Abstract
In primate retinas, the dendrites of DB3 diffuse bipolar cells are known to receive inputs from cones. The goal of this study was to describe the synaptic connections of DB3 bipolar cell axons in the inner plexiform layer. DB3 bipolar cells in midperipheral retina were labeled with antibodies to calbindin, and their axons were analyzed in serial, ultrathin sections by electron microscopy. Synapses were found almost exclusively at the axonal varicosities of DB3 axon terminals. There were 2.14 synaptic ribbons per varicosity. There were 33 varicosities per DB3 cell, giving an average of 71 ribbons per axon terminal. Because there were 1.5 postsynaptic ganglion cell dendrites per DB3 axonal varicosity, we estimate that there is at least 1 synapse per varicosity onto a parasol ganglion cell dendrite. There were 3.4 input synapses from amacrine cells per axonal varicosity. Among these were feedback synapses to the DB3 bipolar cell axon varicosities, which were made by 47% of the postsynaptic amacrine cell processes. Some of the feedback synapses could be from amacrine cells immunoreactive for cholecystokinin precursor or choline acetyltransferase, because both types of amacrine cells costratify with parasol cells and are known to be presynaptic to bipolar cells. AII amacrine cells were both presynaptic and postsynaptic to DB3 axons, a finding consistent with the large rod input to parasol ganglion cells reported in physiological experiments. DB3 bipolar cell axons also made frequent contacts with neighboring DB3 axons, and gap junctions were always found at these sites.
Indexing terms: electron microscopy, gap junctions, amacrine cells, parasol ganglion cells
Bipolar cells convey signals from photoreceptors in the outer retina to amacrine and ganglion cells in the inner retina. In mammals, there are rod bipolar cells, which receive input exclusively from rods, and cone bipolar cells, which receive input from cones (reviewed by Boycott and Wässle, 1991). Although there is only one type of rod bipolar cell (for review, see Chun et al., 1993), there are several distinct types of cone bipolar cells (Kolb et al., 1981; Cohen and Sterling, 1990; Boycott and Wässle, 1991; Mills and Massey, 1992). In primates, cone bipolar cells are divided into midget bipolar cells, which contact a single cone in the central retina, and diffuse bipolar cells, which contact several cones there (Polyak, 1941; Boycott and Dowling, 1969; Kolb et al., 1992). There are two types of midget bipolar cells (Kolb et al., 1969) and at least seven types of diffuse bipolar cells (Mariani, 1981; Mariani, 1984; Boycott and Wässle, 1991; Kolb et al., 1992). One of these, the blue cone bipolar cell, contacts blue cones selectively (Mariani, 1984; Kouyama and Marshak, 1992). The other six types of diffuse bipolar cells (DB1-DB6) contact all the cones within their dendritic fields (Mariani, 1984; Boycott and Wässle, 1991; Kolb et al., 1992). Axons of types DB1, DB2, and DB3 ramify in the outer half of the inner plexiform layer (IPL; sublamina a) along with the dendrites of OFF ganglion cells. Because bipolar cells use glutamate as their neurotransmitter and all glutamate effects on primate ganglion cells are excitatory (Zhou et al., 1994), they are presumed to have OFF responses, themselves. Their dendrites contact cones mainly by basal synapses (Hopkins and Boycott, 1995; Hopkins and Boycott, 1997; Calkins et al., 1998). Types DB4, DB5, and DB6 have axons in the inner half of the IPL (sublamina b) where dendrites of ON ganglion cells are located and, presumably, have ON responses (Dacey and Lee, 1994). Their dendrites contact cones mainly by invaginating synapses (Hopkins and Boycott, 1995; Hopkins and Boycott, 1997; Calkins et al., 1998). In addition to the difference in their response polarity, all of the primate diffuse bipolar cells are thought to carry similar, achromatic visual information. The subtypes are distinguished by differences in the morphology and level of stratification of their axon terminals in the IPL. Each type could make a unique contribution to vision if it interacted with different sets of local circuit neurons and different sets of ganglion cells. To begin studying these local circuits, we analyzed the synaptic connections of the DB3 diffuse bipolar cells in the IPL.
We selected DB3 bipolar cells for this electron microscopic study because they could be labeled with antibodies to the calcium-binding protein calbindin-D28K (CaBP; Martin and Grünert, 1992; Grünert et al., 1994). From analysis of Golgi-stained retinas, DB3 and DB2 bipolar cells are known to be similar in many respects (Boycott and Wässle, 1991), and it was important to determine how they might differ in their synaptic connections. DB3 cells contact 6–11 cones, and about 75% of these basal synapses are triad-associated (Hopkins and Boycott, 1995). On the other hand, approximately half of the DB2 bipolar cell cone synapses are triad-associated (Boycott and Hopkins, 1993; reviewed by Hopkins and Boycott, 1997). The axon terminals of DB3 cells are narrowly stratified in stratum 2 of the IPL, and the axons of DB2 cells ramify more broadly throughout sublamina a of the IPL (Boycott and Wässle, 1991). The boundaries between neighboring DB3 axon terminals are difficult to distinguish because they make extensive appositions (Boycott and Wässle, 1991; Jacoby et al., 1999). Studying serial electron micrographs of labeled DB3 axons, we found that these appositions represent sites of gap junctions between DB3 bipolar cell axons. Gap junctions between bipolar cells of the same type might increase the ratio of signal to noise in their light responses and, possibly, minimize differences between responses of neighboring bipolar cells that contact different numbers of cones (Umino et al., 1994).
DB3 bipolar cells are known to provide input to OFF parasol retinal ganglion cells from electron microscopic studies (Calkins et al., 1995; Jacoby et al., 2000). We found that there were 1.5 postsynaptic ganglion cell dendrites per DB3 axonal varicosity, enough for each varicosity to make at least one synapse onto a parasol cell dendrite.
The synapses of amacrine cells with diffuse bipolar cells had not been described previously, however. We found that most of the postsynaptic elements at the ribbon synapses of DB3 bipolar cells were amacrine cells. Of the postsynaptic amacrine cells, 47% made feedback synapses. Some of the amacrine cells that were both presynaptic and postsynaptic to DB3 cells were AII amacrine cells, which also receive inputs from rod bipolar cells (Wässle et al., 1995).
MATERIALS AND METHODS
A light-adapted macaque (Macaca mulatta) eye was enucleated within 10 minutes after the animal had been overdosed with sodium pentobarbital (50–100 mg/kg, IV) by other investigators at the conclusion of experiments that did not involve the eyes. Animal protocols were approved by the University of Texas Health Science Center Animal Care and Use Committee. The globe was hemisected and fixed by immersion for 60 minutes in 4% paraformaldehyde/0.1% glutaraldehyde in 0.1 M sodium phosphate buffer (PB), pH 7.4 at 37°C. The eyecup was then postfixed overnight in 4% paraformaldehyde in PB, pH 10, at 4°C. After rinsing in phosphate-buffered saline (PBS), pH 7.4, the retina was isolated and treated with 1% sodium borohydride/PBS for 1 hour followed by an ascending and descending series of graded ethanol solutions in PBS (10 minutes each in 10, 25, and 40%; 30 minutes in 50%; 10 minutes each in 40, 25, and 10%). The retina was incubated for 7–10 days in 1:1,000 monoclonal mouse anti-calbindin D-28K (Sigma, St. Louis, MO) in PBS with 0.3% sodium azide, rinsed in PBS, and then incubated for 2 days in 1:100 biotinylated goat anti-mouse IgG (Vector Laboratories, Burlingame, CA) in PBS. The biotin was visualized by using Vector standard avidin-biotin-peroxidase (1:100, overnight at 4°C) and diaminobenzidine (DAB, 0.5 mg/ml) with hydrogen peroxide (0.005%, 60 minutes). The retina was then treated with osmium tetroxide (1% in PB, 60 minutes) and embedded in Epon. Approximately 100-nm serial, vertical sections and random vertical sections from ≈4 mm eccentricity were collected on Formvar-coated, single-hole grids and stained with uranyl acetate (2% in 50% methanol, 60 minutes) and lead citrate (0.2% aqueous, 1–2 minutes). Labeled processes in each section were photographed at 10,000× by using a rotating, goniometer stage to orient the sections and align the synaptic membranes.
