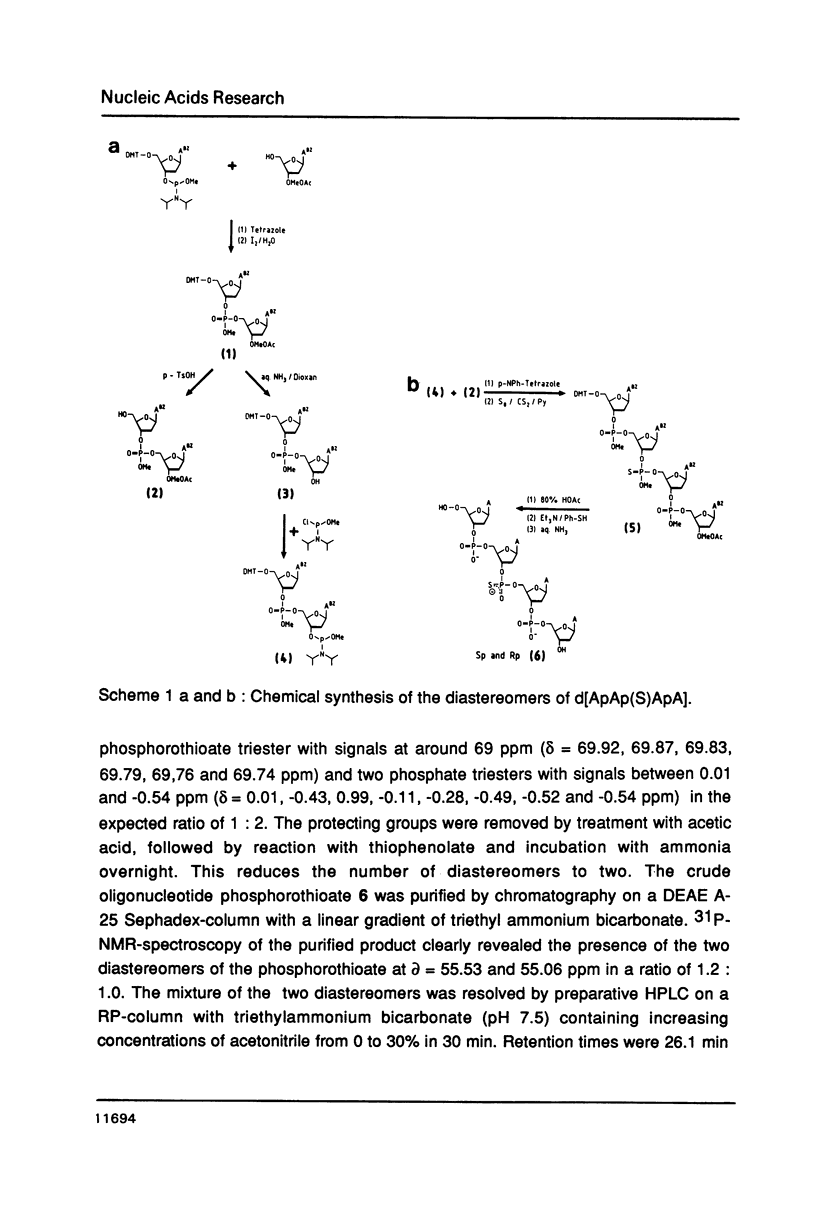
Abstract
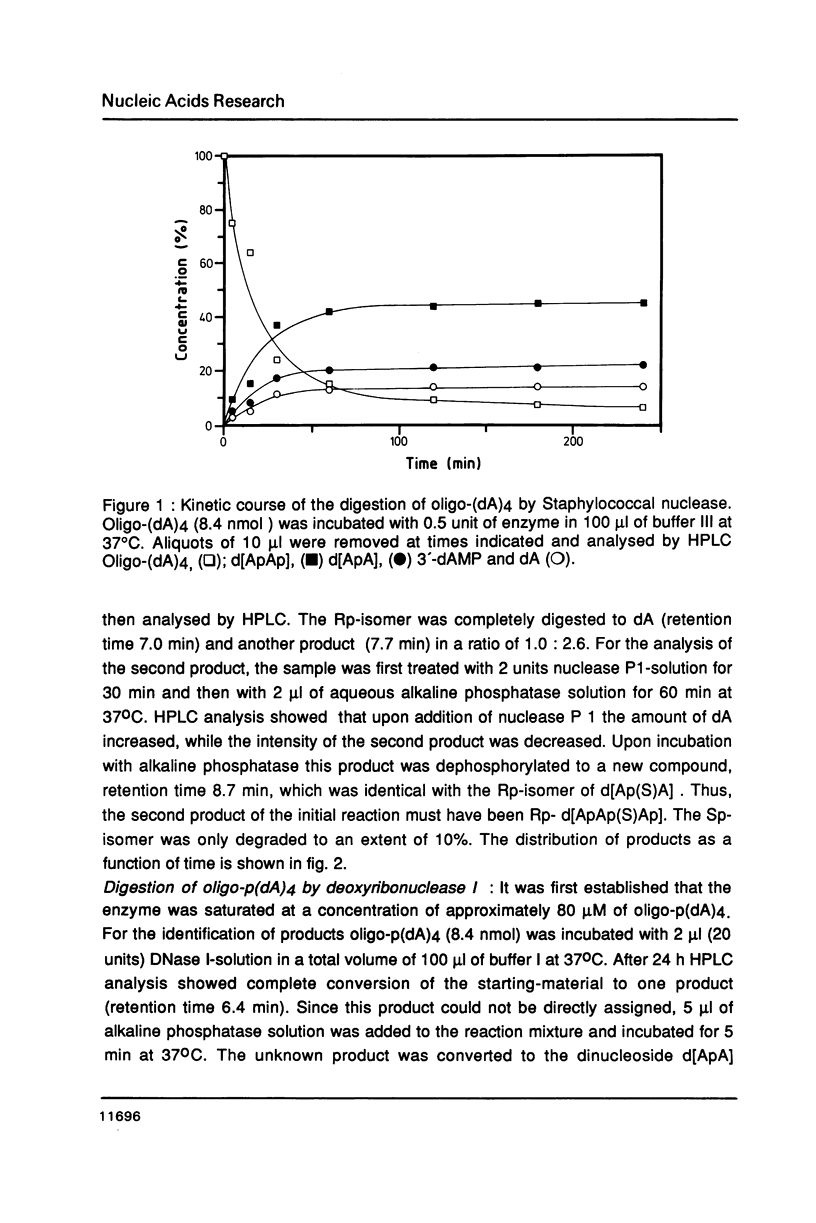
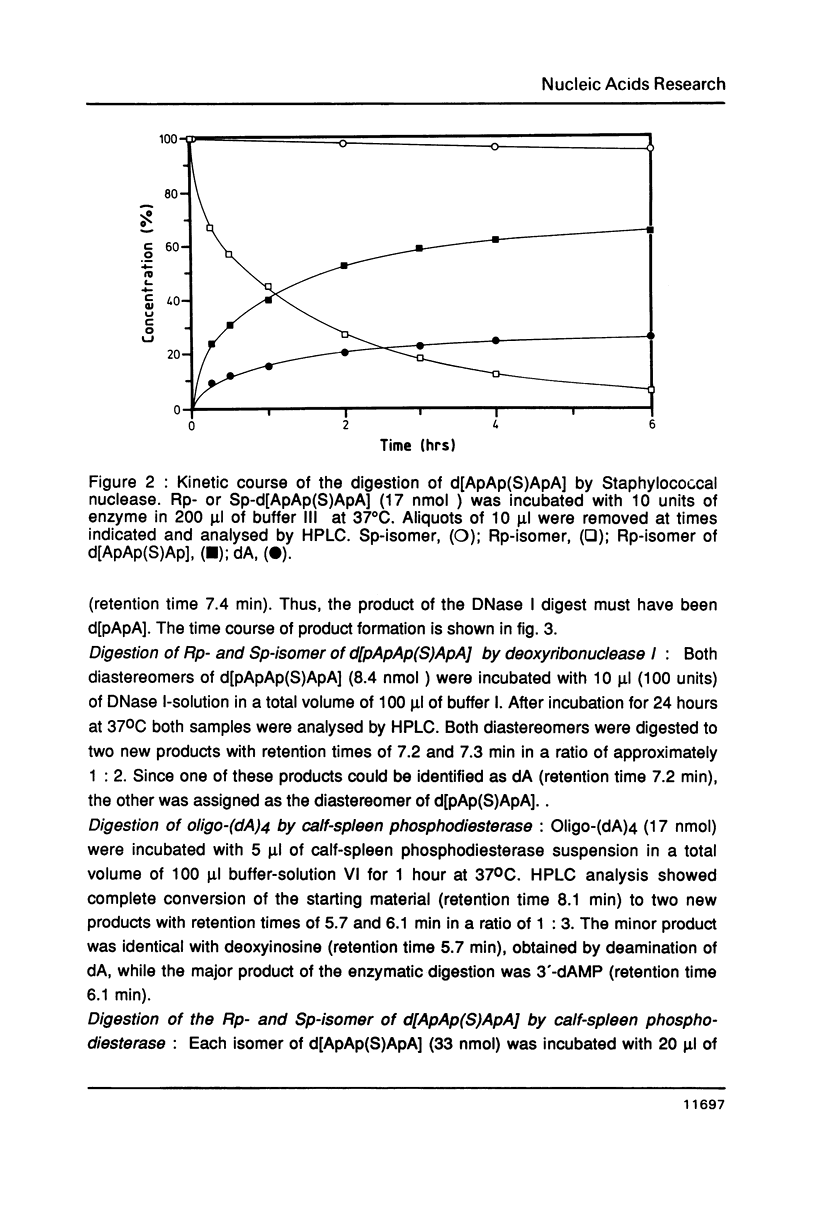
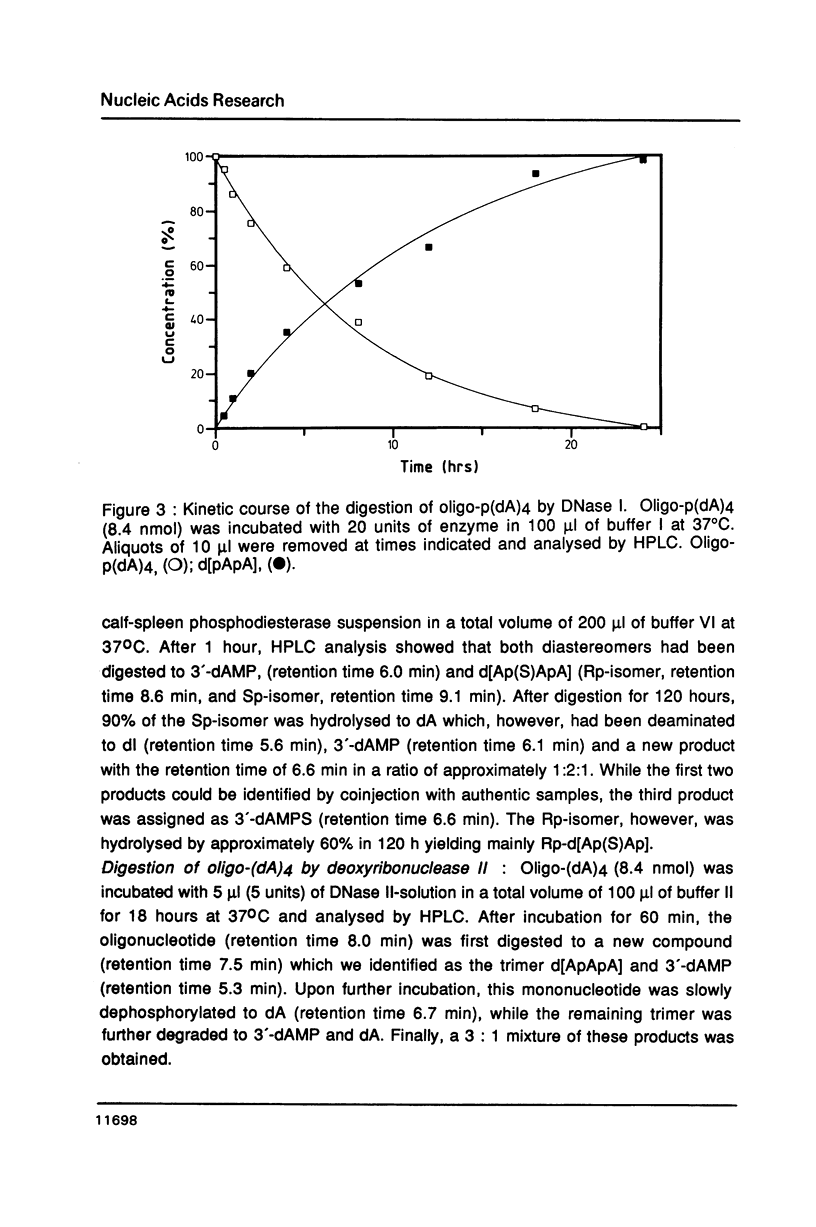
The Rp- and Sp-diastereomers of the phosphorothioate-containing oligonucleotide d[ApAp(S)ApA] have been synthesized. They and the tetramer d[ApApApA] were tested as substrates for staphylococcal nuclease, DNase II and spleen phosphodiesterase. For digestions with DNase I these oligonucleotides were converted to the 5'-phosphorylated derivates. The reactions with the nucleases were analysed by HPLC. The phosphorothioate groups of both diastereomers were resistant to the action of staphylococcal nuclease, DNase I and DNase II. While the phosphorothioate group of the Rp-diastereomer was resistant to the action of spleen phosphodiesterase, the Sp-diastereomer was hydrolysed at an estimated rate 1/100 the rate of cleavage of the unmodified tetramer. The presence of the phosphorothioate group in the center of the molecule affected the rate of hydrolysis of neighbouring phosphate groups for some enzymes. In particular, very slow release of 3'-dAMP from the Rp-diastereomer occurred on incubation with staphylococcal nuclease but the Sp-diastereomer was completely resistant. DNase II produced 3'-dAMP quite rapidly from both diastereomers of d[ApAp(S)ApA] and DNase I released 5'-dAMP from both diastereomers of d[pApAp(S)ApA] only slowly.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Black D. R., Eckstein F., DeClercq E., Merigan T. C. Studies on the toxicity and antiviral activity of various polynucleotides. Antimicrob Agents Chemother. 1973 Feb;3(2):198–206. doi: 10.1128/aac.3.2.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody R. S., Frey P. A. Unambiguous determination of the stereochemistry of nucleotidyl transfer catalyzed by DNA polymerase I from Escherichia coli. Biochemistry. 1981 Mar 3;20(5):1245–1252. doi: 10.1021/bi00508a030. [DOI] [PubMed] [Google Scholar]
