Abstract
Background
Perioperative neurocognitive disorders (PND) result in long-term morbidity and mortality with no effective interventions available. Because interleukin-6 (IL-6), a pro-inflammatory cytokine, is consistently up-regulated by trauma, including after surgery, we determined whether IL-6 is a putative therapeutic target for PND in a mouse model.
Methods
Following institutional approval, adult (12–14 weeks) male C57/Bl6 mice were pretreated with the IL-6 receptor (IL6R) blocking antibody tocilizumab prior to open tibia fracture with internal fixation under isoflurane anaesthesia. Inflammatory and behavioural responses in a trace fear conditioning (TFC) paradigm were assessed postoperatively. Separately, the effects of IL-6 administration or of depletion of bone marrow-derived monocytes (BM-DMs) with clodrolip on the inflammatory and behavioural responses were assessed. Blood brain barrier disruption, hippocampal microglial activation, and infiltration of BM-DMs were each assessed following IL-6 administration.
Results
The surgery-induced decrement in freezing time in the TFC assay, indicative of cognitive decline, was attenuated by tocilizumab (P<0.01). The surgery-induced increase in pro-inflammatory mediators was significantly reduced by tocilizumab. Exogenously administered IL-6 significantly impaired freezing behaviour (P<0.05) and up-regulated pro-inflammatory cytokines; both responses were prevented by depletion of BM-DMs. IL-6 disrupted the blood brain barrier, and increased hippocampal activation of microglia and infiltration of BM-DMs.
Conclusions
IL-6 is both necessary and sufficient to produce cognitive decline. Following further preclinical testing of its perioperative safety, the IL6R blocker tocilizumab is a candidate for prevention and/or treatment of PND.
Keywords: cognitive dysfunction, cytokines, interleukin-6 (IL-6), surgery, tocilizumab
Editor's key points.
-
•
A mouse model of postoperative surgical trauma (tibial fracture) was used to determine the role of interleukin-6 (IL-6) in postoperative cognitive dysfunction.
-
•
Tocilizumab, an approved IL-6 receptor blocking antibody, prevented surgical trauma–induced behavioural deficits and increases in inflammatory mediators.
-
•
Exogenous IL-6 reproduced the behavioural and inflammatory effects of tibial fracture.
-
•
Therapeutic interruption of the IL-6 pathway provides a potential strategy for prevention and treatment of postoperative cognitive disorders.
Perioperative neurocognitive disorders (PND), encompassing both postoperative delirium and postoperative cognitive dysfunction, complicate postoperative recovery with serious consequences.1, 2 The pathophysiologic processes of PND, and hence possible targets for therapeutic intervention, need to be determined. A growing body of evidence, mainly from animal models of PND, suggests that the innate immune system is engaged through trauma-provoked release of damage associated molecular patterns (DAMPS) that initiate a peripheral inflammatory response.3 Circulating pro-inflammatory cytokines are sensed by the central nervous system (CNS) through neural mechanisms,4 resulting in complementary neuroinflammation through a disrupted blood brain barrier,5 and producing microglial activation6 with elaboration and release of pro-inflammatory cytokines and chemokines that are capable of disrupting long-term potentiation (LTP),7 a biologic correlate of learning and memory.8 Recent clinical studies reveal that neuroinflammation also occurs in the immediate postoperative period following peripheral surgery.9, 10, 11
The pro-inflammatory cytokine, interleukin-6 (IL-6), is up-regulated in both the periphery and CNS following experimental trauma in preclinical models,12 and in surgical patients.9 Circulating IL-6 levels positively correlate with deficits in cognition in humans.13, 14 An observational study suggested that up-regulated postoperative IL-6 levels are associated with subsequent development of short- and medium-term impairment of cognitive function after surgery.15 Administration of IL-6 diminished LTP in a hippocampal slice preparation16 and lipopolysaccharide-induced decline in cognitive behaviour was attenuated in IL-6 deficient mice.17 Interestingly, IL-6 neutralizing antibodies can improve postoperative cognitive impairment in aged mice.18 These findings suggest that IL-6 might be an important factor that both facilitates immune-to-brain communication and hippocampal inflammatory mechanisms that negatively affect cognitive processing. We investigated whether IL-6 is both necessary and sufficient to produce cognitive decline in a mouse model of PND. If so, IL-6 could be an important target for therapeutic intervention to prevent or attenuate the development of PND.
Methods
Animals
The Institutional Animal Care and Use Committee of the University of California, San Francisco, USA approved all experimental procedures involving animals in accordance with the National Institutes of Health (Bethesda, MD, USA) Guidelines (approval number 167062-01A). Adult (12–14 weeks) male C57Bl/6J mice (Jackson Laboratory, Bar Harbor, ME, USA) and Ccr2RFP/+ Cx3cr1GFP/+ mice (acquired from Dr. Israel Charo, J. David Gladstone Institute, San Francisco, CA, USA) were used. Standard rodent food and water ad libitum were provided to all animals, which were housed (five per cage) in cages lined with sawdust. A 12-h light/dark schedule was followed in an air-conditioned environment. Before any procedure or treatment, animals were randomly tagged and divided into groups. Group assignments were revealed after the analysis phase to ensure blinding to group allocation. Mice were euthanized according to institutional guidelines.