Of the 100 serial sections analyzed in this study, 70 were used for three-dimensional, graphical reconstruction. The negatives were digitized at a resolution of 600 dpi with a UMAX scanner with a transparency adapter. Individual digitized images were converted into positives, and the contrast was adjusted by using Photoshop 3.0 (Adobe Systems, Mountain View, CA). The calbindin-immunoreactive (CaBP-IR) processes, the surrounding processes that made synapses with them, and 3–6 fiducial marks were outlined in each image and labeled. With use of established, ultrastructural criteria (Dowling and Boycott, 1966; Koontz and Hendrickson, 1987), the unlabeled profiles were identified as amacrine cell processes or ganglion cell dendrites, and the synapses were classified as conventional or ribbon synapses. Consecutive traced images were aligned by using the fiducial marks and reconstructed by using Neurolucida (Microbrightfield, Colchester, VT).
RESULTS
Axon terminals of DB3 diffuse bipolar cells have swellings, or varicosities, at regular intervals separated by narrow connecting regions. We studied six DB3 axon segments varying in length from 3–7 μm from midperipheral retina (≈4 mm eccentricity). It was uncertain how many bipolar cells contributed to these axons because the cells were not reconstructed in their entirety, but there were probably at least six. One hundred serial, 100-nm sections were analyzed, and therefore the portion of stratum 2 we studied was 10 μm deep and 0.5 mm wide. When a rectangle with these dimensions was placed over a CaBP-labeled whole-mount (Jacoby et al., 2000) from the same eccentricity in various orientations, portions of six to nine DB3 axon terminals occupied the area.
Calbindin-immunoreactive (IR) DB3 bipolar cell axons could be identified in the electron microscope by the dense peroxidase reaction product they contained (Fig. 1). Synaptic ribbons and postsynaptic densities were still easily recognized in the labeled profiles, however. The axons were located at 22–48% (mean = 32 ± 5% SD) of the distance from the inner nuclear layer (INL) to the ganglion cell layer (GCL), and there were no other CaBP-IR processes at this depth in the IPL. Other CaBP-IR bipolar cell axons were occasionally seen in the inner half of the IPL at a depth of 70–85%. These were probably from DB5 bipolar cells (Grünert et al., 1994), and they will not be described further here.
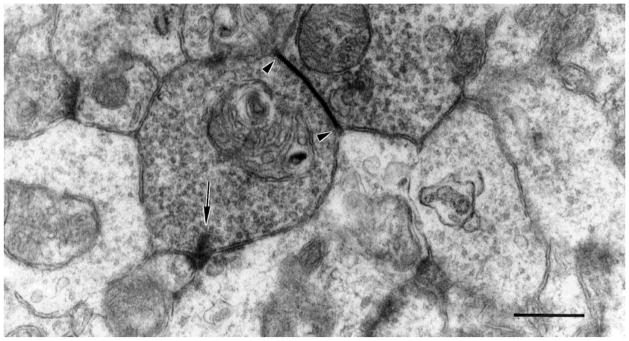
Fig. 1.
A labeled DB3 bipolar cell axon varicosity makes a ribbon synapse (arrowhead) onto an amacrine cell (left) and a ganglion cell dendritic spine (right). Scale bar = 0.5 μm.
Synapses occur at varicosities
Because large, beadlike varicosities are a distinctive feature of DB3 cell axon terminals, we wanted to measure their size and describe their synaptic connections. The axon terminals in our sample had 14 varicosities, of which 2 were reconstructed graphically (Fig. 2). The other varicosities were followed through serial sections but not graphically reconstructed. The varicosities were ovoid in cross section, with the short axis typically oriented vertically, that is, from the photoreceptors to the ganglion cells. At their widest points, the short axes of the varicosities were 1.68 ± 0.3 μm (range, 1.2–2.2 μm) in diameter, and the long axes were 2.41 ± 0.6 μm, on average. The regions of axons between the varicosities ranged in diameter from 0.1 to 0.3 μm at their narrowest points, averaging 0.2 ± 0.08 μm. Therefore, DB3 axon profiles were classified as belonging to a varicosity whenever the short axis diameter was >0.4 μm.
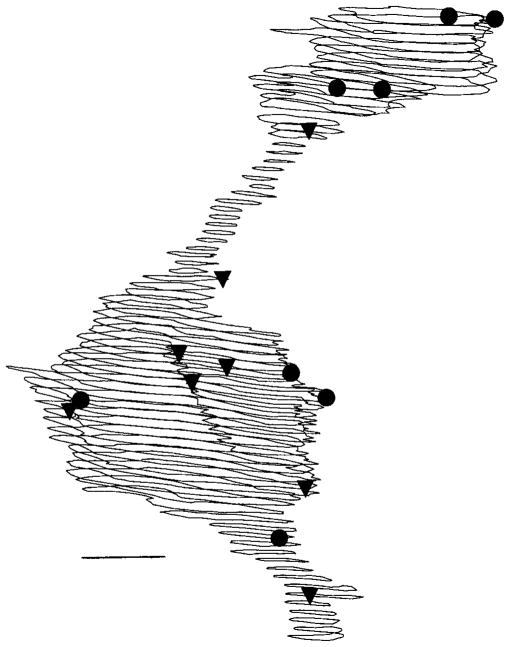
Fig. 2.
Almost all of the synapses with DB3 bipolar cell axons occurred on axonal varicosities. On these three-dimensionally reconstructed DB3 varicosities, output synapses (ribbon synapses) are denoted by filled circles, and input synapses are denoted by filled triangles. Scale bar = 1 μm.
Figure 2 shows the two consecutive DB3 varicosities reconstructed graphically from 70 of the sections in our sample of 100 serial sections. We counted 47 conventional synapses onto DB3 axons, 44 (94%) of which were onto varicosities; the remaining 3 were onto narrower axon regions between varicosities (examples shown in Fig. 2, triangles). All but 1 (29 of 30) of the output, or ribbon, synapses were located inside a varicosity (examples shown in Fig. 2, circles). This appears to be a common feature of bipolar cells, because synapses also are concentrated at terminal swellings of rod bipolar cells (Grünert and Martin, 1991). There were, on average, 3.36 ± 1.78 conventional (input) synapses and 2.14 ± 1.25 synaptic ribbons (output sites) per DB3 varicosity. This resulted in an output synapse to input synapse (O:I) ratio of 0.64. In cat retina, this ratio is generally consistent among bipolar cells of the same type, and it varies between types from 0.9 to 2.6 (McGuire et al., 1984; Cohen and Sterling, 1990). Among primate bipolar cells, rod bipolar cells are the most similar to DB3 cells in this respect, because they have an O:I ratio of 0.5 (Grünert and Martin, 1991). Midget bipolar cells have roughly the same number of input and output synapses, for an O:I ratio of 1 (Kolb and Dekorver, 1991; Calkins et al., 1994), and blue cone bipolar cells have an O:I ratio of 2.4 (Marshak et al., 1990). In the following article, we found that there were 33 varicosities per DB3 cell in peripheral retina (Jacoby et al., 2000). A typical DB3 cell from peripheral retina would, therefore, have 71 synaptic ribbons.
We analyzed all the processes that were apposed to five of the DB3 varicosities to determine how many of them actually made synapses. Each varicosity, on average, was contacted by 14 processes (range, 9–17). This is almost certainly a low estimate because some small or obliquely running processes were probably missed. Nevertheless, only 47% (range, 33–59%) of the apposed processes actually made synapses with the DB3 varicosities. This finding is consistent with results elsewhere in the nervous system, where only half of the contacts between neurons seen with a confocal microscope were found to be synapses using electron microscopy (Mann et al., 1997).
