Abstract
Background
Neurocysticercosis is a parasitic infection of the central nervous system by the larval stage of the pork tapeworm and is a common cause of seizures and epilepsy in endemic areas. Anthelmintics (albendazole or praziquantel) may be given alongside supportive treatment (antiepileptics/analgesia) with the aim of killing these larvae (cysticerci), with or without corticosteroid treatment. However, there are potential adverse effects of these drugs, and the cysticerci may eventually die without directed anthelminthic treatment.
Objectives
To assess the effects of anthelmintics on people with neurocysticercosis.
Search methods
We searched the Cochrane Infectious Diseases Group Specialized Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, LILACS, the WHO ICTRP, and ClinicalTrials.gov, up to 21 October 2020.
Selection criteria
Randomized controlled trials comparing anthelmintics and supportive treatment (+/‐ corticosteroids) with supportive treatment alone (+/‐ corticosteroids) for people with neurocysticercosis.
Data collection and analysis
Two review authors independently screened the title and abstract of all articles identified by the search. We obtained full‐text articles to confirm the eligibility of all studies that passed screening. One review author extracted data, which a second review author checked. Two review authors assessed the risk of bias of each trial and performed GRADE assessments. In cases of disagreement at consensus discussion stage between review authors, we consulted a third review author. We calculated risk ratios (RR) for dichotomous variables, with 95% confidence intervals (CIs) for pooled data from studies with similar interventions and outcomes.
Main results
We included 16 studies in the review. Only two studies investigated praziquantel and did not report data in a format that could contribute to meta‐analysis. Most results in this review are therefore applicable to albendazole versus placebo or no anthelmintic.
The aggregate analysis across all participants with neurocysticercosis did not demonstrate a difference between groups in seizure recurrence, but heterogeneity was marked (RR 0.94, 95% CI 0.78 to 1.14; 10 trials, 1054 participants; I2 = 67%; low‐certainty evidence). When stratified by participants with a single cyst or multiple cysts, pooled analysis suggests that albendazole probably improves seizure recurrence for participants with a single cyst (RR 0.61, 95% CI 0.4 to 0.91; 5 trials, 396 participants; moderate‐certainty evidence). All studies contributing to this analysis recruited participants with non‐viable, intraparenchymal cysts only, and most participants were children. We are uncertain whether or not albendazole reduces seizure recurrence in participants with multiple cysts, as the certainty of the evidence is very low, although the direction of effect is towards albendazole causing harm (RR 2.05, 95% CI 1.28 to 3.31; 2 trials, 321 participants; very low‐certainty evidence). This analysis included a large study containing a highly heterogeneous population that received an assessment of unclear risk for multiple 'Risk of bias' domains.
Regarding radiological outcomes, albendazole probably slightly improves the complete radiological clearance of lesions (RR 1.22, 95% CI 1.07 to 1.39; 13 trials, 1324 participants; moderate‐certainty evidence) and the evolution of cysts (RR 1.27, 95% CI 1.10 to 1.47; 6 trials, 434 participants; moderate‐certainty evidence).
More adverse events appeared to be observed in participants treated with either albendazole or praziquantel compared to those receiving placebo or no anthelmintic. The most commonly reported side effects were headache, abdominal pain, and nausea/vomiting.
Authors' conclusions
For participants with a single cyst, there was less seizure recurrence in the albendazole group compared to the placebo/no anthelmintic group. The studies contributing to this evidence only recruited participants with a non‐viable intraparenchymal cyst. We are uncertain whether albendazole reduces seizure recurrence for participants with multiple cysts. We also found that albendazole probably increases radiological clearance and evolution of lesions. There were very few studies reporting praziquantel outcomes, and these findings apply to albendazole only.
Plain language summary
Anthelmintics for people with neurocysticercosis
What is the aim of this review?
The aim of this review was to explore whether treatment with anthelmintics (drugs that kill worms) can benefit people with neurocysticercosis (an infection of the brain caused by the pork tapeworm). The primary outcome of the review was the impact of treatment on seizures (epilepsy). We collected and analysed all relevant studies (trials) to answer this question and found 16 studies. The most commonly reported outcomes were those relating to seizures and also the number and appearance of lesions caused by viable or degrading cysts (dormant worms) on brain imaging.
Key messages
We found that the anthelmintic albendazole probably reduces the recurrence of seizures in people with neurocysticercosis with a single cyst (moderate‐certainty evidence). We are uncertain whether albendazole reduces seizure recurrence for people with neurocysticercosis with more than one cyst (very low‐certainty evidence). We found little information regarding another anthelmintic drug, praziquantel; therefore these results are applicable to albendazole only. Albendazole treatment also probably increases the clearance and evolution of cysts in people with neurocysticercosis (moderate‐certainty evidence). Evolution of a cyst is progression to a later cyst stage, which is thought to be an improvement towards clearance.
What was studied in the review?
Neurocysticercosis is an infection of the brain with the pork tapeworm Taenia solium, which is caused by eating food or drinking water contaminated with the eggs of the worm. The eggs can travel from the gut to the brain, forming cysts in the brain that can cause various symptoms, the most common of which is seizures/epilepsy. Neurocysticercosis is found mainly in areas where people keep pigs and have poor sanitation facilities, and is a common cause of seizures in areas where it is prevalent.
People with neurocysticercosis may have single or multiple cysts, and their symptoms depend on the position and numbers of these cysts within the brain. Each cyst goes through the natural process of being alive and dormant (viable), degrading (non‐viable), and then it resolves or calcifies. This process can take many years. The number, type, and position of the cysts can be seen on brain imaging (lesions).
Two anthelmintics (drugs to treat worm infections), albendazole and praziquantel, are often used to treat neurocysticercosis. However, it is uncertain whether they reduce or stop seizures and other symptoms, or make them worse. In theory, the body's immune response to cysts dying as a result of treatment could cause more swelling and damage to the brain.
What are the main results of the review?
We included 16 studies in the review. These studies compared treatment with an anthelmintic versus placebo (a mock tablet/pill resembling the anthelmintic) or no anthelmintic treatment in adults or children with neurocysticercosis diagnosed by brain imaging.
For people with a single cyst, treatment with albendazole probably reduces seizure recurrence (moderate‐certainty evidence). Notably, all studies that contributed to this analysis only included people with non‐viable cysts. For people with multiple cysts, the evidence was of very low certainty, therefore we are uncertain whether or not albendazole reduces seizure recurrence for this group of patients. The studies contributing to this finding included participants with cysts that were both viable and non‐viable. We found very little information regarding praziquantel, therefore these results are apply to albendazole only.
Treatment with albendazole probably increases complete clearance of lesions on brain imaging as well as the evolution of cysts (from viable to non‐viable to resolved or calcified) (moderate‐certainty evidence). The studies contributing to this evidence included people with single and multiple cysts, both viable and non‐viable.
More side effects were reported by participants treated with either albendazole or praziquantel compared to those receiving placebo or no anthelmintic. The most commonly reported side effects were headache, abdominal pain, and nausea/vomiting.
How up‐to‐date is this review?
We searched for studies that had been published up to 21 October 2020.
Summary of findings
Summary of findings 1. Albendazole compared with placebo or no anthelmintics for people with neurocysticercosis.
| Albendazole compared with placebo or no anthelmintics for people with neurocysticercosis | ||||||
|
Population: people with neurocysticercosis Settings: any healthcare setting Intervention: albendazole Comparison: placebo or no anthelmintics | ||||||
| Outcomes | Illustrative comparative risks (95% CI)* | Relative effect (95% CI) | Number of participants | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo or no anthelmintic | Albendazole | |||||
| Seizure recurrence ‐ all participants | 28 per 100 |
26 per 100 (22 to 32) |
RR 0.94 (0.78 to 1.14) |
1054 (10 RCTs) |
⊕⊕⊝⊝
Low1,2,3 Due to risk of bias and inconsistency |
Albendazole probably makes little or no difference to seizure recurrence overall (all participants). |
| Seizure recurrence ‐ participants with a single cyst | 23 per 100 |
14 per 100 (9 to 21) |
RR 0.61 (0.40 to 0.91) |
396 (5 RCTs) |
⊕⊕⊕⊝
Moderate4,5 Due to imprecision |
Albendazole probably improves seizure recurrence in participants with a single cyst. |
| Seizure recurrence ‐ participants with multiple (> 1) cysts | 21 per 100 |
43 per 100 (27 to 70) |
RR 2.05 (1.28 to 3.31) |
321 (2 RCTs) |
⊕⊝⊝⊝
Very low6,7,8,9 Due to risk of bias, inconsistency, and indirectness |
We are uncertain whether albendazole reduces seizure recurrence in participants with multiple cysts. |
| Complete radiological clearance of lesions | 34 per 100 |
41 per 100 (36 to 47) |
RR 1.22 (1.07 to 1.39) |
1324 (13 RCTs) |
⊕⊕⊕⊝
Moderate10,11 Due to inconsistency |
Albendazole probably slightly improves the complete radiological clearance of lesions overall (all participants). |
| Evolution of cysts | 64 per 100 |
81 per 100 (70 to 94) |
RR 1.27 (1.10 to 1.47) |
434 (6 RCTs) |
⊕⊕⊕⊝
Moderate12,13 Due to imprecision |
Albendazole probably slightly improves the evolution of cysts overall (all participants). |
| *The assumed risk is from the median control group risk across studies. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval, RCT: randomized controlled trial, RR: risk ratio | ||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded by one level for risk of bias: two studies have 'Risk of bias' domains assessed as high risk (Kalra 2003; Khurana 2012), and Das 2007 is at unclear risk of bias for random sequence generation, allocation concealment, and blinding. Given the findings of Das 2007 and the number of participants, risk of bias would likely seriously alter the results of this outcome. 2Downgraded by one level for inconsistency: substantial heterogeneity and clear heterogeneity in trial design for recruitment. 3Not downgraded for indirectness (all studies include clinical criteria to ensure new‐onset seizures in the context of radiological +/‐ serological neurocysticercosis diagnosis), imprecision (CI does not include RR values that would be considered appreciable benefit or harm), or publication bias. 4Downgraded by one level for imprecision: fewer than 300 events, and CI upper limit includes values that would be considered of little effect. 5Not downgraded for risk of bias (most data are from studies with low risk of bias, and any risk of bias identified is unlikely to alter the results of this outcome), inconsistency (low heterogeneity, and all studies recruited participants with non‐viable intraparenchymal cysts only), indirectness (all studies include clinical criteria to ensure new‐onset seizures in the context of radiological +/‐ serological neurocysticercosis diagnosis), or publication bias. 6Downgraded by one level for risk of bias: Das 2007 is at unclear risk of bias for random sequence generation, allocation concealment, and blinding. Given the findings of Das 2007 and the number of participants, risk of bias would likely seriously alter the results of this outcome. 7Downgraded by one level for inconsistency: substantial heterogeneity and clear heterogeneity between participants. 8Downgraded by one level for indirectness: participants receiving albendazole in Das 2007 also received corticosteroids, whereas participants that did not receive albendazole did not. Given that Das 2007 is the largest trial contributing to this outcome, the pooled results are less directly relevant to this review. 9Not downgraded for imprecision (CI lower limit does not include RR values that would be considered of little or no harm, and RR increase lower limit is greater than 25%) or publication bias. 10Downgraded by one level for inconsistency: substantial heterogeneity. 11Not downgraded for risk of bias (most data are from studies with low risk of bias, and the results are unchanged if studies assessed as having a high risk of bias for any 'Risk of bias' domain, Kalra 2003; Khurana 2012; Singhi 2000; Sotelo 1988, are excluded), indirectness (all studies include clinical criteria to ensure new‐onset seizures in the context of radiological +/‐ serological neurocysticercosis diagnosis), imprecision (over 300 events and large number of participants), or publication bias. 12Downgraded by one level for imprecision: fewer than 300 events, and CI lower limit includes values that would be considered of little effect (RR increase < 25%). 13Not downgraded for risk of bias (most data are from studies with low risk of bias, and any risk of bias identified is unlikely to alter the results of this outcome), inconsistency (very low heterogeneity, and most studies recruited participants with non‐viable intraparenchymal cysts only), indirectness (all studies include clinical criteria to ensure new‐onset seizures in the context of radiological +/‐ serological neurocysticercosis diagnosis), or publication bias.