- Burgers P. M., Eckstein F. A study of the mechanism of DNA polymerase I from Escherichia coli with diastereomeric phosphorothioate analogs of deoxyadenosine triphosphate. J Biol Chem. 1979 Aug 10;254(15):6889–6893. [PubMed] [Google Scholar]
- Darby M. K., Vosberg H. P. Relaxation of supercoiled phosphorothioate DNA by mammalian topoisomerases is inhibited in a base-specific manner. J Biol Chem. 1985 Apr 10;260(7):4501–4507. [PubMed] [Google Scholar]
- Dörper T., Winnacker E. L. Improvements in the phosphoramidite procedure for the synthesis of oligodeoxyribonucleotides. Nucleic Acids Res. 1983 May 11;11(9):2575–2584. doi: 10.1093/nar/11.9.2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckstein F. Nucleoside phosphorothioates. Annu Rev Biochem. 1985;54:367–402. doi: 10.1146/annurev.bi.54.070185.002055. [DOI] [PubMed] [Google Scholar]
- Gerlt J. A., Coderre J. A., Mehdi S. Oxygen chiral phosphate esters. Adv Enzymol Relat Areas Mol Biol. 1983;55:291–380. doi: 10.1002/9780470123010.ch4. [DOI] [PubMed] [Google Scholar]
- Gupta A. P., Benkovic P. A., Benkovic S. J. The effect of the 3',5' thiophosphoryl linkage on the exonuclease activities of T4 polymerase and the Klenow fragment. Nucleic Acids Res. 1984 Jul 25;12(14):5897–5911. doi: 10.1093/nar/12.14.5897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho N. W., Gilham P. T. Action of micrococcal nuclease on chemically modified deoxyribonucleic acid. Biochemistry. 1974 Mar 12;13(6):1082–1087. doi: 10.1021/bi00703a004. [DOI] [PubMed] [Google Scholar]