Gap junctions
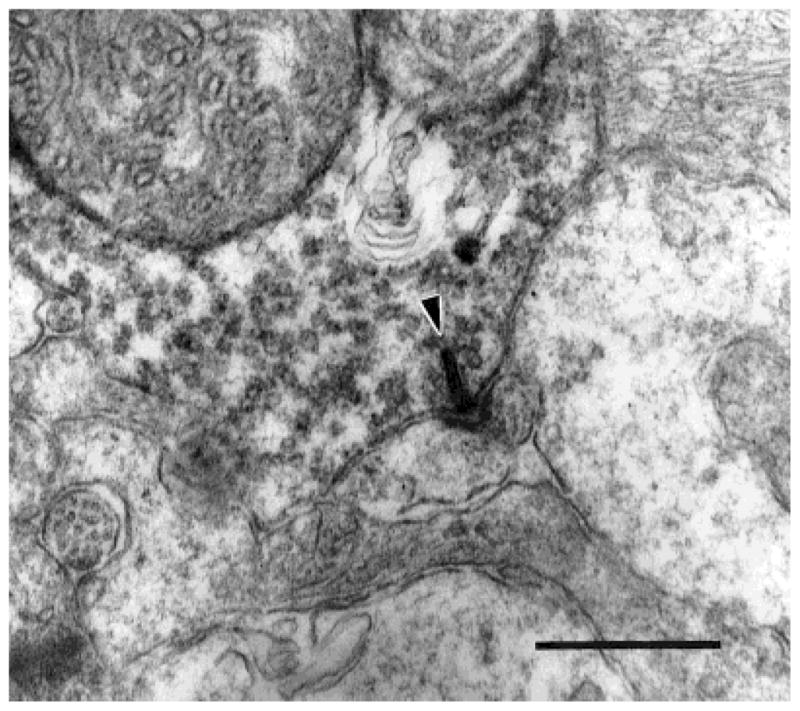
We found that the appositions between neighboring DB3 axon terminals observed in the light microscope were sites of gap junctions (Fig. 3). A densitometric scan (Fig. 4) of a DB3 gap junction shows that the outer leaflets (two central peaks) of the membrane were separated by approximately 2 nm, and the inner leaflets (larger peaks) were approximately 26 nm apart. Two of these homologous gap junctions were found in the axon segments in our sample of serial sections. They occurred at the only two sites in our sample where DB3 axons contacted one another. Several more gap junctions were found in random sections, and the junctions were always as large as the area of contact between the two axons (Fig. 3). The gap junctions from the reconstructed series were 0.227 and 0.279 μm2 in area. It is likely, therefore, that gap junctions occur wherever neighboring DB3 axons come into contact.
Fig. 3.
Neighboring DB3 axon varicosities form a gap junction (arrowheads). Gap junctions were always as large as the area of contact between varicosities. The varicosity on the left also makes a ribbon synapse onto two amacrine cells (arrow). Scale bar = 0.5 μm.
Fig. 4.
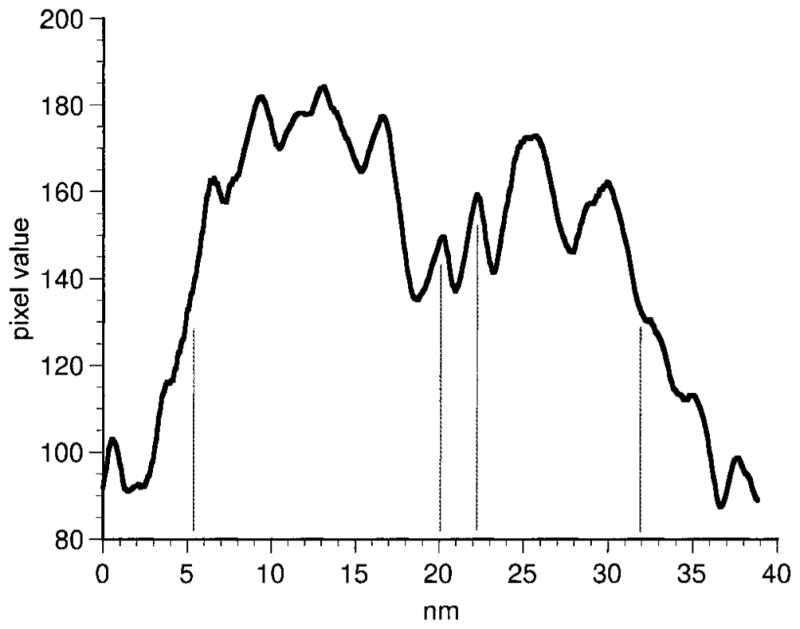
Densitometric scan of a gap junction between DB3 varicosities. The peaks indicate the densest portions of the membrane leaflets. The outer leaflets (central vertical lines) are clearly visible with a separation of approximately 2 nm.
Chemical synapses
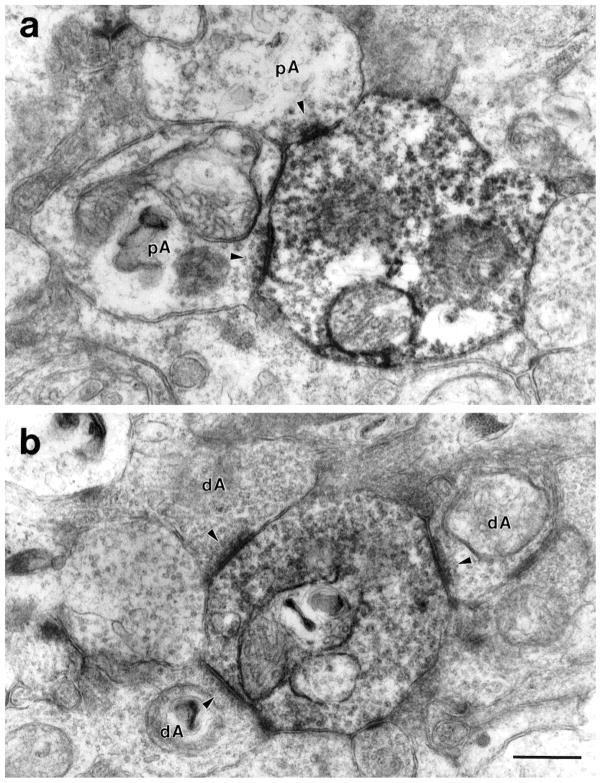
Inputs to DB3 axons came from at least three types of amacrine cells. Some had relatively electron-lucent cytoplasm and very few synaptic vesicles (Fig. 5a, pAs), and others had relatively electron-dense cytoplasm filled with synaptic vesicles (Fig. 5b, dAs). Other amacrine cells had large, lobular processes that were electron-dense, filled with synaptic vesicles (Fig. 6) and are likely to be from AII amacrine cells, based on descriptions of calretinin-IR AII amacrine cells in macaque retina (Wässle et al., 1995). Like AII cells from other mammals, they had large, rugose profiles that were filled with synaptic vesicles and large mitochondria (Famiglietti and Kolb, 1975; Kolb, 1979; Strettoi et al., 1992; Chun et al., 1993).
Fig. 5.
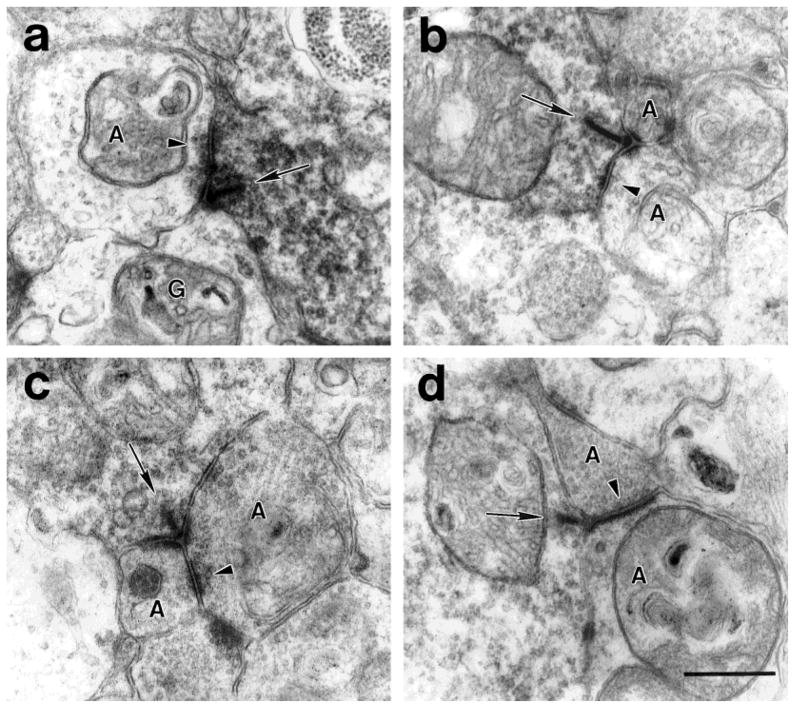
a: Two large, electron-lucent amacrine cells (pA) make conventional synapses (arrowheads) onto a labeled DB3 axon varicosity. These amacrine cells contained few synaptic vesicles. b: Synapses (arrowheads) onto a DB3 varicosity from relatively electron-dense amacrine cells (dA) filled with synaptic vesicles. Clusters of synapses were seen frequently. Note the electron-lucent amacrine cell to the left of the DB3 axon for comparison. Scale bar = 0.5 μm.