Background
Description of the condition
Neurocysticercosis is an infection of the central nervous system (CNS) by the cystic larval stage (cysticercus) of the pork tapeworm Taenia solium. The natural life cycle of T solium is for a human host infected with the adult intestinal worm to shed eggs (ova), which can survive for several months before ingestion, or egg‐filled motile worm segments (gravid proglottids) into the environment. These are subsequently ingested by pigs through contamination of their diet. The ova hatch within the porcine gastrointestinal tract to become oncospheres, invade the mucosa to gain access to the bloodstream, migrate to various tissues and encyst to become cysticerci. When a human ingests encysted pork, the cysticerci attach to the intestinal mucosa and mature into adult tapeworms, completing the life cycle. Human neurocysticercosis is an aberration of this life cycle and occurs when a human (rather than a pig) ingests the ova of T solium. Cysticerci (cysts) form within various human tissues (cysticercosis), much the same as within the porcine host. This review is confined to the treatment of neurocysticercosis, where cysticerci form within the CNS of a human host.
Neurocysticercosis is found where people live in close contact with pigs and where sanitation is poor, allowing for a natural life cycle of T solium to establish. Cases cluster around individuals with taeniasis secondary to T solium, suggesting a larger proportion of human‐to‐human infections via faecal‐oral transmission rather than through environmental contamination (Lescano 2009). It is common in much of South and Central America, China, the Indian subcontinent, South‐East Asia, and sub‐Saharan Africa, and is estimated cause to epilepsy (presenting as recurrent seizures) in over 1% of the population in endemic settings (Coyle 2012; Savioli 2010). It is the leading cause of adult‐onset epilepsy worldwide, and is estimated to cause at least 50,000 deaths each year (Roman 2000). Neurocysticercosis is therefore a significant public health problem, burdening not only healthcare systems with significant costs, but also households and communities with lost productivity through morbidity and mortality.
The cysts in neurocysticercosis naturally evolve over a period of years, beginning with viable encysted larvae and ending with the death of the parasite and either resorption (clearance) or calcification of the cyst. Individuals may have one or more cysticerci in the brain, and multiple cysts can represent one point of contact or cumulative exposure. Depending on the site of the cysts, neurocysticercosis can be intraparenchymal (most frequently between white/grey matter boundary) or extraparenchymal. Extraparenchymal disease can be further subdivided: intraventricular (most frequently in the fourth ventricle); racemose (a severe variant with large, multi‐lobar cysts in the subarachnoid space, often complicated by basal cisterns or Sylvian fissure involvement); and spinal (an extracranial manifestation of subarachnoid disease). Symptoms may or may not occur, depending on the number, location, and stage of the cysts, as well as the extent of the infected individual's immune response.
Although there is a wide spectrum of clinical manifestations of neurocysticercosis, seizures are the most common presentation, followed by headaches, focal neurological deficit, and signs of raised intracranial pressure (Carabin 2011). Intraparenchymal neurocysticercosis has a much more favourable prognosis than extraparenchymal; intraparenchymal disease has a very low mortality, usually presenting as seizures that respond well to antiepileptic medication, whereas extraparenchymal disease has a significant rate of mortality in endemic areas without the availability of optimal surgical treatment (Degiorgio 2002).
Cysts within the CNS can be visualized using computed tomography (CT) or magnetic resonance imaging (MRI); these radiological findings underpin the diagnostic criteria required for definitive diagnosis (Del Brutto 2017). Over the course of an infection, radiological images of a lesion change from 'non‐enhancing' (after intravenous injection of a radiographic contrast), indicating a viable cyst with little or no associated inflammation, to 'ring‐enhancing', indicating a degenerating (non‐viable) cyst with surrounding infiltration secondary to the host's immune response. These cysts can either progress to resolution or calcification (Degiorgio 2004). Symptoms can develop at any point during the course of an infection and can be associated not only with the structural and functional abnormality caused by the cyst and surrounding inflammation, but also the calcification that remains after a cyst has been eliminated (Leite 2000). Infection burden varies widely, and is cumulative with repeat exposure (Garcia 2002).
Description of the intervention
Treatment options depend on the number, size, and location of cysts and the individual's symptoms. Initial symptomatic treatment includes antiepileptic medication for seizures and analgesia for headache. Some extraparenchymal cysts are treated with surgery, either to remove the cyst or to relieve intracranial pressure. Where significant inflammation of the brain is present (usually associated with cyst degeneration), corticosteroids may be administered. Two anthelmintic medications are used for the management of neurocysticercosis: praziquantel and albendazole, available since 1979 and 1987, respectively. Anthelmintics were previously considered only for people with viable cysts, with the aim of killing the live parasites. More recently, however, guidelines authored by expert panels present a stronger recommendation for using anthelmintics for both viable and non‐viable cysts, unless diffuse cerebral oedema or hydrocephalus are present (White 2018). If anthelmintics are used, corticosteroids are often prescribed to prevent inflammation of the brain caused by the host immune response to the co‐ordinated death of multiple parasites.
Why it is important to do this review
The original version of this Cochrane Review found no evidence that the potential benefits of treatment outweigh the potential harms (Salinas 1999). An update in 2010 found that albendazole treatment at a standard dose in children with small numbers of non‐viable intraparenchymal cysts may reduce the risk of recurrence between six to 18 months. In studies recruiting adults with viable cysts, the results suggested that albendazole may increase radiological clearance (Abba 2010).
This latest review update was undertaken as a substantive update of the 2010 Cochrane Review (Abba 2010) to take studies published since 2010 into account. Compared to previous versions (Abba 2010; Salinas 1999), this review focused more on the core question of whether anthelmintics are of benefit or harm in the management of neurocysticercosis compared to no anthelmintic, as there was insufficient evidence in previous versions of the review to answer this question. Comparisons between anthelmintic regimens were not made, and the inclusion criteria were updated to reflect this change.
We considered stratification of participants by age (children, defined as under 16 years of age, and adults), by whether participants had viable or non‐viable cysts (given the natural history and assumptions about when anthelmintics may or may not be effective), by the intraparenchymal/extraparenchymal anatomical site of the cysts, and by number of cysts (single or multiple) for each comparison, presenting the stratified data if the result added to the overall outcome finding.
Objectives
To assess the effects of anthelmintics on people with neurocysticercosis.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials.
Types of participants
People with symptomatic or asymptomatic neurocysticercosis defined by viable or non‐viable cysts in the brain, identified as 'non‐enhancing' or 'enhancing'/'ring‐enhancing' lesions on medical imaging, respectively.
Types of interventions
Intervention
Anthelmintics plus supportive treatment (antiepileptics/analgesia)
Anthelmintics plus corticosteroids plus supportive treatment
Control
Supportive treatment only
Corticosteroids plus supportive treatment
We included trials irrespective of the type of anthelmintic used, or the dosage and duration of treatment.
Types of outcome measures
Primary outcomes
Seizure status at follow‐up
Seizure recurrence
Seizure recurrence after withdrawal of antiepileptics
Time to seizure remission*
Frequency of seizures (< 1/1 to 3/4+ per month)
*Defined as being free from seizures for the previous 12 months or for the duration of follow‐up (if follow‐up was less than 12 months).
Secondary outcomes
Secondary health status indicators at follow‐up
Death (any cause)
Hospital admission (any cause)
Headache
Signs of focal neurological deficit (e.g. paralysis, visual disturbance)
Surgical intervention
Resolution of symptoms
Resumption of normal activities
Radiological findings at follow‐up
Complete radiological clearance of lesions
Reduction of number of lesions
Evolution of cysts (viable to non‐viable/resolved/calcified or non‐viable to resolved/calcified)
Radiological resolution/development of oedema
Radiological resolution/development of raised intracranial pressure
Adverse events associated with treatment (side effects) at follow‐up
Frequency and nature of adverse events
Adverse event requiring withdrawal of anthelmintics
Search methods for identification of studies
We aimed to identify all relevant studies regardless of language or publication status (published or unpublished, in press, or in progress).
Electronic searches
We searched the following databases using the search terms and strategy described in Appendix 1 up to 21 October 2020: Cochrane Infectious Diseases Group Specialized Register; the Cochrane Central Register of Controlled Trials (CENTRAL), published in the Cochrane Library, Issue 10 of 12, October 2020; MEDLINE (PubMed, from 1966); Embase (Ovid, from 1947); and LILACS (Latin American and Caribbean Health Science Information database) (BIREME, from 1982). We also searched the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP; www.who.int/clinical-trials-registry-platform) and US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov/ct2/home) for trials in progress, using “neurocysticercosis ” and “Taenia solium” as search terms.
Searching other resources
We checked the reference lists of all studies, articles, and reviews identified by the above search terms for additional eligible studies.
Data collection and analysis
Selection of studies
Two review authors (EJMM and either KA or LNR) independently screened all citation titles and abstracts using a selection criteria eligibility form to identify all relevant studies. Where it was unclear if a study met our eligibility criteria or it appeared to be eligible, we obtained the full‐text article for assessment. Any differences of opinion were resolved by consensus discussion or by consulting the third review author if necessary. Studies that did not meet the criteria at any point in assessment were excluded.
Data extraction and management
One review author (EJMM) extracted data using a tailored data extraction form in which data were stratified by age (child/adult), cyst viability (viable/non‐viable/mixed), and anatomical position (intraparenchymal/extraparenchymal/mixed) for each outcome. The form also captured the studies' intervention/control regimens in detail, the inclusion/exclusion criteria of each study, and the follow‐up duration of each result extracted. A second review author (KA) checked the extracted data, with any disagreements resolved by discussion. The extracted data were entered into Review Manager 5 (Review Manager 2020).
Assessment of risk of bias in included studies
Two review authors (EJMM and either KA or LNR) independently assessed the risk of bias of the included studies using a pro forma based on Cochrane's tool for assessing risk of bias, described in the Cochrane Handbook for Systematic Reviews of Interventions (Boutron 2021). We categorized the generation of the allocation sequence and allocation concealment as adequate, unclear, or inadequate (Jüni 2001). We assessed whether the participants, care providers, and investigators were blinded to the drug regimen received by participants (intervention or control) as well as staff reporting imaging. For all outcomes, we assessed whether incomplete outcome data had been adequately addressed; if less than 85% of participants were included, adequate steps must have been documented to demonstrate that this did not lead to biased results to not be considered at unclear or high risk of bias. We also examined the articles for any evidence of selective reporting of outcomes or any other issues that could have biased the results. In cases of disagreement after consensus discussions, the third review author was consulted.
Measures of treatment effect
We used risk ratio (RR) as the measure of treatment effect for analysis.
Unit of analysis issues
We extracted data at the participant‐level for all outcomes, rather than at the lesion‐level. If studies reported radiological outcomes at lesion‐level only, they were included in the results narrative but not the meta‐analysis.
Dealing with missing data
If loss to follow‐up was less than 15%, we considered studies to be at low risk of bias for incomplete data. We considered studies with loss to follow‐up of 15% to 20% to be at low, unclear, or high risk of bias, depending on the circumstances and reporting of reasons for loss to follow‐up. We considered studies with loss to follow‐up of over 20% to be at high risk of bias, unless the loss was clearly accounted for in the articles, with no evidence of bias. Where data were presented in a manner that did not allow for stratification by our variables of interest, we approached the corresponding study authors for additional details. If unpublished data were provided, we have stated this in the Included studies section of the References section.