Surgical trauma
Mice were subjected to open left tibia fracture with intramedullary fixation under aseptic conditions during general anaesthesia with isoflurane 2% as described.3, 5, 6, 12 Buprenorphine 0.1 mg kg−1 was administered s.c. for analgesia immediately after anaesthetic induction and prior to surgery. Surgical anaesthesia was established by loss of pedal reflex. During surgery, body temperature was maintained at 37.0°C [Standard deviation (sd) 0.5 °C] by a heating lamp and warming pad. Sham mice for bone fracture (controls) received the same anaesthesia and analgesia as for tibia fracture. The full procedure from induction of anaesthesia to end of surgery lasted no longer than 15 min.
Trace-fear conditioning
Trace-fear conditioning (TFC) was used to assess learning and memory as previously described.3, 5, 6, 12 Briefly, animals were trained to associate a conditional stimulus (tone) with an aversive, unconditional stimulus (foot-shock). Aversive memory is associated with freezing behaviour when the mouse is re-exposed to the same context. The behavioural study was conducted using a conditioning chamber (Med Associates Inc., St. Albans, VT, USA) and an unconditional stimulus (two periods of 2-s foot-shock of 0.75 mA). Behaviour was captured with an infrared video camera (Video Freeze; Med Associates Inc.). After a particular intervention, animals underwent a training session and were then returned to their cage. Mice underwent a context test 72 h after training, during which no tones or foot-shocks were delivered. Lack of movement, indicating freezing behaviour, of video-recordings was analysed. Memory impairment is indicated by a decrease in freezing time.
Systemic inflammatory response
Blood was collected transcardially 6 h after aseptic surgical trauma via thoracotomy under terminal isoflurane anaesthesia. Blood was collected into heparin-coated syringes and samples were centrifuged at 1200× g for 10 min; plasma was collected and stored at −80°C until assayed. Commercially available enzyme-linked immunosorbent assay (ELISA) kits were used to measure plasma IL-6, IL-1β, and monocyte chemoattractant protein-1 (MCP-1) according to manufacturer's instructions (R&D Systems, Minneapolis, Minnesota, USA).
Neuroinflammatory response
Mice were perfused3, 5, 6, 12 with phosphate buffered saline and the hippocampus harvested 6 h after surgery. Samples were rapidly extracted and stored at −80°C until assayed. IL-6, IL-1β, and MCP-1 levels in the hippocampus were measured with ELISA assays and total protein concentration was assessed using the Pierce BCA Protein Assay Kit (Thermo Scientific, Rockford, IL, USA).
Permeability of the blood brain barrier
The brain was homogenized with RIPA Lysis Buffer (Cell Signaling Technology, Danvers, Massachusetts, USA) plus protease inhibitor (Halt Protease Inhibitor Single-Use Cocktail, Thermo Fisher Scientific, Waltham, Massachusetts, USA) and phenylmethanesulfonyl fluoride (Cell Signaling Technology) and sonicated. For immunoblotting, sample buffer was prepared by adding 950 μl of 2× Laemmli Sample Buffer to 50 μl of 2-mercaptoethanol (Bio-Rad, Hercules, California, USA), and mixed in a 1:1 ratio with samples. After boiling for 5 min, 20 μg of protein was subjected to 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and then transferred onto nitrocellulose transfer membranes. After the membranes were incubated with blocking buffer (LICOR®, BD Biosciences, San Jose California, USA) for 1 h at room temperature, these were incubated with rabbit antibody directed against murine albumin (ab207327, Abcam, Burlingame, California, USA) at 1:1000 dilution overnight at 4°C. For loading control, rabbit monoclonal antibody against murine Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (ab181602, Abcam) was used. After washing four times with Tris buffered saline (TBS) containing 0.1% Tween 20 (TBST), membranes were incubated with 1:10 000 IRDye 680RD or 800RD labelled goat anti-rabbit antibody (LICOR®) for 1 h at room temperature. Membranes were washed three times with TBST and once with TBS, and images were captured and quantified using a LI-COR Imager (LICOR®, Biosciences).
Hippocampal infiltration of CCR2+ cells and activation of microglia
Ccr2RFP/+ Cx3cr1GFP/+ mice were administered IL-6 50 mg/kg and 6 h later were sacrificed and perfused with saline followed by 4% paraformaldehyde in 100 mM phosphate buffer to assess infiltration of CCR2-expressing cells and activation of microglia as described.6
Interventions
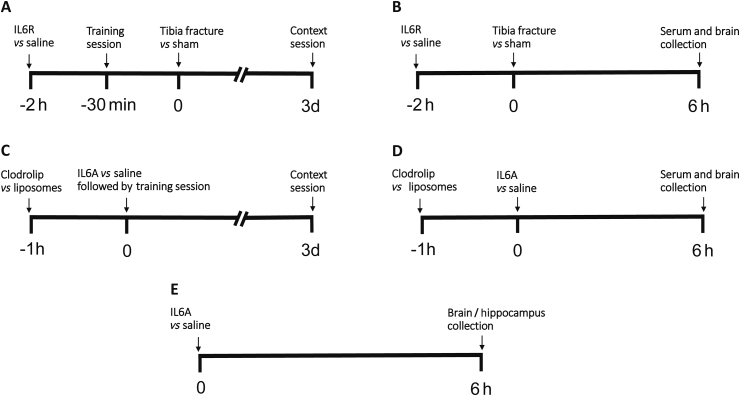
The experimental protocol is depicted in Fig. 1.
Fig 1.