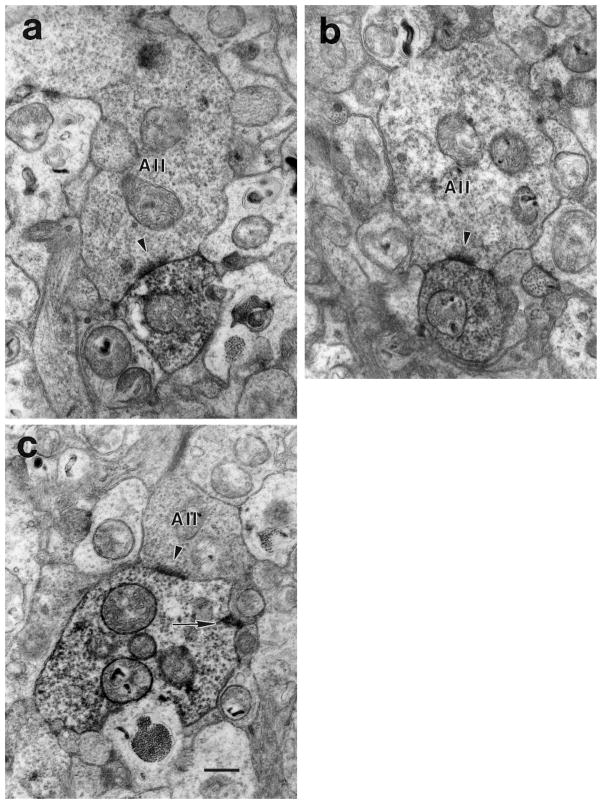
Fig. 6.
a: A large, lobular AII amacrine cell process (above) makes a synapse (arrowhead) onto a DB3 axon (below). The AII process contained large mitochondria and was filled with synaptic vesicles. b: An AII amacrine cell (above) makes a synapse (arrowhead) onto a DB3 axon (below). c: An AII amacrine cell is presynaptic (arrowhead) and postsynaptic (arrow) to a DB3 axon. Scale bar = 0.5 μm.
AII amacrine cells were both presynaptic and postsynaptic to DB3 bipolar cell axons. The electron-lucent amacrine cells also made feedback synapses (Fig. 7a,b). Over half (58%) of the feedback synapses were in the same sections as the ribbon input synapses. The average number of sections separating a feedback synapse made by an amacrine cell and its input from a synaptic ribbon was 2.3 (range, 0–8), which corresponds to a distance of 0.23 μm. However, most of the input to DB3 cells, 75%, was from amacrine cells that did not receive input from DB3 bipolar cells within the area we studied. These were classified as nonreciprocal synapses, although it is possible that some of these amacrine cells got input from DB3 cells outside of the analyzed area. The average maximum diameter of an input synapse was 0.25 ± 0.01 μm, both for feedback and nonreciprocal synapses.
Fig. 7.
a: A DB3 axon varicosity makes a ribbon synapse (arrow) onto a relatively electron-lucent amacrine cell (A) and a ganglion cell (G). The amacrine cell makes a feedback synapse (arrowhead) onto the DB3 axon. b: A DB3 axon makes a ribbon synapse (arrow) onto two amacrine cell processes (A). One of the amacrine cells makes a feedback synapse (arrowhead) onto the DB3 axon. c: A DB3 axon makes a ribbon synapse (arrow) onto two amacrine cell processes (A). One of the amacrine cells makes a feedforward synapse (arrowhead) onto the other amacrine cell in the dyad. d:A DB3 axon makes a ribbon synapse (arrow) onto an electron lucent amacrine cell (bottom) and an electron-dense amacrine cell (top). The electron-dense amacrine cell also makes a feedforward synapse (arrowhead) onto the larger, electron-lucent amacrine cell. Scale bar = 0.5 μm.
An average of 3.36 (range, 1–7) amacrine cell processes was found presynaptic at each DB3 axonal varicosity, and typically each originated from a different amacrine cell. In only one case did the same amacrine cell process make two synapses onto the same varicosity. Because there were 33 varicosities per DB3 cell, there would be 111 (3.36 × 33) chemical synapses onto a peripheral DB3 cell.
Two postsynaptic processes were opposed to the DB3 axons at ribbon synapses, forming dyads (Dowling and Boycott, 1966). There was a pair of amacrine cells at 27% of the dyads, and there was one amacrine and one ganglion cell at 53% of the dyads. There were two ganglion cell dendrites at 7% of the dyads and 13% of the dyads contained at least one unidentified process (Table 1). Overall, 57% of the output from DB3 cells was directed to amacrine cells (see Table 2).
TABLE 1.
Composition of DB3 Bipolar Cell Dyads1
| No. (%) | |
|---|---|
| Amacrine-amacrine | 8 (27) |
| Amacrine-ganglion | 16 (53) |
| Ganglion-ganglion | 2 (7) |
| ≥1 Unidentified | 4 (13) |
| Total | 30 |
All the synapses where DB3 bipolar cells were presynaptic had two postsynaptic elements. Approximately half had one amacrine cell process and one ganglion cell dendrite. Dyads with two identified ganglion cell dendrites were uncommon, but some of the small, unidentified postsynaptic elements may have been dendritic spines of ganglion cells.
TABLE 2.
Synaptic Contacts of DB3 Bipolar Cells
| No. | Total Synapses (%)1 | Input (%) | |
|---|---|---|---|
| Presynaptic | |||
| Amacrine reciprocal | 12 | 11 | 25 |
| Amacrine nonreciprocal | 35 | 33 | 75 |
| Total inputs | 47 | ||
| Output (%)
|
|||
| Postsynaptic | |||
| Amacrine cells | 34 | 32 | 57 |
| Ganglion cells | 22 | 20 | 37 |
| Unidentified | 4 | 4 | 6 |
| Total outputs | 60 | ||
The percentage of total synapses column indicates the proportion of each kind of synapse relative to the total number of synapses made by the reconstructed DB3 axons. The percentage of input column indicates the proportion of each kind of process presynaptic to the DB3 cell, and the percentage of output column indicates the proportion of each type of process postsynaptic to the DB3 cell.
About half (47%) of the postsynaptic amacrine cells made feedback synapses to the DB3 axons (Fig. 7a,b). The postsynaptic amacrine cell processes that provided feedback onto the DB3 axons were significantly larger than those that did not (t-test, P = 0.015). Processes making feedback synapses averaged 0.91 ± 0.43 μm, and those not making feedback synapses averaged 0.61 ± 0.34 μm in diameter at the dyads. Five (15%) of the postsynaptic amacrine cells made feedforward synapses onto the neighboring process at the dyads (Fig. 7c,d). Two of these feedforward synapses were onto amacrine cells, and three were onto ganglion cells. Of the postsynaptic processes tentatively identified as ganglion cell dendrites in our material, 47% were classified as dendritic spines because they were less than 0.2 μm in diameter (Fig. 1). The synaptic ribbons of the DB3 bipolar cells were 0.18 ± 0.07 μm long, on average.
Ganglion cell dendrites made up 37% of the postsynaptic processes (Table 2). At each DB3 axonal varicosity, there were 1.5 (range, 0–3) postsynaptic ganglion cell dendrites, on average. In only one instance did the same ganglion cell dendrite receive two synapses from the same varicosity. Therefore, with 33 varicosities, a DB3 cell would be expected to have 50 synapses onto ganglion cells. In the central retina, a reconstructed DB3 bipolar cell provided 9% of its output synapses to a small-field bistratified ganglion cell (Calkins et al., 1998). If peripheral DB3 cells also follow this pattern, then they would make 13 synapses (0.09 × 142 postsynaptic elements) onto small-field bistratified ganglion cells, accounting for 26% of the synapses onto ganglion cells. This leaves 37 output synapses per DB3 cell, or slightly more than one synapse per axonal varicosity, available for parasol ganglion cells and other ganglion cell types.
