 2000;59:561–574. doi: 10.1093/jnen/59.7.561. [DOI] [PubMed] [Google Scholar]
- Strazielle N, Preston JE. Transport across the choroid plexuses in vivo and in vitro. Methods Mol Med. 2003;89:291–304. doi: 10.1385/1-59259-419-0:291. [DOI] [PubMed] [Google Scholar]
- Szmydynger-Chodobska J, Fox LM, Lynch KM, Zink BJ, Chodobski A. Vasopressin amplifies the production of proinflammatory mediators in traumatic brain injury. J Neurotrauma. 2010;27:1449–1461. doi: 10.1089/neu.2010.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szmydynger-Chodobska J, Strazielle N, Zink BJ, Ghersi-Egea JF, Chodobski A. The role of the choroid plexus in neutrophil invasion after traumatic brain injury. J Cereb Blood Flow Metab. 2009;29:1503–1516. doi: 10.1038/jcbfm.2009.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M, Galligan C, Tessarollo L, Yoshimura T. Monocyte chemoattractant protein-1 (MCP-1), not MCP-3, is the primary chemokine required for monocyte recruitment in mouse peritonitis induced with thioglycollate or zymosan A. J Immunol. 2009;183:3463–3471. doi: 10.4049/jimmunol.0802812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibeault I, Laflamme N, Rivest S. Regulation of the gene encoding the monocyte chemoattractant protein 1 (MCP-1) in the mouse and rat brain in response to circulating LPS and proinflammatory cytokines. J Comp Neurol. 2001;434:461–477. doi: 10.1002/cne.1187. [DOI] [PubMed] [Google Scholar]
- Toft-Hansen H, Buist R, Sun XJ, Schellenberg A, Peeling J, Owens T. Metalloproteinases control brain inflammation induced by pertussis toxin in mice overexpressing the chemokine CCL2 in the central nervous system. J Immunol. 2006;177:7242–7249. doi: 10.4049/jimmunol.177.10.7242. [DOI] [PubMed] [Google Scholar]
- Yamasaki Y, Matsuo Y, Zagorski J, Matsuura N, Onodera H, Itoyama Y, Kogure K. New therapeutic possibility of blocking cytokine-induced neutrophil chemoattractant on transient ischemic brain damage in rats. Brain Res. 1997;759:103–111. doi: 10.1016/s0006-8993(97)00251-5. [DOI] [PubMed] [Google Scholar]