Assessment of heterogeneity
We assessed the heterogeneity for each outcome by considering the I2 statistic. We considered values of > 60% as indicative of substantial heterogeneity, 40% to 60% moderate heterogeneity, and < 40% low heterogeneity. We explored heterogeneity further in subgroup analysis.
Assessment of reporting biases
We assessed the probability of publication bias by examining a funnel plot for asymmetry for the primary outcome with the largest number of contributing studies, as well as the overall outcome with the largest number of contributing studies, given the different bias implications in clinical seizure and radiological reporting.
Data synthesis
We analysed extracted data using Review Manager 5 (Review Manager 2020). We calculated RR for dichotomous data and mean difference (MD) for continuous data. We measured precision using 95% confidence intervals (CIs). Where more than one study included similar participants and interventions, without significant clinical or methodological diversity, we undertook a meta‐analysis using a fixed‐effect model. Highly skewed data (where the standard deviation was greater than the mean) were presented in the text, as were any data from trials that could not be extracted due to the reporting format.
In studies with more than one follow‐up point, the latest follow‐up point was used for analysis unless the follow‐up points (within the same study) differed by more than 12 months, in which case the most similar follow‐up point (compared to the follow‐up of the other contributing studies) was included in the analysis. The same participant was not included at two points in time for any individual analysis.
Subgroup analysis and investigation of heterogeneity
We investigated potential sources of heterogeneity for each outcome by comparing the I2 statistic between subgroups and the primary (pooled) analysis. We performed subgroup analyses by age (child/adult), type of lesion (viable/non‐viable/mixed cysts), the anatomical position of cysts (intraparenchymal/extraparenchymal/mixed), number of cysts (single/multiple), and variations in intervention regimen (e.g. corticosteroids/no corticosteroids), where reported, for all outcomes and presented the results where they changed the interpretation of the data. Studies in which the data could not be stratified were not included in subgroup analysis.
Sensitivity analysis
Where sufficient trial data were available, we undertook sensitivity analyses by excluding studies without adequate reported allocation concealment. We also performed sensitivity analysis to address peculiarities of studies under investigation as they arose during the review process. These analyses were performed for all outcomes and were reported where the results could potentially change the interpretation of the data.
Summary of findings and assessment of the certainty of the evidence
We chose which outcomes to present in the 'Summary of findings' table based on two factors: the clinical significance of the outcome, and the frequency at which an outcome is measured/considered by clinicians in neurocysticercosis diagnosis and follow‐up. These were thought to be the resolution of seizures, given that epilepsy is the most common presentation of neurocysticercosis, and radiological resolution/improvement of lesions (complete radiological clearance of lesions and evolution of cysts). We decided which outcomes to include also in the context of the results of this review in order to include any unexpected findings of importance.
We used the GRADE approach to formally assess the certainty of the evidence for all outcomes, based on an assessment of the risk of bias, inconsistency, indirectness, imprecision, and publication bias of the collective evidence contributing to each outcome (Ryan 2016; Schünemann 2021). Two review authors (EJMM and KA) independently conducted this assessment, with any disagreements resolved by discussion. The results of the GRADE assessment for each of the outcomes included in our Table 1 are discussed in the table's footnotes.
Results
Description of studies
Results of the search
Our database search identified 628 articles that met our search terms. After title/abstract screening, we assessed 39 full‐text articles. We excluded 17 articles after full‐text assessment: six because they did not report trials (commentaries, case reports, and reviews). The remaining 11 excluded articles and the reasons for their exclusion are presented in the Characteristics of excluded studies. Figure 1 shows the screening and assessment flow of articles identified by the search.
1.
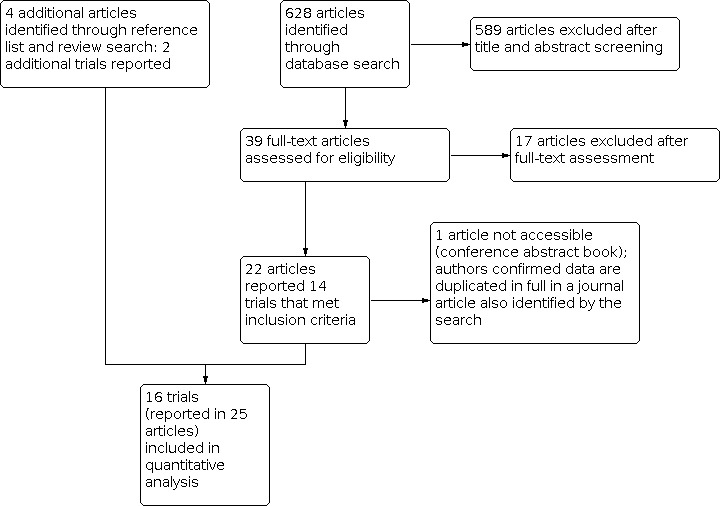
Study flow diagram.
Of the 22 articles that met our inclusion criteria (reporting data from 14 studies), we were unable to access one (a conference abstract). We confirmed with the corresponding study author that this article reported data that were duplicated by a journal article also identified through the search.
Four additional articles, reporting data from two additional studies, were identified through searching of reference lists of included studies and published reviews identified by the search. We included these in the review, contributing to a total of 25 accessible articles reporting data from 16 studies.
Our search identified no relevant ongoing trials recruiting or yet to recruit.
Included studies
Sixteen studies matched our inclusion criteria and are included in this review (Figure 1, Characteristics of included studies). We extracted data from 25 articles that reported the outcomes of these studies. A trial ID was established for each study for reference throughout this review using the first author's surname and year of publication on the primary reference article.
We have provided a description of the included randomized controlled trials in Table 2. Three of these trials are new since the last update of this review (Chaurasia 2010; Foyaca‐Sibat 2001; Khurana 2012), whilst the remaining 13 studies contributed to the previous version of this review (Abba 2010).
1. Summary of characteristics of included studies.
| Trial ID | Country | Year | Setting | Age | Position of cysts | Number of cysts | Viability of cysts | Imaging modality | Anthelmintic regimen (intervention arms only) | Steroid |
| Alarcon 2001 | Ecuador | 1989 to 1996 | Tertiary | All | Intraparenchymal | 1 to 6 | Viable | CT | Albendazole | No |
| Baranwal 1998 | India | Not stated | Tertiary | 2 to 12 years | Intraparenchymal | 1 | Non‐viable | CT | Albendazole | Yes |
| Carpio 2008 | Ecuador | 2001 to 2005 | Tertiary | All | Intraparenchymal and extraparenchymal | ≥ 1 | Viable or non‐viable, or both | CT | Albendazole | Yes |
| Chaurasia 2010 | India | 2007 to 2008 | Tertiary | All | Intraparenchymal | 1 | Non‐viable | CT | Albendazole | No |
| Das 2007 | India | 1997 to 2005 | Tertiary | Not stated, but all participants ≥ 18 years | Intraparenchymal and extraparenchymal | ≥ 2 | Non‐viable +/‐ viable | CT and MRI | Albendazole | Yes (intervention arm only) |
| de Souza 2009 | India | 2002 to 2006 | Tertiary | All | Intraparenchymal | 1 | Non‐viable | CT and MRI | Albendazole | No |
| Foyaca‐Sibat 2001 | South Africa | Not stated | Tertiary | Not stated, but all participants ≥ 12 years | Not stated | Not stated | Viable or non‐viable, or both | CT | Praziquantel | Yes (intervention arm only) |
| Garcia 2004 | Peru | 1997 to 2001 | Tertiary | ≥ 16 years | Intraparenchymal | 1 to 20 | Viable | CT and MRI | Albendazole | Yes (intervention arm only) |
| Gogia 2003 | India | 2000 to 2001 | Tertiary | ≤ 12 years | Intraparenchymal | ≥ 1 | Non‐viable | CT | Albendazole | Yes |
| Kalra 2003 | India | Not stated | Tertiary | 1 to 14 years | Intraparenchymal | 1 to 2 | Non‐viable | CT | Albendazole | Yes (intervention arm only) |
| Khurana 2012 | India | 2010 to 2011 | Tertiary | All | Intraparenchymal | 1 | Non‐viable | CT and MRI | Albendazole | No |
| Padma 1994 | India | Not stated | Tertiary | All | Intraparenchymal | 1 | Non‐viable | CT | Albendazole | Not stated |
| Padma 1995 | India | Not stated | Tertiary | All | Intraparenchymal and extraparenchymal | ≥ 2 | Viable or non‐viable, or both | CT | Aldendazole | Not stated |
| Singhi 2000 | India | 1994 to 1998 | Tertiary | ≤ 14 years | Intraparenchymal | 1 | Non‐viable | CT and MRI | Albendazole | Unclear |
| Singhi 2004 | India | Not stated | Tertiary | 1 to 14 years | Intraparenchymal | 1 | Non‐viable | CT | Albendazole | Yes |
| Sotelo 1988 | Mexico | Not stated | Not stated | Not stated, but all participants ≥ 18 years | Intraparenchymal | ≥ 1 | Viable | CT | Albendazole and praziquantel | Yes |
CT: computed tomography
MRI: magnetic resonance imaging
Geographical location and time period
The included studies were mostly conducted in India (11 trials: Baranwal 1998; Chaurasia 2010; Das 2007; de Souza 2009; Gogia 2003; Kalra 2003; Khurana 2012; Padma 1994; Padma 1995; Singhi 2000; Singhi 2004), or the Americas (four trials: two in Ecuador (Alarcon 2001; Carpio 2008), one in Mexico (Sotelo 1988), and one in Peru (Garcia 2004)). One study was conducted in South Africa (Foyaca‐Sibat 2001). Recruitment for most included studies was conducted in the 1990s and 2000s, with only two studies including recruitment in the late 1980s (Alarcon 2001; Sotelo 1988), and one study recruiting participants in the early 2010s (Khurana 2012).
Participants
All participants were enrolled into the included studies on the basis of CT or MRI radiological diagnosis of neurocysticercosis. In studies reporting seizure outcomes, seizures prior to enrolment were also an essential inclusion criteria. Two studies included serological confirmation in addition to radiological findings (Das 2007; Garcia 2004).
The included studies differed in their population inclusion/exclusion criteria based on age, cyst viability, position of cysts (intraparenchymal/extraparenchymal), and number of cysts. These are detailed in the Characteristics of included studies tables and summarized in Table 2. Participant age, cyst viability, and cyst position required for recruitment are also demonstrated in Figure 2. Seven studies recruited participants with single cysts only (Baranwal 1998; Chaurasia 2010; de Souza 2009; Khurana 2012; Padma 1994; Singhi 2000; Singhi 2004). Das 2007 and Padma 1995 recruited participants with more than one cyst only. Participants were recruited in three studies with 1 or more cysts (Carpio 2008; Gogia 2003; Sotelo 1988); one study with 1 to 2 cysts (Kalra 2003); one study with 1 to 6 cysts (Alarcon 2001); and one study with 1 to 20 cysts (Garcia 2004). Foyaca‐Sibat 2001 did not state how many cysts were required for recruitment.
2.
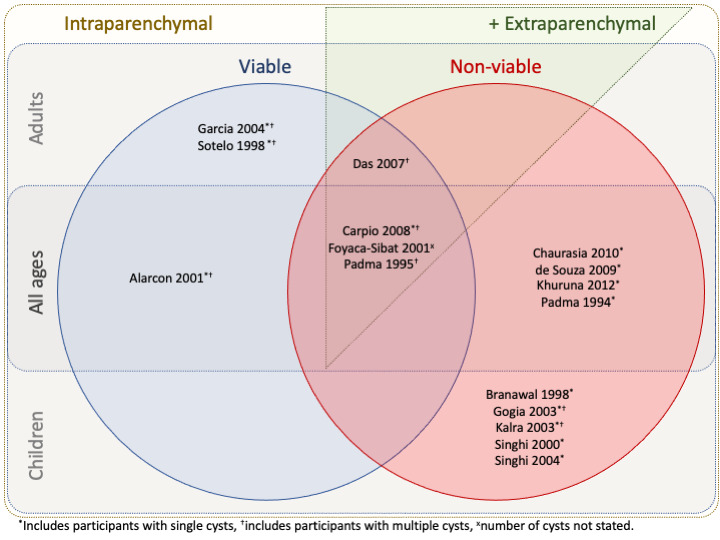
Venn diagram of study characteristics according to age of participants, viability of cysts, position of cysts, and number of cysts. Children are defined as being < 16 years of age.
Interventions
All but one study compared albendazole administration versus placebo or no anthelmintic (Foyaca‐Sibat 2001). Two studies compared praziquantel administration versus placebo or no anthelmintic (Foyaca‐Sibat 2001; Sotelo 1988). The anthelmintic dosing regimen and concurrent steroid and/or antiepileptic therapy for each study varied greatly and is summarized in Table 3. Albendazole regimens varied from 3 to 28 days. Praziquantel regimens varied from 1 to 14 days.
2. Anthelmintic dose regimens and supportive care used in included studies.
| Trial ID | Anthelmintic | Dose regimen | Control arm | Steroid | Antiepileptic |
| Alarcon 2001 | Albendazole | Group 1: 15 mg/kg daily for 3 days Group 2: 15 mg/kg daily for 8 days |
Group 3: no albendazole | None | Participants with seizures: various (including phenytoin and carbamazepine) as monotherapy |
| Baranwal 1998 | Albendazole | Group 1: 15 mg/kg daily in 2 divided doses for 28 days | Group 2: placebo (dextrose) capsules | All participants: prednisolone 1 to 2 mg/kg/day for 5 days | Participants with seizures: carbamazepine (phenytoin if carbamazepine was unaffordable to participant's family) |
| Carpio 2008 | Albendazole | Group 1: 400 mg twice daily for 8 days (if ≥ 50 kg) or 15 mg/kg daily (if < 50 kg, including children) | Group 2: placebo tablets | All participants: prednisolone 75 mg daily for 8 days, followed by 50 mg daily for 1 week, followed by 25 mg daily for 1 week (if ≥ 50 kg) or 1.5 mg/kg/day for 8 days, followed by 1.0 mg/kg/day for 1 week, followed by 0.5 mg/kg/day for 1 week (if < 50 kg) | Participants with seizures: phenytoin (carbamazepine if phenytoin is contraindicated or seizure control not achieved) |
| Chaurasia 2010 | Albendazole | Group 1: 15 mg/kg daily in 2 divided doses for 3 days | Group 2: placebo tablets | None | All participants: phenytoin, carbamazepine, or oxcarbazepine as monotherapy |
| Das 2007 | Albendazole | Group 1: 15 mg/kg day daily for 14 days plus dexamethasone | Group 2: placebo | Group 1 only: dexamethasone 2 mg orally 3 times a day for 14 days and then tapered | All participants: phenytoin, carbamazepine, or sodium valproate as monotherapy |
| de Souza 2009 | Albendazole | Group 1: 15 mg/kg daily in 2 divided doses for 28 days | Group 2: no albendazole | None | All participants: carbamazepine, oxcarbazepine, phenytoin, or phenobarbital with dual therapies used (18.5%) |
| Foyaca‐Sibat 2001 | Praziquantel | Group 1: 100 mg/kg daily in 4 divided doses for 1 day | Group 2: no praziquantel | Group 1 only: prednisone 40 mg daily for 5 days | All participants: 400 mg phenytoin at night |
| Garcia 2004 | Albendazole | Group 1: 400 mg twice daily for 10 days | Group 2: 2 placebos | Group 1 only: dexamethasone 2 mg orally 3 times a day for 10 days | All participants: monotherapy as per the participant's attending neurologist, or phenytoin if not already receiving treatment |