Experimental protocol. (A) Four groups of randomly-assigned mice were treated with either saline or an intraperitoneal (i.p.) injection of tocilizumab, an antibody directed against the interleukin-6 (IL-6) receptor (IL6R) followed by tibial fracture or sham surgery 2 h later. Thirty min prior to tibia fracture/sham, the training session for trace fear conditioning (TFC) was performed and context testing was undertaken 72 h later. (B) Four groups of mice were prepared as for A. Six h after surgery or sham, mice were killed and blood and brain were harvested. (C) Mice were randomly assigned into four groups and treated with i.p. injection of clodrolip in a liposome emulsion or liposome emulsion only 1 h before TFC training session. This was followed by an i.p injection of IL-6 (IL6A) or saline control after training session and thereafter context testing was performed 72 h later. (D) Four groups of mice were prepared as for C. Six h after IL6A, mice were killed and blood and brain were harvested.
Depletion of bone marrow-derived monocytes
Clodrolip is a liposomal formulation of encapsulated clodronate (dichloromethylene bisphosphonic acid) that is ingested and digested by phagocytes, followed by intracellular release and accumulation of clodronate. Clodronate induces apoptosis of the phagocytes that includes bone marrow-derived monocytes (BM-DMs). Some 280 μl of clodrolip, 5 mg ml−1, (Liposoma BV, Amsterdam, the Netherlands) was injected intraperitoneally (i.p.) 60 min before administration of IL-6 50 mg/kg. Control animals received 280 μl of liposomal solution without the encapsulated clodronate.
Administration of IL-6 receptor blocker
Two h before surgery, the IL-6 receptor (IL6R) blocker tocilizumab (a gift from Genentech, South San Francisco CA, USA) 4 mg was administered i.p. Animals receiving the same volume of vehicle (saline) served as controls.
Administration of IL-6
IL6 50 μg kg−1 (Peprotech, Rocky Hill, New Jersey, USA) was administered i.p. Animals receiving the same volume of the vehicle (saline) served as controls.
Statistical analysis
GraphPad Prism version 5.01 (GraphPad Software, San Diego, CA, USA) statistical package and Stata 11.2 software (StataCorp, College Station, TX, USA) were used for statistical analysis. Results are expressed as mean (SD). Data were compared using analysis of variance whenever there was a possibility of interaction between independent treatment variables, and prior to performing individual pairwise comparisons by Newman-Keuls post hoc test wherever appropriate. In each case, P<0.05 was considered significant.
Results
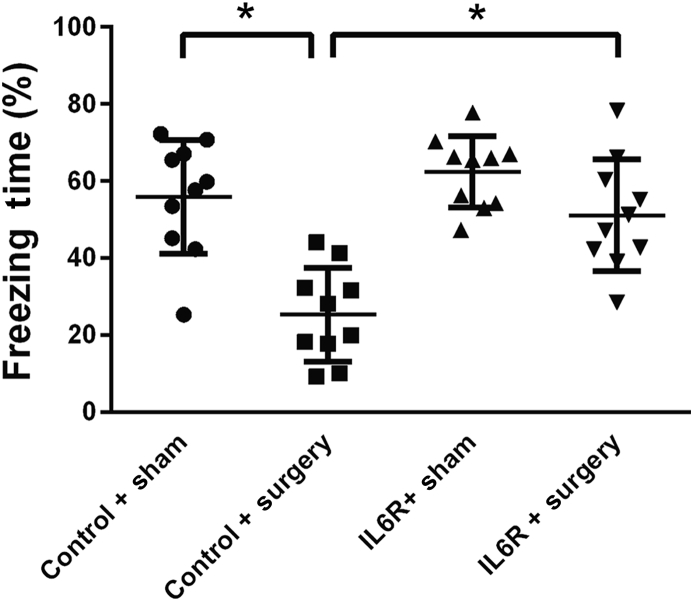
IL6R blocker can abolish cognitive decline induced by surgery
Cognitive decline was induced by surgery as evidenced by a significant reduction in freezing time [25 (12)% vs 56 (15)%, P<0.01; Fig. 2). Pre-emptive exposure to tocilizumab (IL6R antibody) attenuated surgery-induced freezing behaviour [51 (14)% vs 25 (12)%, P<0.01; Fig. 2), and the response to tocilizumab alone was not significantly different from that of the sham control group [62 (9)% vs 56 (15)%; Fig. 2).
Fig 2.
Pre-emptive blockade of interleukin-6 (IL-6) receptor (IL6R) prevents postoperative decrement in freezing behaviour. Four groups of randomly-assigned mice (n=10) were treated with either saline or an intraperitoneal injection of tocilizumab, an antibody directed against the IL-6 receptor (IL6R) followed by tibia fracture or sham surgery 2 h later. Thirty min prior to tibia fracture/sham, the training session for TFC was performed and context testing for freezing behaviour was undertaken 72 h later. Data are expressed as means (SEM) and compared by one-way analysis of variance and Student-Newman-Keuls test. *P<0.01.