DISCUSSION
Ribbon synapses from DB3 diffuse bipolar cells provide a substantial proportion of the input to parasol ganglion cells in the macaque retina. Another diffuse bipolar cell type, the more broadly stratified DB2 type, also provides input to parasol ganglion cells (Calkins, 1999). Because both types of bipolar cells are also presynaptic to the small-field bistratified ganglion cells, it is important to determine how the two bipolar cells differ from one another and how each contributes to the light responses of the postsynaptic ganglion cells.
Previous reports have indicated some differences between DB2 and DB3 bipolar cells (Calkins et al., 1995; Hopkins and Boycott, 1995; Hopkins and Boycott, 1997; Calkins et al., 1998; Calkins, 1999). In the parafovea, Calkins et al. (1995) found that DB2-like bipolar cells formed 43–51 ribbon synapses and that DB3-like cells formed 65–72 ribbons. By calculating the average number of synapses made by reconstructed DB3 axonal varicosities in this study, we could estimate the total number of synapses made by DB3 bipolar cells. Our estimate of 71 ribbons per peripheral DB3 cell is similar to the number from the abstract of Calkins et al. (1995) for a foveal DB3. This finding suggests that the number of output synapses per DB3 cell stays roughly constant with increasing eccentricity. Because bipolar cell axon terminal size increases with eccentricity (Boycott and Wässle, 1991; Wässle et al., 1994; Mills and Massey, 1992; Massey and Mills, 1996) and the larger axon terminals in the periphery have the same number of output synapses as smaller ones in central retina, the density of DB3 bipolar cell ribbons in the IPL should decrease with eccentricity. It appears that DB3 bipolars contain a larger number of ribbons than DB2 cells. It is possible, therefore, that the number of ribbons can be used to distinguish diffuse bipolar cells in primates, as is the case for midget bipolar cells in the macaque fovea. In foveal retina midget bipolar cells can be subdivided into a group containing 30 ribbons and another with 50 ribbons, irrespective of the level of their terminations in the IPL (Calkins et al., 1994). In addition, blue cone-selective bipolar cells in the parafovea make an average of 42 ribbon synapses in the IPL (Calkins et al., 1998), and rod bipolar cells at 1–2 mm eccentricity make roughly 20 ribbon synapses (Grünert and Martin, 1991). Thus, each type of primate bipolar cell analyzed to date appears to make a characteristic number of ribbon synapses. In order of increasing number of ribbons, there are: rod bipolar cells (20 ribbons), one midget bipolar cell type (30 ribbons), blue cone bipolar cells (42 ribbons), DB2 bipolar cells (43–51 ribbons), the other midget bipolar cell type (50 ribbons), and DB3 bipolar cells (65–72 ribbons).
Our results predict that bipolar cell ribbon density in the IPL decreases with eccentricity. This decrease has been demonstrated directly in the cat retina. Kier et al. (1995) found that the density of bipolar cell inputs to ganglion cells per unit area of dendritic field, or retinal density, decreased with increasing eccentricity, although the density of bipolar cell inputs remained constant as a function of dendritic membrane area of the ganglion cells. As with cat ganglion cell dendrites, DB3 axons branch more sparsely as they increase in size. Therefore, the number of ribbons per axonal membrane area could remain constant with eccentricity and still allow the retinal density of bipolar cell ribbons to decrease. If so, this result might explain the inverse relationship between the peak sensitivity of macaque ganglion cells and their dendritic field size. Croner and Kaplan (1994) found that although the peak sensitivity of both P (midget) and M (parasol) ganglion cells decreases as dendritic field size increases, the integrated sensitivity remains constant. The investigators suggested that larger ganglion cells may have a lower retinal density of synaptic inputs, due to their more sparsely branching dendrites, and, therefore, they would be less sensitive than smaller cells to small spots of light falling on their receptive fields. Our results support this hypothesis.
DB3 cells made fewer synapses onto ganglion cells (37% of total output) than other known types of cone bipolar cells in primates. At 27% of the dyad synapses, there were two amacrine cells postsynaptic. Midget bipolar cells provide 54% of their output to amacrine cells, and only 9% of their dyads have two amacrine cells (Kolb and Dekorver, 1991; Calkins et al., 1994). The remaining midget bipolar cell dyads have one amacrine cell and one ganglion cell. For blue cone bipolar cells, 48% of the postsynaptic processes were amacrine cells, and 11% of the dyads had two amacrine cells postsynaptic (Marshak et al., 1990). Rod bipolar cells, however, do not make any synapses onto ganglion cells (Grünert and Martin, 1991).
Synapses with amacrine cells
The majority of both input and output synapses of DB3 axons were with amacrine cells. Some of these had relatively electron-lucent cytoplasm and contained only a small number of synaptic vesicles. Two kinds of wide-field amacrine cells that costratify with DB3 axons are those containing immunoreactive choline acetyltransferase (ChAT) and those containing immunoreactive cholecystokinin precursor (G6-gly; Jacoby et al., 1996). Cholinergic amacrine cells receive most of their input from bipolar cells (Mariani and Hersh, 1988). Recently, synapses have been found from cholinergic amacrine cells onto bipolar cells, and nicotinic acetylcholine receptor immunoreactivity has been localized to a subpopulation of bipolar cells in macaque retina (Yamada et al., 1998). G6-gly-IR amacrine cells also receive synapses from bipolar cells, although fewer than the cholinergic cells receive, and they make synapses onto bipolar cells (Marshak et al., 1990).
It is likely that the remainder of the electron-lucent amacrine cells we observed in this study is either GABAergic or glycinergic. Most of the synapses between amacrine cells and bipolar cells in macaque IPL involve GABA-IR amacrine cells (Koontz and Hendrickson, 1990). In fact, Enz et al. (1996) showed that the ρ subunit of the GABAC receptor was localized to 38% of DB3 axonal varicosities. CaBP-IR DB3 varicosities had from 0 to 3 ρ-immunoreactive puncta (0.5 per varicosity, on average). Assuming that each punctum represents a single GABAergic input synapse, there would be space for about three additional input synapses per varicosity, because we found 3.36 input synapses per varicosity, overall. This is consistent with results from rat retina, where the putative DB3 homolog, the type 5 cone bipolar cell (Euler and Wässle 1995: Jacoby et al., 2000), was shown to have a much smaller contribution from GABAC receptors than from GABAA receptors relative to other bipolar cells (Euler and Wässle, 1998). Because large amacrine cells in macaque retina typically contain GABA (Kalloniatis et al., 1996), G6-gly-IR amacrine cells are likely to release GABA, in addition to cholecystokinin, which also has inhibitory actions on briskly responding ganglion cells such as parasol cells (Thier and Bolz, 1985).
Other amacrine cell processes that made synapses with DB3 axons in this study resembled the glycinergic AII amacrine cells in macaques (Wässle et al., 1995). Their ultrastructure generally matched the original descriptions of AII amacrine cells from cat (Famiglietti and Kolb, 1975; Kolb, 1979), and those from rabbit (Strettoi et al., 1992) and rat (Chun et al., 1993). One difference between AII amacrine cells in macaques and those in cat retina is the size of their output synapses. AII amacrine cells make small, punctate synapses in the cat (Famiglietti and Kolb, 1975). The synapses from AII cells onto DB3 axons, however, were not noticeably smaller than synapses from other amacrine cells. Wässle et al. (1995) also reported that some synapses between calretinin-IR AII amacrine cells and cone bipolar cell axons in the outer half of the macaque IPL were “quite large.”
Putative AII amacrine cells were also postsynaptic at DB3 ribbon synapses. AII amacrine cells receive a significant amount of input from cone bipolar cells in the distal half of the IPL of cat (McGuire et al., 1984), rabbit (Strettoi et al., 1992; Merighi et al., 1996), rat (Chun et al., 1993), human (Marc and Liu, 1985), and macaque (Wässle et al., 1995; Grünert, 1997) retinas. There is also electrophysiological evidence for OFF cone bipolar cell input to AII amacrine cells. In macaque retina, blockade of ON responses with 2-amino-4-phosphonobutyrie acid (APB) unmasks a cone-driven OFF response in AII amacrine cells (Stone et al., 1997). OFF cone bipolar input to an AII cell would create an inhibitory feedback circuit, which could make the bipolar cell’s response after light decrements more transient (Strettoi et al., 1992). This feedback could also sensitize the AII cell by keeping it closer to its threshold for firing action potentials, which may be important for sensing small rod bipolar cell signals under dark-adapted conditions (Chun et al. 1993).