| Gogia 2003 | Albendazole | Group 1: 15 mg/kg daily in 2 divided doses for 28 days | Group 2: placebo | All participants: prednisolone 2 mg/kg/day for 3 days | All participants: phenytoin 5 mg/kg daily |
| Kalra 2003 | Albendazole | Group 1: 15 mg/kg daily for 28 days | Group 2:no albendazole | Group 1 only: dexamethasone 0.15 mg/kg in 2 to 3 divided doses for 5 days | All participants: monotherapy as per the participant's attending neurologist, or phenytoin if not already receiving treatment |
| Khurana 2012 | Albendazole | Group 1: 15 mg/kg daily in 2 divided doses for 3 days Group 2: 15 mg/kg daily in 2 divided doses for 15 days |
Group 3: no albendazole | None | All participants: oxcarbazepine |
| Padma 1994 | Albendazole | Group 1: 15 mg/kg daily for 7 days | Group 2: placebo | Not stated | Not stated |
| Padma 1995 | Albendazole | Group 1: 15 mg/kg daily in 2 divided doses for 7 days | Group 2: placebo tablets | Not stated | Participants with seizure history: antiepileptic medication "continued" |
| Singhi 2000 | Albendazole | Group 1: 15 mg/kg daily for 28 days | Group 2: no albendazole | Unclear: Group 1 may have received prednisolone 2 mg/kg daily for 5 days | Participants with seizures: carbamazepine, phenytoin, or phenobarbital |
| Singhi 2004 | Albendazole | Group 1: 15 mg/kg daily for 28 days Group 2: 15 mg/kg daily for 28 days plus prednisolone for 7 days |
Group 3: no albendazole plus prednisolone | Group 2: prednisolone 2 mg/kg daily for 5 days Group 3: prednisolone 2 mg/kg daily for 21 days |
All participants: carbamazepine or phenytoin |
| Sotelo 1988 | Albendazole or praziquantel | Group 1: albendazole 15 mg/kg daily for 1 month Group 2: praziquantel 50 mg/kg daily for 14 days |
Group 3: no albendazole or praziquantel | All participants: dexamethasone 5 mg intramuscularly 3 times daily for "intense" adverse reactions | All participants: antiepileptic medication continued for those receiving it |
Corticosteroids were administered to all participants in three studies (Baranwal 1998; Carpio 2008; Gogia 2003), and to participants receiving the intervention only in five studies (Das 2007; Foyaca‐Sibat 2001; Garcia 2004; Kalra 2003; Singhi 2004). In one study, participants in both the intervention and control arm received corticosteroids only if adverse events occurred that indicated their use (Sotelo 1988). Steroids were not administered in four studies (Alarcon 2001; Chaurasia 2010; de Souza 2009; Khurana 2012). In one study, it was unclear whether participants receiving the intervention only received corticosteroids (Singhi 2000). Steroid administration was not stated in two studies (Padma 1994; Padma 1995). Corticosteroid regimens varied from 3 to 21 days (Table 3).
Antiepileptic medications were administered to all participants in 10 studies (Chaurasia 2010; Das 2007; de Souza 2009; Foyaca‐Sibat 2001; Garcia 2004; Gogia 2003; Kalra 2003; Khurana 2012; Singhi 2004; Sotelo 1988), and to participants receiving the intervention only in four studies (Alarcon 2001; Baranwal 1998; Carpio 2008; Singhi 2000). One study continued antiepileptic medication if participants suffering from seizures had already started treatment (Padma 1995), and one study made no mention of antiepileptics (Padma 1994). The antiepileptics used are described in Table 3.
Follow‐up
All included studies had follow‐up points within 12 months of recruitment. Seven studies included longer‐term follow‐up past 12 months (Alarcon 2001; Baranwal 1998; Carpio 2008; Das 2007; de Souza 2009; Garcia 2004; Singhi 2004). Table 4 summarizes follow‐up points for each included trial, stratified by short term (up to 12 months) and long term (> 12 months) follow‐up.
3. Follow‐up duration of included studies.
| Trial ID | Short‐term follow‐up points | Long‐term follow‐up points |
| Alarcon 2001 | 3 months 12 months |
≥ 24 months |
| Baranwal 1998 | 1 month 3 months |
15 months |
| Carpio 2008 | 2 weeks 1, 2, 3, 4, 5, 6 months 9 months 12 months |
15 months 18 months 24 months |
| Chaurasia 2010 | 6 months | ‐ |
| Das 2007 | 1, 2, 3, 4, 5, 6 months 9 months 12 months |
Every 3 months between 15 and 60 months |
| de Souza 2009 | 3 months 6 months 12 months |
24 months Telephone follow‐up October 2006 (Follow‐up mean of 31.4 months, +/‐ 14.8 months) |
| Foyaca‐Sibat 2001 | 1 month | ‐ |
| Garcia 2004 | 30 days 60 days 90 days 6 months 9 months 12 months |
15 months 18 months 24 months 30 months |
| Gogia 2003 | 6 months | ‐ |
| Kalra 2003 | 15 days 3 months 6 months |
‐ |
| Khurana 2012 | 1, 2, 3, 4, 5, 6 months | ‐ |
| Padma 1994 | 1 week 1 month 3 months |
‐ |
| Padma 1995 | 1 month 3 months |
‐ |
| Singhi 2000 | Between 3 and 6 months (mean 3.5) | ‐ |
| Singhi 2004 | 3 months 6 months 9 months 12 months |
15 months 18 months |
| Sotelo 1988 | 3 months (Groups 1 and 2: intervention) 4 months (Group 3: control) |
‐ |
Outcome measures
Twelve studies reported seizure recurrence as an outcome. Ten of these studies had data that could be included in meta‐analysis (Alarcon 2001; Baranwal 1998; Carpio 2008; Chaurasia 2010; Das 2007; Garcia 2004; Gogia 2003; Kalra 2003; Khurana 2012; Singhi 2004); the remaining two studies reported data on seizure recurrence suitable for discussion only (de Souza 2009; Foyaca‐Sibat 2001).
Thirteen studies reported complete radiological clearance of lesions as an outcome, all of which had data that could be included in meta‐analysis (Alarcon 2001; Baranwal 1998; Carpio 2008; Chaurasia 2010; Das 2007; de Souza 2009; Garcia 2004; Gogia 2003; Kalra 2003; Khurana 2012; Singhi 2000; Singhi 2004; Sotelo 1988). Eleven studies reported evolution of cysts as an outcome, six of which had data that could be included in meta‐analysis (Baranwal 1998; Carpio 2008; Chaurasia 2010; Gogia 2003; Khurana 2012; Padma 1994), whilst the remaining five included studies reported data on evolution of cysts suitable for discussion only (Das 2007; de Souza 2009; Garcia 2004; Kalra 2003; Singhi 2000).
Table 5 summarizes the specific outcomes reported in each trial, according to reporting of seizure occurrence/pattern, reporting of additional health status indicators, reporting of radiological findings, and reporting of adverse events.
4. Output measures of interest of included studies.
| Trial ID | Seizure status | Additional health status indicators | Radiological findings | Adverse events |
| Alarcon 2001 | Seizure recurrence | Surgical intervention | Complete radiological clearance of lesions | Frequency and nature of adverse events* |
| Baranwal 1998 | Seizure recurrence | ‐ | Complete radiological clearance of lesions Evolution of cysts |
Frequency and nature of adverse events* |
| Carpio 2008 | Seizure recurrence Frequency of seizures* |
Death (all cause) Headache Signs of focal neurological deficit |
Complete radiological clearance of lesions Reduction of number of lesions Evolution of cysts |
Frequency and nature of adverse events* |
| Chaurasia 2010 | Seizure recurrence | ‐ | Complete radiological clearance of lesions Evolution of cysts |
Frequency and nature of adverse events* |
| Das 2007 | Seizure recurrence | Death (all cause) Hospital admission (all cause) Headache* |
Complete radiological clearance of lesions Evolution of cysts* |
‐ |
| de Souza 2009 | Seizure recurrence* Time to seizure remission* |
‐ | Complete radiological clearance of lesions Evolution of cysts* Radiological resolution/development of oedema* |
‐ |
| Foyaca‐Sibat 2001 | Seizure recurrence* Frequency of seizures* |
‐ | ‐ | ‐ |
| Garcia 2004 | Seizure recurrence Frequency of seizures* |
‐ | Complete radiological clearance of lesions Evolution of cysts* |
Frequency and nature of adverse events* |
| Gogia 2003 | Seizure recurrence | ‐ | Complete radiological clearance of lesions Evolution of cysts |
‐ |
| Kalra 2003 | Seizure recurrence | ‐ | Complete radiological clearance of lesions Evolution of cysts* |
Frequency and nature of adverse events* |
| Khurana 2012 | Seizure recurrence | ‐ | Complete radiological clearance of lesions Evolution of cysts |
Frequency and nature of adverse events* |
| Padma 1994 | ‐ | ‐ | Evolution of cysts | ‐ |
| Padma 1995 | ‐ | ‐ | Reduction of number of lesions Radiological resolution/development of oedema |
‐ |
| Singhi 2000 | ‐ | ‐ | Complete radiological clearance of lesions Evolution of cysts* |
‐ |
| Singhi 2004 | Seizure recurrence Seizure recurrence after withdrawal of antiepileptics* |
‐ | Complete radiological clearance of lesions | ‐ |
| Sotelo 1988 | ‐ | ‐ | Complete radiological clearance of lesions Reduction of number of lesions |
Frequency and nature of adverse events* |
*Results not suitable for meta‐analysis, but included in the narrative of results.
Excluded studies
The reasons for exclusion of the 18 excluded studies are provided in the Characteristics of excluded studies table. Eleven of these were identified by the database search and excluded on full‐text assessment as previously discussed. Other studies identified by the database search and excluded through title/abstract screening are not included in the table.
An additional seven studies which were included in previous versions of this review, Abba 2010; Salinas 1999, but excluded at title/abstract screening in this version, as they compared anthelmintic treatment regimens without a supportive treatment comparison group, are also described in Characteristics of excluded studies. This was the result of a change in the comparison definitions for this version of the review (Appendix 2); a total of eight studies included in previous citations of this review were not included in this version of the review for this reason (Alarcon 1989; Cruz 1995; Del Brutto 1999; Garcia 1997; Gongora‐Rivera 2006; Kaur 2009; Singhi 2003; Sotelo 1990).
Risk of bias in included studies
A 'Risk of bias' table is provided for each included study in the Characteristics of included studies section. The results of the 'Risk of bias' assessment are summarized across all included trials in Figure 3 and Figure 4.
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
4.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Ten included studies reported adequate methods of randomization, employing computer‐generated number sequences or random number tables, and were assessed as being at a low risk of bias (Baranwal 1998; Carpio 2008; Chaurasia 2010; de Souza 2009; Foyaca‐Sibat 2001; Garcia 2004; Gogia 2003; Kalra 2003; Khurana 2012; Singhi 2004). The remaining studies did not report the method of randomization and were assessed as at unclear risk of bias.
Five studies reported adequate allocation concealment and were assessed as being at a low risk of bias (Baranwal 1998; Carpio 2008; Garcia 2004; Gogia 2003; Kalra 2003), whilst the remaining studies did not clearly describe allocation concealment methods and were judged as at unclear risk of bias. All of the included studies that adequately reported allocation concealment also reported an adequate method of randomization. No trials were excluded as part of a sensitivity analysis due to allocation, as none of the included studies were assessed as being at high risk of bias.
Blinding
Eight included studies reported adequate blinding of participants, research staff, and outcome reporting clinicians (Baranwal 1998; Carpio 2008; Chaurasia 2010; Foyaca‐Sibat 2001; Garcia 2004; Gogia 2003; Padma 1994; Padma 1995). In three studies, clinicians reporting radiological outcomes were described as blinded, but participants and research staff receiving/administering the intervention or placebo/control, or both, were not blinded (Alarcon 2001; de Souza 2009; Singhi 2004). We judged each of these studies as at high risk of performance bias. We considered radiological outcomes as at low risk and seizure status outcomes as at high risk of detection bias.
Four included studies were unblinded and assessed as being at a high risk of both performance and detection bias (Kalra 2003; Khurana 2012; Singhi 2000; Sotelo 1988). One included study did not describe blinding methods, and was therefore assessed as having an unclear risk of both performance and detection bias (Das 2007).
Incomplete outcome data
Eleven included studies reported data from over 85% of their randomized participants and were assessed as at low risk of attrition bias (Alarcon 2001; Baranwal 1998; Carpio 2008; Chaurasia 2010; Das 2007; Garcia 2004; Gogia 2003; Khurana 2012; Padma 1994; Padma 1995; Sotelo 1988). de Souza 2009 reported data for 84% of randomized participants and was considered as at low risk of attrition bias after assessment of their reporting, whilst Singhi 2004 reported data for 83% of randomized participants and was assessed as having an unclear risk of attrition bias.
Three included studies reported data on fewer than 80% of randomized participants and were assessed as being at high risk of attrition bias: data were reported for 79% of randomized participants in Foyaca‐Sibat 2001, 75% of randomized participants in Kalra 2003, and 46% of randomized participants in Singhi 2000.
Selective reporting
We did not detect any selective reporting in the 16 included studies, although we did not have access to original protocols. Trials with data reported by several articles, some of which spanned a number of years, correlated without discrepancy.
Other potential sources of bias
We did not detect any significant additional sources of bias within the included studies. All trials based the inclusion of participants on a radiological diagnosis of neurocysticercosis and, if reporting of seizure outcomes was planned, the presence of seizures in a defined time frame prior to recruitment.
Regarding publication bias, Figure 5 is a funnel plot for seizure recurrence (the primary outcome with the largest number of contributing studies); values below one favoured albendazole. Figure 6 is a further funnel plot for complete radiological clearance of lesions (the overall outcome with the largest number of contributing studies); values above one favoured albendazole. There was no obvious evidence of publication bias through asymmetry, though the number of included trials was low.
5.