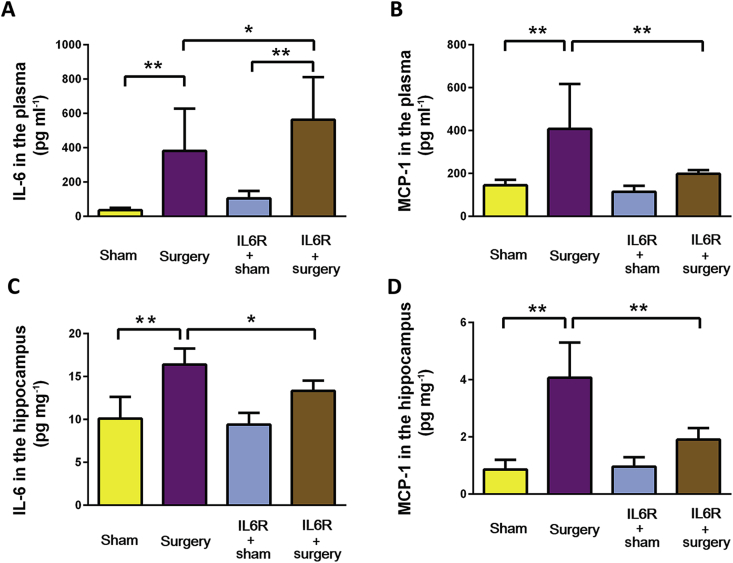
IL6R blocker reduces systemic and neuroinflammatory response to surgery
After surgery, there was a significant (P<0.01) increase of both plasma IL-6 (Fig. 3A) and MCP-1 (Fig. 3B) at 6 h. While IL6R blockade with tocilizumab prevented the surgery-induced increase in plasma MCP-1 (Fig. 3B), there was a further increase in circulating IL-6 (Fig. 3A). Surgery significantly (P<0.01) increased hippocampal IL-6 (Fig. 3C) and MCP-1 (Fig. 3D); both responses were prevented by IL6R blockade.
Fig 3.
IL-6 Receptor blocker reduces inflammatory response after surgery. Four groups of randomly-assigned mice (n=8) were treated with either saline or an intraperitoneal (i.p.) injection of tocilizumab, an antibody directed against the interleukin-6 (IL-6) receptor (IL6R) followed by tibia fracture or sham surgery 2 h later. Six h after surgery, mice were killed and blood and brain were harvested and assayed for circulating IL-6 (A), circulating MCP-1 (B), hippocampal IL-6 (C), and hippocampal monocyte chemoattractant protein-1 (MCP-1) (D). Data were analysed by one-way analysis of variance and Student-Newman-Keuls test. *P<0.05; **P<0.01.
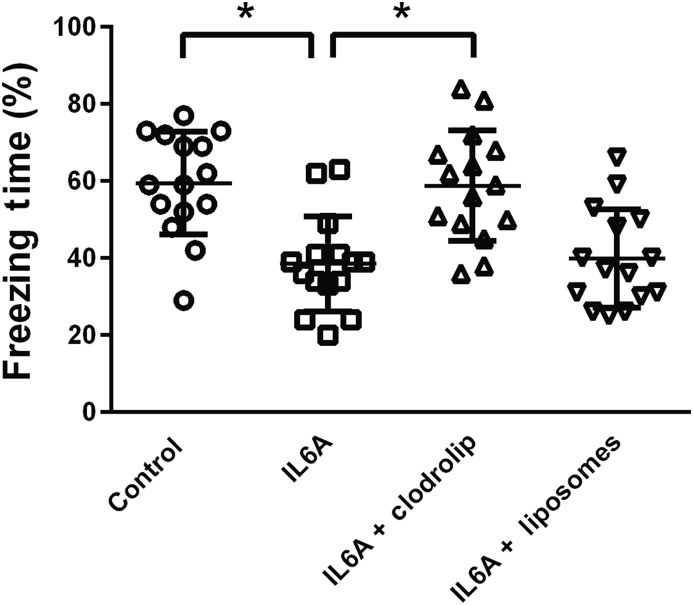
IL-6 causes cognitive decline through involvement of BM-DMs
At 3 days after IL-6 50 μg kg−1 there was a decrement in freezing behaviour [59 (13)% vs 39 (12)%; P<0.01) that was prevented by prior depletion of BM-DMs by clodrolip pretreatment; control liposomes alone had no reversal effect (Fig. 4).
Fig 4.
Depletion of bone marrow-derived monocytes prevents interleukin-6 (IL-6) induced decrement in freezing behaviour. Four groups each of randomly-assigned mice (n=15) were treated with either saline, IL-6 (IL6A), liposomes containing clodronate (clodrolip)+IL6A, or liposomes+IL6A. One h later the training session for trace fear conditioning (TFC) was performed and context testing for freezing behaviour was undertaken 72 h later. Data are expressed as means (SEM) and compared by one-way analysis of variance and Student-Newman-Keuls test. *P<0.01.