- Kitts P. A., Nash H. A. Homology-dependent interactions in phage lambda site-specific recombination. Nature. 1987 Sep 24;329(6137):346–348. doi: 10.1038/329346a0. [DOI] [PubMed] [Google Scholar]
- Knowles J. R. Enzyme-catalyzed phosphoryl transfer reactions. Annu Rev Biochem. 1980;49:877–919. doi: 10.1146/annurev.bi.49.070180.004305. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Eckstein F., Mildvan A. S., Koplitz R. M., Loeb L. A. Deoxynucleoside [1-thio]triphosphates prevent proofreading during in vitro DNA synthesis. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6734–6738. doi: 10.1073/pnas.78.11.6734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsukura M., Shinozuka K., Zon G., Mitsuya H., Reitz M., Cohen J. S., Broder S. Phosphorothioate analogs of oligodeoxynucleotides: inhibitors of replication and cytopathic effects of human immunodeficiency virus. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7706–7710. doi: 10.1073/pnas.84.21.7706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray A. W., Atkinson M. R. Adenosine 5'-phosphorothioate. A nucleotide analog that is a substrate, competitive inhibitor, or regulator of some enzymes that interact with adenosine 5'-phosphate. Biochemistry. 1968 Nov;7(11):4023–4029. doi: 10.1021/bi00851a032. [DOI] [PubMed] [Google Scholar]
- Nakamaye K. L., Eckstein F. Inhibition of restriction endonuclease Nci I cleavage by phosphorothioate groups and its application to oligonucleotide-directed mutagenesis. Nucleic Acids Res. 1986 Dec 22;14(24):9679–9698. doi: 10.1093/nar/14.24.9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niewiarowski W., Uznanski B. Substrate specificity and stereospecificity of calf spleen phosphodiesterase towards deoxyribonucleosidyl 3'-(4-nitrophenyl phosphates) and phosphorothioates. Eur J Biochem. 