Funnel plot of comparison: 1 Albendazole versus placebo or no anthelmintic, outcome: 1.1 Seizure recurrence.
6.

Funnel plot of comparison: 1 Albendazole versus placebo or no anthelmintic, outcome: 1.12 Complete radiological clearance of lesions.
Effects of interventions
See: Table 1
Comparison: albendazole versus placebo or no anthelmintic
Outcome of interest: seizure status at follow‐up
Recurrence of seizures
Ten included studies reported on the recurrence of seizures (Figure 7). Heterogeneity of these studies with regard to participant recruitment criteria is shown in Figure 2. Alarcon 2001 and Garcia 2004 exclusively included participants with viable cysts; Carpio 2008 and Das 2007 included participants with viable or non‐viable cysts, or both, and all other studies included participants exclusively with non‐viable cysts. With regard to the anatomical position of cysts, Carpio 2008 and Das 2007 included participants with extraparenchymal cysts, whilst all other studies included participants with intraparenchymal cysts only. Baranwal 1998, Gogia 2003, Kalra 2003, and Singhi 2004 only included children (< 16 years); Das 2007 and Garcia 2004 only included adults (≥ 16 years); and the remaining studies included both children and adults. There was substantial heterogeneity, with an I2 statistic of 67%. Subgroup analyses of this outcome are discussed below.
7.