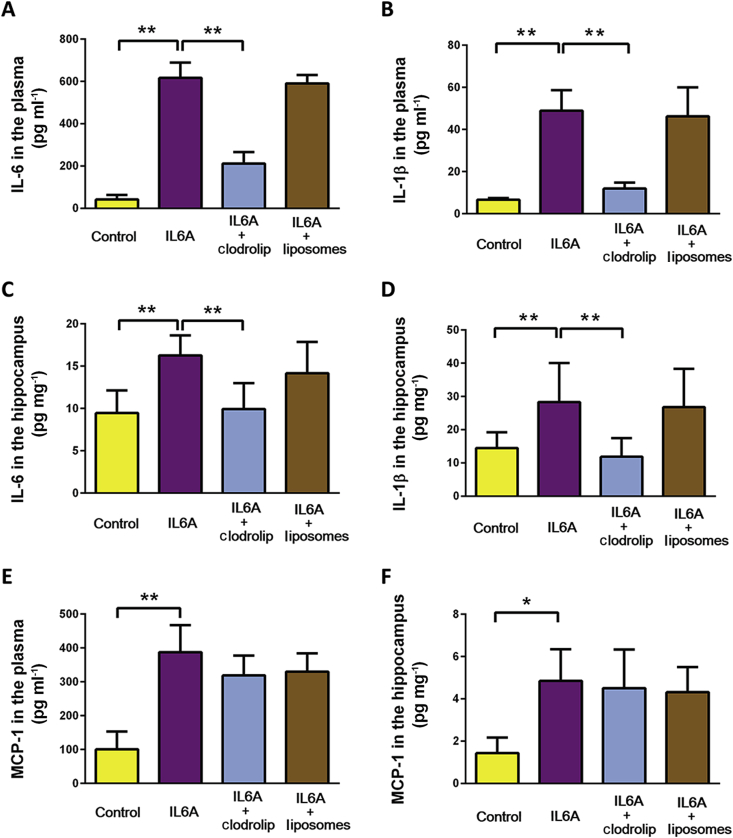
IL-6 produces systemic and hippocampal inflammation through involvement of BM-DMs
At 6 h after administration of 50 μg kg−1 IL-6, circulating IL-6 (Fig. 5A), circulating IL-1β (Fig. 5B), hippocampal IL-6 (Fig. 5C), and hippocampal IL-1β (Fig. 5C) were each significantly increased. In each case the IL-6-induced incremental change was precluded by depletion of BM-DMs with clodrolip. Interestingly, although both circulating (Fig. 5E) and hippocampal (Fig. 5F) MCP-1 were each increased by IL-6, the incremental change was not attenuated by clodrolip.
Fig 5.
Depletion of bone marrow-derived monocytes prevents interleukin-6 (IL-6) induced inflammatory response. Two groups of randomly-assigned mice (n=8) were treated with either saline or an intraperitoneal (i.p.) injection of IL-6 (IL6A). Two groups of randomly-assigned mice (n=8) were pre-treated with liposomes containing clodronate (clodrolip) or liposomes without clodronate followed by injection of IL-6 (IL6A) 1 h later. Six h after treatment, mice were killed and blood and brain were harvested and assayed for circulating IL-6 (A), circulating IL-1β (B), hippocampal IL-6 (C), and hippocampal IL-1β (D), circulating MCP-1 (E), and hippocampal monocyte chemoattractant protein-1 (MCP-1) (F). Data were analysed by one way analysis of variance followed by Student-Newman-Keuls test. *P<0.05; **P<0.01.
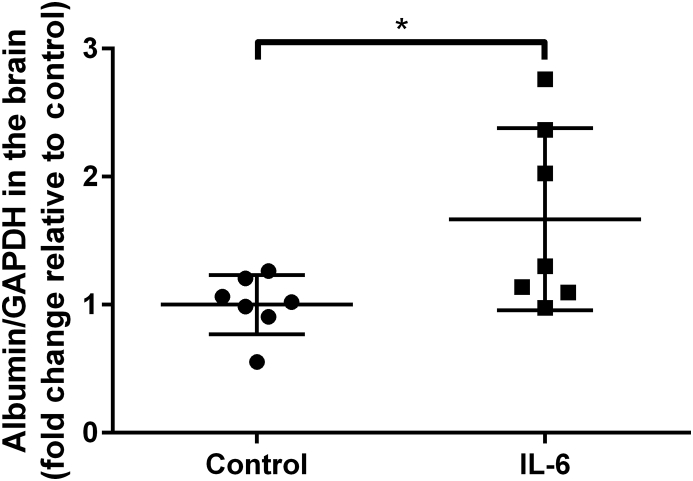
IL-6 disrupts the blood brain barrier
At 6 h after administration of 50 μg kg−1 IL-6, the amount of albumin that penetrated into the brain was significantly greater than in control mice (Fig. 6).
Fig 6.
Peripheral injection of interleukin-6 (IL-6) induces disruption of the blood brain barrier. Two groups of randomly-assigned mice (n=7) were treated with vehicle (control) or 50 μg kg−1 IL-6. Six h after treatment, mice were sacrificed and the brains were harvested for immunoblotting of albumin expression. Data are expressed as mean (SD) relative to control and were analysed by two way t-test. *P<0.05.
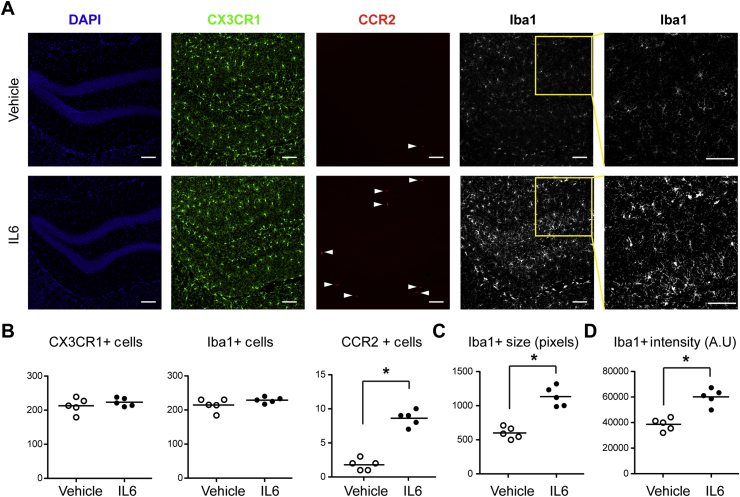
IL-6 promotes hippocampal infiltration of CCR2-expressing monocytes and activation of microglia
At 6 h after administration of 50 μg kg−1 IL-6, there was an increase in the number of CCR2-expressing BM-DMs with no change in the number of CX3CR1-expressing microglia (Fig. 7A and B). The number of large (Fig. 7C) and high intensity (Fig. 7D) Iba1-stained microglia, indicative of transition to an activated pro-inflammatory state, increased following IL-6 administration.