1985 Nov 15;153(1):145–153. doi: 10.1111/j.1432-1033.1985.tb09280.x. [DOI] [PubMed] [Google Scholar]
- Ott J., Eckstein F. Protection of oligonucleotide primers against degradation by DNA polymerase I. Biochemistry. 1987 Dec 15;26(25):8237–8241. doi: 10.1021/bi00399a032. [DOI] [PubMed] [Google Scholar]
- POTTER J. L., LAURILA U. R., LASKOWSKI M. Studies of the specificity of deoxyribonuclease I. I. Hydrolysis of a trinucleotide. J Biol Chem. 1958 Oct;233(4):915–916. [PubMed] [Google Scholar]
- Potter B. V., Connolly B. A., Eckstein F. Synthesis and configurational analysis of a dinucleoside phosphate isotopically chiral at phosphorus. Stereochemical course of Penicillium citrum nuclease P1 reaction. Biochemistry. 1983 Mar 15;22(6):1369–1377. doi: 10.1021/bi00275a008. [DOI] [PubMed] [Google Scholar]
- Potter B. V., Eckstein F., Uznański B. A stereospecifically 18O-labelled deoxydinucleoside phosphate block for incorporation into an oligonucleotide. Nucleic Acids Res. 1983 Oct 25;11(20):7087–7103. doi: 10.1093/nar/11.20.7087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter B. V., Romaniuk P. J., Eckstein F. Stereochemical course of DNA hydrolysis by nuclease S1. J Biol Chem. 1983 Feb 10;258(3):1758–1760. [PubMed] [Google Scholar]
- Putney S. D., Benkovic S. J., Schimmel P. R. A DNA fragment with an alpha-phosphorothioate nucleotide at one end is asymmetrically blocked from digestion by exonuclease III and can be replicated in vivo. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7350–7354. doi: 10.1073/pnas.78.12.7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RALPH R. K., SMITH R. A., KHORANA H. G. Studies on polynucleotides. XV. Enzymic degradation. The mode of action of pancreatic deoxyribonuclease on thymidine, deoxycytidine, and deoxyadenosine polynucleotides. Biochemistry. 1962 Jan;1:131–137. doi: 10.1021/bi00907a020. [DOI] [PubMed] [Google Scholar]
- Romaniuk P. J., Eckstein F. A study of the mechanism of T4 DNA polymerase with diastereomeric phosphorothioate analogues of deoxyadenosine triphosphate. J Biol Chem. 1982 Jul 10;257(13):7684–7688. [PubMed] [Google Scholar]
- Sayers J. R., Schmidt W., Wendler A., Eckstein F. Strand specific cleavage of phosphorothioate-containing DNA by reaction with restriction endonucleases in the presence of ethidium bromide. Nucleic Acids Res. 1988 Feb 11;16(3):803–814. doi: 10.1093/nar/16.3.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber G., Koch E. M., Neubert W. J. Selective protection of in vitro synthesized cDNA against nucleases by incorporation of phosphorothioate-analogues. Nucleic Acids Res. 1985 Nov 11;13(21):7663–7672. doi: 10.1093/nar/13.21.7663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. W., Schmidt W., Cosstick R., Okruszek A., Eckstein F. The use of phosphorothioate-modified DNA in restriction enzyme reactions to prepare nicked DNA. Nucleic Acids Res. 1985 Dec 20;13(24):8749–8764. doi: 10.1093/nar/13.24.8749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uznanski B., Niewiarowski W., Stec W. J. Activity of acid deoxyribonuclease towards diastereoisomers of thymidyl 3'-(4-nitrophenyl phosphorothioate). Stereochemistry of transnucleotidylation reaction. J Biol Chem. 1986 Jan 15;261(2):592–598. [PubMed] [Google Scholar]