Forest plot of comparison: 1 Albendazole versus placebo or no anthelmintic, outcome: 1.1 Seizure recurrence.
Overall, there was no evidence of benefit or harm of albendazole versus placebo or no anthelmintic (risk ratio (RR) 0.94, 95% confidence interval (CI) 0.78 to 1.14; 10 trials, 1054 participants; Analysis 1.1).
1.1. Analysis.

Comparison 1: Albendazole versus placebo or no anthelmintic, Outcome 1: Seizure recurrence
A further included study, de Souza 2009, reported no significant difference in months seizure‐free at the end of follow‐up for participants administered albendazole (22.2 months, 95% CI 6.4 to 38.0; 50 participants) and those who did not receive an anthelmintic (27.9 months, 95% CI 12.3 to 43.5; 53 participants). We did not include these data in the meta‐analysis as they were not in a comparable format.
The denominator from the albendazole intervention arm of Das 2007 differed slightly (150 versus 148) to a previous version of this review (Abba 2010), as two participants that died within the first three months of follow‐up were included. This was because the follow‐up point of data extraction was three months. The numerator and denominator from the no‐anthelmintic comparison arm of Singhi 2004 differed to a previous version of this review (Abba 2010), as data were extracted for participants prior to stopping of antiepileptic medication. The origin of the previous values in Abba 2010 are unclear from the article; however, they are not relevant to this version of the review.
Seizure recurrence after withdrawal of antiepileptics
One included study described seizure recurrence in participants after stopping of antiepileptics. Singhi 2004 reported no significant difference in recurrence of seizures in 2/37 (5.4%) participants receiving albendazole alone, 2/35 (5.7%) participants receiving albendazole and corticosteroids, and 3/38 (7.9%) participants receiving corticosteroids alone.
Time to seizure remission
One included study, de Souza 2009, reported the time taken for participants to become seizure‐free; participants administered albendazole became seizure‐free after a mean of 6 months (95% CI −5.9 to 17.9; 50 participants), whereas participants that did not receive an anthelmintic became seizure‐free after a mean of 5.3 months (95% CI −6.3 to 16.9; 53 participants). The difference between these findings was not significant.
Frequency of seizures
Two included studies reported on the frequency of seizures as an outcome (Carpio 2008; Garcia 2004). Carpio 2008 reported a reduction in seizure frequency at 12 months in participants with generalized seizures who received albendazole compared to participants who received placebo (adjusted RR 0.21, 95% CI 0.04 to 0.96). The study was not powered sufficiently to support a reduction in seizure frequency at 24 months (adjusted RR 0.13, 95% CI 0.01 to 1.39). There was no difference for participants with focal seizures at 24 months (adjusted RR 3.96, 95% CI 0.54 to 26.28).
Garcia 2004 also reported findings for participants with generalized and focal (partial) seizures. A reduction in seizure frequency was reported at 30 months of follow‐up in participants with generalized seizures who received albendazole compared to participants who received placebo (adjusted RR 0.33, 95% CI 0.16 to 0.68). There was no difference for participants with partial seizures (adjusted RR 0.66, 95% CI 0.24 to 1.79).
Seizure definitions and follow‐up periods differed significantly between these two studies, therefore data were not subjected to meta‐analysis.
Sensitivity analysis: seizure status at follow‐up
We excluded studies with an unclear risk of bias for allocation concealment from a sensitivity analysis of seizure recurrence (Alarcon 2001; Chaurasia 2010; Das 2007), which showed an apparent benefit of albendazole versus placebo or no anthelmintic (RR 0.71, 95% CI 0.57 to 0.88; 7 trials, 629 participants; I2 = 50%). As none of the excluded studies had a high risk of selection bias (only unclear risk), and moderate heterogeneity remained, we have not reported this sensitivity analysis as the main results of this review.
It is important to note that Das 2007 was one of the excluded studies in this sensitivity analysis, which is a large study that introduced considerable heterogeneity to the meta‐analysis by including adult participants with multiple cysts only, with mixed viability and mixed anatomical position (intraparenchymal and extraparenchymal). When purposefully removed from the primary analysis, there was evidence of benefit of albendazole versus placebo or no anthelmintic (RR 0.73, 95% CI 0.59 to 0.91; 9 trials, 754 participants). The I2 statistic with Das 2007 removed from the analysis was 39%.
Although Das 2007 was assessed as at unclear risk of selection, performance, and detection bias, no 'Risk of bias' domain was assessed as high risk for this study, therefore we have continued to include it in our main analysis, given that it meets our inclusion criteria and we cannot justify exclusion based on bias assessment. Given the heterogeneity of participants, Das 2007 could not be included in the majority of subgroup analyses.
Subgroup analyses of note: seizure status at follow‐up
Subgroup analysis for the recurrence of seizures was conducted, and the results for stratification by number of cysts (single versus multiple) are presented in detail below. A brief description of other subgroup analyses is also included. It should be noted that all studies that presented data from participants with a single cyst did so for participants with non‐viable intraparenchymal cysts.
Recurrence of seizures: participants with a single cyst
Five studies reported data from participants with a single cyst (Figure 8). Baranwal 1998, Khurana 2012, and Singhi 2004 included only children (< 16 years) with single, non‐viable intraparenchymal cysts, whereas Chaurasia 2010 and Gogia 2003 included participants of all ages with single, non‐viable intraparenchymal cysts. There was low heterogeneity (I2 = 16%).
8.