Our finding of synapses between AII amacrine cells and DB3 bipolar cells is consistent with previous studies of AII circuitry and glycine receptor localization in macaque retinas. Roughly 60% of the cone bipolar cell inputs to AII cells and 68% of the outputs from AII cells to cone bipolar cells were found in stratum 2 (20–40% depth) of the IPL (Wässle et al., 1995), where DB3 cells ramify (mean = 32% depth). Punctate immunoreactivity for the alpha-1 subunit of the glycine receptor was also found at the same level of the IPL, and furthermore, some of the puncta were localized to calbindin immunoreactive DB3 axons in stratum 2 of the IPL (Grünert and Wässle, 1996).
The amount of feedback from amacrine cells to bipolar cells may help distinguish bipolar cell types in macaques, as it does in the cat retina (Cohen and Sterling, 1990). We found that 47% of the amacrine cells postsynaptic to DB3 axons made feedback synapses to the same DB3 axon, and, overall, 25% of the amacrine cell inputs were via feedback synapses. On the other hand, a much higher percentage (77%) of the amacrine cells postsynaptic to midget bipolar cells make feedback synapses, and 73% of the amacrine cell inputs to midget bipolar cells are via feedback synapses (Calkins and Sterling, 1996). Early studies of the macaque IPL suggested that all of the amacrine cell to bipolar cell synapses may be feedback synapses (Dowling and Boycott, 1966), but more recent evidence from specific bipolar cell types analyzed using serial sections indicate that this is not the case. Rod bipolar cells in primates have a smaller proportion of postsynaptic amacrine cells that give feedback, 25% (Marc and Liu, 1985; Grünert and Martin, 1991). However, these feedback synapses on rod bipolars account for about the same percentage of the amacrine cell input as for DB3 cells. Overall, 23% of the amacrine cell inputs to rod bipolar cells are feedback synapses. For blue cone bipolar cells, 60% of the postsynaptic amacrine cell processes make feedback synapses (Calkins et al., 1998). Thus, all primate bipolar cells studied so far appear to use feedback circuits, but each type uses them to a different extent. In order of increasing percentages of postsynaptic amacrine cells that give feedback, they are: rod bipolar cells, DB3 bipolar cells, blue cone bipolar cells, and midget bipolar cells.
Homologs of OFF diffuse cone bipolar cells from the cat have been studied extensively (Kolb et al., 1981; McGuire et al., 1984). Table 3 summarizes the similarities between macaque DB3 bipolar cells and three types of cat bipolar cells that ramify narrowly in sublamina a of the IPL. Each cat bipolar cell has some features in common with DB3 cells, but no single type obviously corresponds to primate DB3 cells based on its ultrastructure, alone.
TABLE 3.
Mammalian Bipolar Cells1
| Species
|
||||
|---|---|---|---|---|
| Macaque | Cat | |||
| Reference2 | 1 | 2 | 3, 4 | 3 |
| Type | DB3 | Cb2 | CBa1* | CBa4 |
| Cone synapses | Mostly basal | Basal | ? | ? |
| Axon stratification | Narrow | Narrow | Narrow | Narrow |
| Axon depth in IPL | s.l. a | s.l. a | s.l. a | s.l. a |
| Postsynaptic GC types | Parasol | α, β | α, β | ? |
| Input from AII ACs | Yes | Yes | Yes | Few |
| Output to AII ACs | Yes | Yes | Yes | Yes |
| Gap junctions with other BCs | Yes | Yes | ? | ? |
| Output to ACs (%) | 61 | 48 | 50 | 60 |
| Postsynaptic ACs that feed back (%) | 47 | 17 | 5 | 56 |
| Feedback as percent of total input (%) | 25 | 12 | 7 | 59 |
Comparisons between macaque DB3 bipolar cells and cat bipolar cells with similar morphology. One of the two CBa1 cells of McGuire et al. (1984) had narrowly stratified axons in the IPL; only its synapses are shown here. The percentage data from Kolb (1979) were obtained by counting synapses made by the smooth, flat cone bipolar axon reconstructed in her Fig. 16. The percentage of output to amacrine cells reflects the proportion of postsynaptic elements that are amacrine cells. The percentage of postsynaptic amacrine cells that give feedback is the proportion of postsynaptic amacrine cells that make synapses onto the same bipolar cells that provide their inputs. The feedback as a percentage of total input is the proportion of amacrine cell synapses onto the bipolar cells that are feedback synapses.
1, Boycott and Wässle, 1991; Hopkins and Boycott, 1997. 2, Kolb, 1979; Kolb et al., 1981. 3, McGuire et al., 1984 (*cell 14). 4, Pourcho and Goebel, 1987. DB, diffuse bipolar; Cb, cone bipolar; IPL, inner plexiform layer; s.l., sublamina; GC, ganglion cell; AC, amacrine cell; BC, bipolar cell.
Gap junctions
Gap junctions were found wherever two neighboring DB3 axons came into contact. This is the first report of homologous gap junctions between primate bipolar cell axons, but our results are consistent with earlier freeze fracture studies of macaque retina. With use of this technique, gap junctions have been found on the axons (Raviola and Raviola, 1982) and dendrites (Raviola and Gilula, 1975) of bipolar cells, but at that time, it was not clear what other types of cells were contacted. In whole-mount preparations, the boundaries of individual DB3 axon terminals are difficult to discern because of the frequent appositions between neighboring axons (Boycott and Wässle, 1991; Grünert et al., 1994; Jacoby et al., 2000). Because these contacts are the sites of gap junctions, other bipolar cell types that make contacts with bipolar cells of the same type may also form gap junctions. By using this criterion, flat midget bipolar cells are not likely to make junctions with other midget bipolar cells, because neighboring terminals do not contact each other at any eccentricity (Wässle et al., 1994). Fewer data are available for the other bipolar cell types. Boycott and Wässle (1991) presented drawings from Golgi-stained whole-mounts showing groups of neighboring DB1, DB2, and DB5 bipolar cells, but they are not adequate to come to a conclusion as to whether they might make homologous gap junctions. Jeon and Masland (1995) found appositions between wide-field bipolar cell axons in the rabbit retina.
Gap junctions have been reported between bipolar cells in other species. In cat retina, type CBb4 cone bipolar cells of Cohen and Sterling (1990) make homologous gap junctions and gap junctions with CBb3 bipolar cells in the inner sublamina of the IPL. Two types of cat bipolar cells with axons ramifying in the outer sublamina, cb1 and cb2, also make gap junctions with other bipolar cells (Kolb, 1979), a finding that supports the hypothesis that primate DB3 cells and cat cb2 cells are homologous. Mills and Massey (1996) reported that DAPI-Ba3 bipolar cells from rabbit retina were tracer-coupled to other DAPI-Ba3 cells and to DAPI-Ba1 bipolar cells. Bipolar cell axons form homologous gap junctions in salamanders (Wong-Riley, 1974) and fish (Witkovsky and Stell, 1973; Van Haesendonck and Misotten, 1983; Kujiraoka and Saito, 1986; Marc et al., 1988). Umino et al. (1994) also reported gap junctions between the dendrites of neighboring bipolar cells in fish retinas.
The function of these gap junctions between bipolar cells has been investigated in fish. Simultaneous intracellular recordings from neighboring bipolar cells showed that current injected into one cell elicited a sign-conserving, sustained potential change in the other cell. The coupling was thought to account for the finding that receptive field centers of bipolar cells are larger than their dendritic fields (Kujiraoka and Saito, 1986). By modeling a coupled network of bipolar cells, Umino et al. (1994) found that electrical coupling of bipolar cells could minimize the effects of differences between the numbers of cones presynaptic to neighboring bipolar cells. Thus, bipolars of the same type that get input from different numbers of cones would, nevertheless, have similar sensitivity to light. Because macaque DB3 bipolar cells receive input from as few as 6 or as many as 11 cones (Boycott and Wässle, 1991; Calkins et al., 1998), this could be an important function of the homologous gap junctions. Electrical coupling may also improve signal-to-noise ratios by averaging weak cone signals and reducing photoreceptor noise (Yamada and Saito, 1997). Thus, electrical coupling between the presynaptic DB3 bipolar cells may account, at least in part, for the high luminance contrast sensitivity of parasol ganglion cells.