Fig 7.
Peripheral injection of interleukin-6 (IL-6) induces hippocampal microglia activation and recruitment of CCR2-expressing cells. (A) Representative immunofluorescence images taken from CX3CR1-GFP/+: CCR2-RFP/+ mice 6 h following intraperitoneal injection of 50 μg kg−1 IL-6, showing the matching of anatomical locations using nuclear counterstaining with 4-6-diamidino-2-phenylindole (DAPI), infiltration of CCR2-expressing cells (red), and increased Iba1 expression (grey) among hippocampal microglia in response to IL-6 injection (scale bars=50 μm). Iba1+ cells also exhibit a transition from a quiescent morphology to one indicative of inflammatory activation showing increased size and fluorescence intensity (inset). (B) Quantification of data from (A) showing CX3CR1+, CCR2+, and Iba1+ cell number. (C–D) Quantification of size (C), and fluorescence intensity (D) of Iba1- expressing cells in anatomically matched hippocampal sections from the same groups of mice represented in A (n=5). *P<0.05 vs vehicle.
Discussion
IL-6 is both necessary and sufficient to produce postoperative neurologic dysfunction
As DAMPs, cytokines, and chemokines are up-regulated by the aseptic trauma of surgery,3, 5, 6, 9, 12 we have addressed which are necessary and sufficient to produce the co-ordinated response that results in postoperative neurologic dysfunction (PND). We now report a requirement for the cytokine IL-6, because interruption of its signalling pathway through a neutralizing antibody to the IL6R (tocilizumab) prevents PND. Furthermore, since administration of IL-6 alone produces PND, this cytokine is sufficient to produce the post-surgical phenotype of cognitive decline. These data do not rule out that other molecules up-regulated following trauma might also be necessary and sufficient. In fact, we have demonstrated that the same is true for the DAMP high molecular group box protein 1 (HMGB1).3 Why are different molecules performing the same function? It is possible that the timing at which these are released (HMGB1 within 1 h and IL-6 from 6 h)3, 5, 12 results in a more concerted response. Furthermore, because the inflammatory response to surgery is so crucial, some redundancy might be required to ensure that these processes are always activated.
IL6R antibody decreases inflammatory response without changing circulating IL-6 expression
Because systemic and hippocampal inflammation are required for the development of PND and because tocilizumab prevents the development of PND, it was anticipated that IL6R blockade would dampen the inflammatory response to surgery. However, surgery-induced upregulation of circulating IL-6 levels was further enhanced by tocilizumab. A possible explanation is that an intervention that interferes with IL-6 signalling induces a feed-forward response to overcome the blockade. Another possibility is the cytokine release syndrome that has been observed clinically when certain types of immunotherapy are administered.19 However, there is preliminary evidence that tocilizumab may be used to ameliorate cytokine release syndrome induced by other biologics.20
Depletion of BM-DMs can prevent IL-6 induced PND and inflammation
We have reported a role for circulating BM-DMs in the development of trauma-induced21 and HMGB1-induced PND.3 It is relevant that elimination of these cells prevents IL-6 induced PND and inflammatory markers in the circulation and hippocampus. Yet MCP-1, the crucial chemokine that attracts BM-DMs into brain to propagate neuroinflammation, was not down-regulated by a perturbation that prevents hippocampal inflammation. This observation is consistent with our previous report that the up-regulated MCP-1 levels that are evident after surgery9 are unaltered by depletion of BM-DMs in an HMGB1-induced surgical phenotype.3 Therefore, these data provide further evidence that MCP-1 does not originate from BM-DMs. Our recent description of the interaction between BM-DMs and microglia raises the possibility that MCP-1 originates from microglia, although the signal that initiates this remains elusive.6
IL-6 promotes hippocampal activation of microglia and infiltration of CCR2+ BM-DMs
Previously, we reported that peripheral surgery disrupts the blood brain barrier and promotes infiltration of CCR2-expressing BM-DMs5 and activation of microglia.6 Now we demonstrate that these crucial steps necessary for PND can be mediated by exogenously administered IL-6.
Limitations
Tocilizumab is a humanized anti-IL6R monoclonal antibody with cross-reactivity against the murine IL6R with no untoward effects reported. IL-6 is an animal-free recombinant protein produced in Escherichia coli. Notwithstanding their longstanding use, these reagents could have hitherto unknown off-target effects giving rise to spurious findings.
IL-6 signalling pathways
Transmembrane signal transduction of IL-6 can be propagated by at least two discrete pathways. In classic signalling, IL-6 stimulates a limited number of target cells22 including hepatocytes, neutrophils, and CD4+ T-cells23, 24 that express membrane-bound IL6R (mIL6R), which, following ligand binding, dimerizes with the signalling receptor protein gp130 leading to regenerative and protective effects. Most cells express gp130 and not mIL6R, and are therefore unresponsive to IL-6 alone. Circulating soluble IL6R (sIL6R) constitutes an alternative binding target for IL-6. The resulting IL-6/sIL6R complex binds to gp130 to initiate the ‘trans-signalling’ pathway resulting in pro-inflammatory effects.25, 26 Neurones and astrocytes respond to the IL-6/sIL6R complex but not to IL-6 alone, suggesting that trans-signalling is crucial for mediating the pro-inflammatory effects of IL-6 on these cells.27, 28, 29 In contrast, microglia respond well to IL-6 alone via classic signaling.30 As tocilizumab blocks the effects of both mIL6R and sIL6R31 we can only speculate that it is blockade of sIL6R, and hence the trans-signalling pathway, that results in the ameliorative effects of tocilizumab on PND.