Forest plot of comparison: 1 Albendazole versus placebo or no anthelmintic, outcome: 1.2 Seizure recurrence subgroup analysis: number of cysts.
Overall, the direction of effect indicated a benefit of albendazole versus placebo or no anthelmintic (RR 0.61, 95% CI 0.4 to 0.91; 5 trials, 396 participants; Analysis 1.2). As described above, all participants included in this analysis had non‐viable cysts.
1.2. Analysis.

Comparison 1: Albendazole versus placebo or no anthelmintic, Outcome 2: Seizure recurrence subgroup analysis: number of cysts
Recurrence of seizures: participants with multiple (> 1) cysts
Two studies reported data from participants with multiple cysts (Figure 8). Das 2007 included participants with viable or non‐viable cysts, or both, regardless of intraparenchymal/extraparenchymal position anatomically, whereas Gogia 2003 included participants with non‐viable intraparenchymal cysts only. Both studies included participants of all ages. There was substantial heterogeneity (I2 = 64%).
Overall, the direction of effect indicated a possible harm of albendazole versus placebo or no anthelmintic (RR 2.05, 95% CI 1.28 to 3.31; 2 trials, 321 participants; Analysis 1.2).
Recurrence of seizures: other subgroup analyses
Stratification by cyst viability: there was no evidence of benefit or harm of albendazole versus placebo or no anthelmintic for participants with viable cysts (RR 0.91, 95% CI 0.62 to 1.33; 2 trials, 174 participants; Analysis 1.3). There was low heterogeneity (I2 = 0%). For participants with non‐viable cysts, there was evidence of a benefit of albendazole versus placebo or no anthelmintic (RR 0.55, 95% CI 0.38 to 0.78; 6 trials, 507 participants; Analysis 1.3). There was low heterogeneity (I2 = 5%).
1.3. Analysis.