Acknowledgments
We thank Mrs. Lillemor Krosby for excellent technical assistance, Ms. Amy Mason for help with the serial reconstruction, and Prof. Brian Boycott for his very helpful comments on the manuscript.
Grant sponsor: National Eye Institute; Grant numbers: EY06472, EY07024, and ET10608; Grant sponsor: National Institute of Mental Health; Grant number: MH10957.
LITERATURE CITED
- Boycott BB, Dowling J. Organization of the primate retina: light microscopy. Phil Trans R Soc Lond [Biol] 1969;255:109–184. doi: 10.1098/rspb.1966.0086. [DOI] [PubMed] [Google Scholar]
- Boycott BB, Hopkins JM. Cone synapses of a flat diffuse cone bipolar cell in the primate retina. J Neurocytol. 1993;22:765–778. doi: 10.1007/BF01181322. [DOI] [PubMed] [Google Scholar]
- Boycott BB, Wässle H. Morphological classification of bipolar cells of the primate retina. Eur J Neurosci. 1991;3:1069–1088. doi: 10.1111/j.1460-9568.1991.tb00043.x. [DOI] [PubMed] [Google Scholar]
- Calkins DJ, Schein SJ, Tsukamoto Y, Sterling P. M and L cones in macaque fovea connect to midget ganglion cells by different numbers of excitatory synapses. Nature. 1994;371:70–72. doi: 10.1038/371070a0. [DOI] [PubMed] [Google Scholar]
- Calkins D, Schein S, Sterling P. Cone inputs to three types of non-midget ganglion cell in macaque fovea. Invest Ophthalmol Vis Sci. 1995;36:S4. [Google Scholar]
- Calkins DJ, Sterling P. Absence of spectrally specific lateral inputs to midget ganglion cells in primate retina. Nature. 1996;381:613–615. doi: 10.1038/381613a0. [DOI] [PubMed] [Google Scholar]
- Calkins DJ, Tsukamoto Y, Sterling P. Microcircuitry and mosaic of a blue-yellow ganglion cell in the primate retina. J Neurosci. 1998;18:3373–3385. doi: 10.1523/JNEUROSCI.18-09-03373.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins DJ. Synaptic organization of cone pathways in the primate retina. In: Gegenfurtner K, Sharpe L, editors. Color vision: from genes to perception. New York: Cambridge University Press; 1999. [Google Scholar]
- Chun MH, Han SH, Chung JW, Wässle H. Electron microscopic analysis of the rod pathway of the rat retina. J Comp Neurol. 1993;332:421–432. doi: 10.1002/cne.903320404. [DOI] [PubMed] [Google Scholar]
- Cohen E, Sterling P. Demonstration of cell types among cone bipolar neurons of cat retina. Proc R Soc Lond [Biol] 1990;330:305–321. doi: 10.1098/rstb.1990.0201. [DOI] [PubMed] [Google Scholar]
- Croner LJ, Kaplan E. Receptive fields of P and M ganglion cells across the primate retina. Vision Res. 1994;35:7–24. doi: 10.1016/0042-6989(94)e0066-t. [DOI] [PubMed] [Google Scholar]
- Dacey DM, Lee BB. The “blue-on” opponent pathway in primate retina originates from a distinct bistratified ganglion cell type. Nature. 1994;367:731–735. doi: 10.1038/367731a0. [DOI] [PubMed] [Google Scholar]
- Dowling JE, Boycott BB. Organization of the primate retina: electron microscopy. Proc R Soc Lond [Biol] 1966;166:80–111. doi: 10.1098/rspb.1966.0086. [DOI] [PubMed] [Google Scholar]
- Enz R, Brandstatter JH, Wässle H, Bormann J. Immunocytochemical localization of the GABAc receptor ρ subunits in the mammalian retina. J Neurosci. 1996;16:4479–4490. doi: 10.1523/JNEUROSCI.16-14-04479.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euler T, Wässle H. Immunocytochemical identification of cone bipolar cells in the rat retina. J Comp Neurol. 1995;361:461–478. doi: 10.1002/cne.903610310. [DOI] [PubMed] [Google Scholar]
- Euler T, Wässle H. Different contributions of GABA(A) and GABA(C) receptors to rod and cone bipolar cells in a rat retinal slice preparation. J Neurophys. 1998;79:1384–1395. doi: 10.1152/jn.1998.79.3.1384. [DOI] [PubMed] [Google Scholar]
- Famiglietti EV, Jr, Kolb H. A bistratified amacrine cell and synaptic circuitry in the inner plexiform layer of the retina. Brain Res. 1975;84:293–300. doi: 10.1016/0006-8993(75)90983-x. [DOI] [PubMed] [Google Scholar]
- Grünert U, Martin PR. Rod bipolar cells in the macaque monkey retina: immunoreactivity and connectivity. J Neurosci. 1991;11:2742–2758. doi: 10.1523/JNEUROSCI.11-09-02742.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grünert U, Martin PR, Wässle H. Immunocytochemical analysis of bipolar cells in the macaque monkey retina. J Comp Neurol. 1994;348:607–627. doi: 10.1002/cne.903480410. [DOI] [PubMed] [Google Scholar]
- Grünert U, Wässle H. Glycine receptors in the rod pathway of the macaque monkey retina. Vis Neurosci. 1996;13:101–115. doi: 10.1017/s0952523800007161. [DOI] [PubMed] [Google Scholar]
- Grünert U. Anatomical evidence for rod input to the parvocellular pathway in the visual system of the primate. Eur J Neurosci. 1997;9:617–621. doi: 10.1111/j.1460-9568.1997.tb01638.x. [DOI] [PubMed] [Google Scholar]
- Hopkins JM, Boycott BB. Synapses between cones and diffuse bipolar cells of a primate retina. J Neurocytol. 1995;24:680–694. doi: 10.1007/BF01179818. [DOI] [PubMed] [Google Scholar]
- Hopkins JM, Boycott BB. The cone synapses of cone bipolar cells of primate retina. J Neurocytol. 1997;26:313–325. doi: 10.1023/a:1018504718282. [DOI] [PubMed] [Google Scholar]
- Jacoby R, Stafford D, Kouyama N, Marshak D. Synaptic inputs to ON parasol ganglion cells in the primate retina. J Neurosci. 1996;16:8041–8056. doi: 10.1523/JNEUROSCI.16-24-08041.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby RA, Wiechmann AF, Amara SG, Leighton BH, Marshak DW. Diffuse bipolar cells provide input to OFF parasol ganglion cells in the macaque retina. J Comp Neurol. 2000;416:6–18. doi: 10.1002/(sici)1096-9861(20000103)416:1<6::aid-cne2>3.0.co;2-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon C-J, Masland RH. A population of wide-field bipolar cells in the rabbit’s retina. J Comp Neurol. 1995;360:403–412. doi: 10.1002/cne.903600304. [DOI] [PubMed] [Google Scholar]
- Kalloniatis M, Marc RE, Murry RF. Amino acid signatures in the primate retina. J Neurosci. 1996;16:6807–6829. doi: 10.1523/JNEUROSCI.16-21-06807.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kier CK, Buchsbaum G, Sterling P. How retinal microcircuits scale for ganglion cells of different size. J Neurosci. 1995;15:7673–7683. doi: 10.1523/JNEUROSCI.15-11-07673.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb H, Boycott BB, Dowling JE. A second type of midget bipolar cell in the primate retina. Phil Trans R Soc Lond [Biol] 1969;255:177–184. [Google Scholar]
- Kolb H. The inner plexiform layer in the retina of the cat: electron microscopic observations. J Neurocytol. 1979;8:295–329. doi: 10.1007/BF01236124. [DOI] [PubMed] [Google Scholar]
- Kolb H, Nelson R, Mariani A. Amacrine cells, bipolar cells and ganglion cells of the cat retina: a Golgi study. Vision Res. 1981;21:1081–1114. doi: 10.1016/0042-6989(81)90013-4. [DOI] [PubMed] [Google Scholar]
- Kolb H, Dekorver L. Midget ganglion cells of the parafovea of the human retina: a study by electron microscopy and serial section reconstructions. J Comp Neurol. 1991;303:617–636. doi: 10.1002/cne.903030408. [DOI] [PubMed] [Google Scholar]