Future studies
Tocilizumab is clinically available and has been used in a variety of inflammation-related diseases, yet its possible future application in the perioperative setting to prevent PND needs to await further preclinical investigations. Firstly, it will be important to establish whether tocilizumab is effective when administered after the initiation of the injury. If not, then its pre-emptive utility will be limited to surgical patients whose high risk for PND is established by their underlying immunological phenotype. Secondly, like all biologics, it needs to be established whether perioperative use of tocilizumab increases the risk for acquiring and responding appropriately to infections. Finally, inflammation is a sine qua non for healing. Therefore, the effects of tocilizumab on surgical wound healing will need to be investigated.
Conclusions
IL-6 is necessary and sufficient to produce postoperative neurological dysfunction in mice. Even though tocilizumab is licensed for indications such as rheumatoid arthritis and giant cell arteritis, the clinical application of strategies designed to disable IL6R signalling must await further preclinical efficacy and safety testing.
Authors' contributions
Performed the bulk of the experimental procedures assays and analysis of data: J.H., X.F.
Performed some of the experimental procedures and assays: D.L., Y.U., M.V., S.K.K.
Acquired the funding, conceptualized and designed the study, and wrote the manuscript: M.M.
Acknowledgements
The authors thank Genentech for the generous gift of tocilizumab, and Dr. Israel Charo for providing the Ccr2RFP/+ Cx3cr1GFP/+ mice. The authors wish to thank Mr. Leng Lai for help with figures and Mr. Christopher Chang for help with preparation of the manuscript.
Editorial decision: November 5, 2017
Handling editor: H.C. Hemmings Jr
Footnotes
This article is accompanied by an editorial: One to rule them all? by Terrando & Eckenhoff, Br J Anesth 2018:120:428–430, doi: 10.1016/j.bja.2017.12.006
Declaration of interest
None declared.
Funding
This work was supported by a grant from the United States National Institutes of Health to M.M. (R01GM104194).
References
- 1.Crocker E., Beggs T., Hassan A. Long-term effects of postoperative delirium in patients undergoing cardiac operation: a systematic review. Ann Thorac Surg. 2016;102:1391–1399. doi: 10.1016/j.athoracsur.2016.04.071. [DOI] [PubMed] [Google Scholar]
- 2.Steinmetz J., Christensen K.B., Lund T., Lohse N., Rasmussen L.S., Group I. Long-term consequences of postoperative cognitive dysfunction. Anesthesiology. 2009;110:548–555. doi: 10.1097/ALN.0b013e318195b569. [DOI] [PubMed] [Google Scholar]
- 3.Vacas S., Degos V., Tracey K.J., Maze M. High-mobility group box 1 protein initiates postoperative cognitive decline by engaging bone marrow-derived macrophages. Anesthesiology. 2014;120:1160–1167. doi: 10.1097/ALN.0000000000000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chavan S.S., Pavlov V.A., Tracey K.J. Mechanisms and therapeutic relevance of neuro-immune communication. Immunity. 2017;46:927–942. doi: 10.1016/j.immuni.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Terrando N., Eriksson L.I., Ryu J.K. Resolving postoperative neuroinflammation and cognitive decline. Ann Neurol. 2011;70:986–995. doi: 10.1002/ana.22664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feng X., Valdearcos M., Uchida Y., Lutrin D., Maze M., Koliwad S.K. Microglia mediate postoperative hippocampal inflammation and cognitive decline in mice. JCI Insight. 2017;2 doi: 10.1172/jci.insight.91229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Terrando N., Gomez-Galan M., Yang T. Aspirin-triggered resolvin D1 prevents surgery-induced cognitive decline. FASEB J. 2013;27:3564–3571. doi: 10.1096/fj.13-230276. [DOI] [PubMed] [Google Scholar]
- 8.Kukushkin N.V., Carew T.J. Memory takes time. Neuron. 2017;95:259–279. doi: 10.1016/j.neuron.2017.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirsch J., Vacas S., Terrando N. Perioperative cerebrospinal fluid and plasma inflammatory markers after orthopedic surgery. J Neuroinflammation. 2016;13:211. doi: 10.1186/s12974-016-0681-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forsberg A., Cervenka S., Jonsson Fagerlund M. The immune response of the human brain to abdominal surgery. Ann Neurol. 2017;81:572–582. doi: 10.1002/ana.24909. [DOI] [PubMed] [Google Scholar]
- 11.Cape E., Hall R.J., van Munster B.C. Cerebrospinal fluid markers of neuroinflammation in delirium: a role for interleukin-1beta in delirium after hip fracture. J Psychosom Res. 2014;77:219–225. doi: 10.1016/j.jpsychores.2014.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cibelli M., Fidalgo A.R., Terrando N. Role of interleukin-1beta in postoperative cognitive dysfunction. Ann Neurol. 2010;68:360–368. doi: 10.1002/ana.22082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mooijaart S.P., Sattar N., Trompet S. Circulating interleukin-6 concentration and cognitive decline in old age: the PROSPER study. J Intern Med. 2013;274:77–85. doi: 10.1111/joim.12052. [DOI] [PubMed] [Google Scholar]