- Kolb H, Linberg KA, Fisher SK. Neurons of the human retina: a Golgi study. J Comp Neurol. 1992;318:147–187. doi: 10.1002/cne.903180204. [DOI] [PubMed] [Google Scholar]
- Koontz MA, Hendrickson AE. Stratified distribution of synapses in the inner plexiform layer of primate retina. J Comp Neurol. 1987;263:581–592. doi: 10.1002/cne.902630409. [DOI] [PubMed] [Google Scholar]
- Koontz MA, Hendrickson AE. Distribution of GABA-immunoreactive amacrine cell synapses in the inner plexiform layer of macaque monkey retina. Vis Neurosci. 1990;5:17–28. doi: 10.1017/s0952523800000043. [DOI] [PubMed] [Google Scholar]
- Kouyama N, Marshak DW. Bipolar cells specific for blue cones in the macaque retina. J Neurosci. 1992;12:1233–52. doi: 10.1523/JNEUROSCI.12-04-01233.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujiraoka T, Saito T. Electrical coupling between bipolar cells in carp retina. Proc Natl Acad Sci USA. 1986;83:4063–4066. doi: 10.1073/pnas.83.11.4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann PT, Southwell BR, Young HM, Furness JB. Appositions made by axons of descending interneurons in the guinea-pig small intestine, investigated by confocal microscopy. J Chem Neuroanat. 1997;12:151–164. doi: 10.1016/s0891-0618(96)00189-5. [DOI] [PubMed] [Google Scholar]
- Marc RE, Liu WL. (3H) glycine-accumulating neurons of the human retina. J Comp Neurol. 1985;232:241–260. doi: 10.1002/cne.902320209. [DOI] [PubMed] [Google Scholar]
- Marc RE, Liu WL, Muller JF. Gap junctions in the inner plexiform layer of the goldfish retina. Vision Res. 1988;28:9–24. [PubMed] [Google Scholar]
- Mariani AP. A diffuse, invaginating cone bipolar cell in primate retina. J Comp Neurol. 1981;197:661–671. doi: 10.1002/cne.901970408. [DOI] [PubMed] [Google Scholar]
- Mariani AP. Bipolar cells in monkey retina selective for the cones likely to be blue-sensitive. Nature. 1984;308:184–186. doi: 10.1038/308184a0. [DOI] [PubMed] [Google Scholar]
- Mariani AP, Hersh LB. Synaptic organization of cholinergic amacrine cells in the rhesus monkey retina. J Comp Neurol. 1988;267:269–280. doi: 10.1002/cne.902670209. [DOI] [PubMed] [Google Scholar]
- Marshak DW, Aldrich LB, Del Valle J, Yamada T. Localization of immunoreactive cholecystokinin precursor to amacrine cells and bipolar cells of the macaque monkey retina. J Neurosci. 1990;10:3045–3055. doi: 10.1523/JNEUROSCI.10-09-03045.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PR, Grünert U. Spatial density and immunoreactivity of bipolar cells in the macaque monkey retina. J Comp Neurol. 1992;323:269–287. doi: 10.1002/cne.903230210. [DOI] [PubMed] [Google Scholar]
- Massey SC, Mills SL. A calbindin-immunoreactive cone bipolar cell type in the rabbit retina. J Comp Neurol. 1996;366:15–33. doi: 10.1002/(SICI)1096-9861(19960226)366:1<15::AID-CNE2>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- McGuire BA, Stevens JK, Sterling P. Microcircuitry of bipolar cells in cat retina. J Neurosci. 1984;4:2920–2938. doi: 10.1523/JNEUROSCI.04-12-02920.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merighi A, Raviola E, Dacheux RF. Connections of two types of flat cone bipolars in the rabbit retina. J Comp Neurol. 1996;371:164–178. doi: 10.1002/(SICI)1096-9861(19960715)371:1<164::AID-CNE10>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Mills SL, Massey SC. Morphology of bipolar cells labeled by DAPI in the rabbit retina. J Comp Neurol. 1992;321:133–149. doi: 10.1002/cne.903210112. [DOI] [PubMed] [Google Scholar]
- Mills SL, Massey SC. Tracer coupling between mammalian bipolar cells. Invest Ophthalmol Vis Sci. 1996;37:S675. [Google Scholar]
- Polyak SL. The Retina. Chicago: The University of Chicago Press; 1941. [Google Scholar]
- Pourcho RG, Goebel DJ. A combined Golgi and autoradiographic study of 3H-glycine-accumulating cone bipolar cells in the cat retina. J Neurosci. 1987;7:1178–1188. doi: 10.1523/JNEUROSCI.07-04-01178.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raviola E, Gilula NB. Intramembrane organization of specialized contacts in the outer plexiform layer of the retina: a freeze-fracture study in monkeys and rabbits. J Cell Biol. 1975;65:192–222. doi: 10.1083/jcb.65.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raviola E, Raviola G. Structure of the synaptic membranes in the inner plexiform layer of the retina: a freeze-fracture study in monkeys and rabbits. J Comp Neurol. 1982;209:233–48. doi: 10.1002/cne.902090303. [DOI] [PubMed] [Google Scholar]
- Stone S, Buck SL, Dacey DM. Pharmacological dissection of rod and cone bipolar input to the AII amacrine in macaque retina. Invest Ophthalmol Vis Sci. 1997;38:S689. [Google Scholar]
- Strettoi E, Raviola E, Dacheux RF. Synaptic connections of the narrow-field, bistratified rod amacrine cell (AII) in the rabbit retina. J Comp Neurol. 1992;325:152–168. doi: 10.1002/cne.903250203. [DOI] [PubMed] [Google Scholar]
- Thier P, Bolz J. Cholecystokinin in the cat retina: action of exogenous CCK8 and localization of cholecystokinin-like immunoreactivity. Invest Ophthalmol Vis Sci. 1985;26:266–272. [PubMed] [Google Scholar]
- Umino O, Maehara M, Hidaka S, Kita S, Hashimoto Y. The network properties of bipolar-bipolar cell coupling in the retina of teleost fishes. Vis Neurosci. 1994;11:533–548. doi: 10.1017/s0952523800002443. [DOI] [PubMed] [Google Scholar]
- Van Haesendonck E, Missotten L. Interbipolar contacts in the dorsal inner plexiform layer in the retina of Callionymus lyra L. J Ultrastruct Res. 1983;83:303–311. doi: 10.1016/s0022-5320(83)90137-5. [DOI] [PubMed] [Google Scholar]
- Wässle H, Grünert U, Martin P, Boycott BB. Immunocytochemical characterization and spatial distribution of midget bipolar cells in the macaque monkey retina. Vision Res. 1994;34:561–579. doi: 10.1016/0042-6989(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Wässle H, Grünert U, Chun MH, Boycott BB. The rod pathway of the macaque monkey retina: identification of AII-amacrine cells with antibodies against calretinin. J Comp Neurol. 1995;361:537–551. doi: 10.1002/cne.903610315. [DOI] [PubMed] [Google Scholar]
- Witkovsky P, Stell WK. Retinal structure in the smooth dogfish Mustelus canis: electron microscopy of serially sectioned bipolar cell synaptic terminals. J Comp Neurol. 1973;150:147–167. doi: 10.1002/cne.901500204. [DOI] [PubMed] [Google Scholar]
- Wong-Riley MT. Synaptic organization of the inner plexiform layer in the retina of the tiger salamander. J Neurocytol. 1974;3:1–33. doi: 10.1007/BF01111929. [DOI] [PubMed] [Google Scholar]
- Yamada ES, Keyser KT, Dimitrieva N, Lindstrom JM, Marshak DW. Cholinergic input to bipolar cell axon terminals in the macaque retina. Soc Neurosci Abstr. 1998;24:520. [Google Scholar]
- Yamada M, Saito T. Dual component in receptive field centers of bipolar cells in carp retina. Vision Res. 1997;37:2331–2338. doi: 10.1016/s0042-6989(97)00042-4. [DOI] [PubMed] [Google Scholar]
- Zhou ZJ, Marshak DM, Fain GL. Amino acid receptors of midget and parasol ganglion cells in primate retina. Proc Natl Acad Sci USA. 1994;91:4907–4911. doi: 10.1073/pnas.91.11.4907. [DOI] [PMC free article] [PubMed] [Google Scholar]