- 14.Balschun D., Wetzel W., Del Rey A. Interleukin-6: a cytokine to forget. FASEB J. 2004;18:1788–1790. doi: 10.1096/fj.04-1625fje. [DOI] [PubMed] [Google Scholar]
- 15.Hudetz J.A., Gandhi S.D., Iqbal Z., Patterson K.M., Pagel P.S. Elevated postoperative inflammatory biomarkers are associated with short- and medium-term cognitive dysfunction after coronary artery surgery. J Anesth. 2011;25:1–9. doi: 10.1007/s00540-010-1042-y. [DOI] [PubMed] [Google Scholar]
- 16.Li A.J., Katafuchi T., Oda S., Hori T., Oomura Y. Interleukin-6 inhibits long-term potentiation in rat hippocampal slices. Brain Res. 1997;748:30–38. doi: 10.1016/s0006-8993(96)01283-8. [DOI] [PubMed] [Google Scholar]
- 17.Bluthe R.M., Michaud B., Poli V., Dantzer R. Role of IL-6 in cytokine-induced sickness behavior: a study with IL-6 deficient mice. Physiol Behav. 2000;70:367–373. doi: 10.1016/s0031-9384(00)00269-9. [DOI] [PubMed] [Google Scholar]
- 18.Dong Y., Xu Z., Huang L., Zhang Y., Xie Z. Peripheral surgical wounding may induce cognitive impairment through interleukin-6-dependent mechanisms in aged mice. Med Gas Res. 2016;6:180–186. doi: 10.4103/2045-9912.196899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee D.W., Gardner R., Porter D.L. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124:188–195. doi: 10.1182/blood-2014-05-552729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanaka T., Narazaki M., Kishimoto T. Immunotherapeutic implications of IL-6 blockade for cytokine storm. Immunotherapy. 2016;8:959–970. doi: 10.2217/imt-2016-0020. [DOI] [PubMed] [Google Scholar]
- 21.Degos V., Vacas S., Han Z. Depletion of bone marrow-derived macrophages perturbs the innate immune response to surgery and reduces postoperative memory dysfunction. Anesthesiology. 2013;118:527–536. doi: 10.1097/ALN.0b013e3182834d94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rose-John S., Scheller J., Elson G., Jones S.A. Interleukin-6 biology is coordinated by membrane-bound and soluble receptors: role in inflammation and cancer. J Leukoc Biol. 2006;80:227–236. doi: 10.1189/jlb.1105674. [DOI] [PubMed] [Google Scholar]
- 23.Gauldie J., Richards C., Harnish D., Lansdorp P., Baumann H. Interferon beta 2/B-cell stimulatory factor type 2 shares identity with monocyte-derived hepatocyte-stimulating factor and regulates the major acute phase protein response in liver cells. Proc Natl Acad Sci U S A. 1987;84:7251–7255. doi: 10.1073/pnas.84.20.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oberg H.H., Wesch D., Grussel S., Rose-John S., Kabelitz D. Differential expression of CD126 and CD130 mediates different STAT-3 phosphorylation in CD4+CD25- and CD25high regulatory T cells. Int Immunol. 2006;18:555–563. doi: 10.1093/intimm/dxh396. [DOI] [PubMed] [Google Scholar]
- 25.Scheller J., Chalaris A., Schmidt-Arras D., Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011;1813:878–888. doi: 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 26.Jones S.A., Scheller J., Rose-John S. Therapeutic strategies for the clinical blockade of IL-6/gp130 signaling. J Clin Investig. 2011;121:3375–3383. doi: 10.1172/JCI57158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marz P., Herget T., Lang E., Otten U., Rose-John S. Activation of gp130 by IL-6/soluble IL-6 receptor induces neuronal differentiation. Eur J Neurosci. 1997;9:2765–2773. doi: 10.1111/j.1460-9568.1997.tb01705.x. [DOI] [PubMed] [Google Scholar]
- 28.Marz P., Heese K., Dimitriades-Schmutz B., Rose-John S., Otten U. Role of interleukin-6 and soluble IL-6 receptor in region-specific induction of astrocytic differentiation and neurotrophin expression. Glia. 1999;26:191–200. doi: 10.1002/(sici)1098-1136(199905)26:3<191::aid-glia1>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 29.Van Wagoner N.J., Oh J.W., Repovic P., Benveniste E.N. Interleukin-6 (IL-6) production by astrocytes: autocrine regulation by IL-6 and the soluble IL-6 receptor. J Neurosci. 1999;19:5236–5244. doi: 10.1523/JNEUROSCI.19-13-05236.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin H.W., Levison S.W. Context-dependent IL-6 potentiation of interferon- gamma-induced IL-12 secretion and CD40 expression in murine microglia. J Neurochem. 2009;111:808–818. doi: 10.1111/j.1471-4159.2009.06366.x. [DOI] [PubMed] [Google Scholar]
- 31.Mihara M., Kasutani K., Okazaki M. Tocilizumab inhibits signal transduction mediated by both mIL-6R and sIL-6R, but not by the receptors of other members of IL-6 cytokine family. Int Immunopharmacol. 2005;5:1731–1740. doi: 10.1016/j.intimp.2005.05.010. [DOI] [PubMed] [Google Scholar]