Comparison 1: Albendazole versus placebo or no anthelmintic, Outcome 3: Seizure recurrence subgroup analysis: viability of cysts
Stratification by anatomical position of cysts: there was evidence of a benefit of albendazole versus placebo or no anthelmintic for participants with intraparenchymal cysts (RR 0.66, 95% CI 0.51 to 0.86; 8 trials, 681 participants; Analysis 1.4). There was moderate heterogeneity (I2 = 20%). No studies recruited participants with extraparenchymal cysts only.
1.4. Analysis.

Comparison 1: Albendazole versus placebo or no anthelmintic, Outcome 4: Seizure recurrence subgroup analysis: position of cysts
Stratification by participant age (children/adults): there was evidence of a benefit of albendazole versus placebo or no anthelmintic for participants under 16 years of age (RR 0.46, 95% CI 0.30 to 0.69; 4 trials, 335 participants; Analysis 1.5). There was low heterogeneity (I2 = 0%). Two studies that recruited adult participants showed evidence of harm of albendazole versus placebo (RR 1.45, 95% CI 1.06 to 1.98; 2 trials, 416 participants; Analysis 1.5). Das 2007 was included in this analysis of adults, and likely contributed to the high heterogeneity (I2 = 89%).
1.5. Analysis.

Comparison 1: Albendazole versus placebo or no anthelmintic, Outcome 5: Seizure recurrence subgroup analysis: age of participants
Stratification by corticosteroid administration: there was no evidence of benefit or harm of albendazole versus placebo or no anthelmintic for participants with the same corticosteroid regimen given to all participants (RR 0.83, 95% CI 0.61 to 1.13; 3 trials, 208 participants; Analysis 1.6). There was also no evidence of benefit or harm of albendazole versus placebo or no anthelmintic for participants receiving no corticosteroids (RR 0.97, 95% CI 0.50 to 1.88; 3 trials, 230 participants; Analysis 1.6). There was low heterogeneity in both analyses, with an I2 statistic of 9% and 0%, respectively. We did not include studies in which the corticosteroid regimen differed between albendazole and placebo or no anthelmintics groups in these analyses.
1.6. Analysis.

Comparison 1: Albendazole versus placebo or no anthelmintic, Outcome 6: Seizure recurrence subgroup analysis: corticosteroid use
Outcomes of interest: secondary health status indicators at follow‐up
Death (any cause)
Two included studies reported deaths (Carpio 2008; Das 2007). Four of 234 (1.7%) participants in the albendazole group and 5 of 236 (2.1%) participants in the placebo group from these trials died during follow‐up of two to five years, with no significant difference between albendazole and placebo treatment groups (Analysis 1.7).
1.7. Analysis.

Comparison 1: Albendazole versus placebo or no anthelmintic, Outcome 7: Death (any cause)
Hospital admission (any cause)
One included study reported hospital admissions, following up participants for five years after enrolment (Das 2007). Data were extractable for analysis at three months (Analysis 1.8), during which time participants treated with albendazole had a higher risk of hospital admission (RR 2.50, 95% CI 1.52 to 4.11; 300 participants). At two, three, four, and five years of follow‐up, there was no significant difference in hospital admission between participants that received albendazole or placebo at enrolment.
1.8. Analysis.

Comparison 1: Albendazole versus placebo or no anthelmintic, Outcome 8: Hospital admission (any cause)
Headache
Two included studies reported headache as an outcome (Carpio 2008; Das 2007). Carpio 2008 reported that within the first 12 months of treatment, 74/79 (93.7%) of participants who received albendazole reported headache, and 78/82 (95.1%) of participants who received placebo reported headache, with no significant difference between groups (Analysis 1.9). In this trial, of the 173 participants initially recruited, 124 (71.7%) reported headache at baseline, with no difference between the albendazole arm (60/88) and the placebo arm (64/90).
1.9. Analysis.

Comparison 1: Albendazole versus placebo or no anthelmintic, Outcome 9: Headache
Das 2007 reported encephalopathy (defined as vomiting/headache/altered sensorium) in participants over five years, though due to the presentation of these data, reliable extraction was only possible at three months: 45/148 (30.4%) participants receiving albendazole and 15/150 (10%) participants receiving placebo reported symptoms of encephalopathy. This difference was significant, despite the exclusion of two participants from the albendazole arm prior to analysis who reportedly died from intractable seizures and severe encephalopathy during this period. Headache‐specific data were not reported and therefore not available for meta‐analysis.
Signs of focal neurological deficit
One included study reported data for signs of focal neurological involvement (Carpio 2008). Limb weakness/gait disturbance were the symptoms/signs captured: there was no difference between these signs/symptoms in participants who received albendazole and those who received placebo at 12 months (RR 0.89, 95% CI 0.65 to 1.23; 161 participants; Analysis 1.10).
1.10. Analysis.

Comparison 1: Albendazole versus placebo or no anthelmintic, Outcome 10: Signs of focal neurological deficit
Surgical intervention
One included study reported surgical intervention (Alarcon 2001). One event was reported amongst participants receiving albendazole, and none amongst participants who did not receive albendazole (RR 1.64, 95% CI 0.07 to 38.94; 83 participants; Analysis 1.11).
1.11. Analysis.

Comparison 1: Albendazole versus placebo or no anthelmintic, Outcome 11: Surgical intervention
Resolution of symptoms/resumption of normal activities
These outcomes were not addressed by any of the included studies.
Outcomes of interest: radiological findings at follow‐up
Complete radiological clearance of lesions
Thirteen included studies reported lesions resolving completely (Figure 9). Data were extracted at the participant level (as per our protocol), not at the lesion level. Figure 2 depicts the heterogeneity of the recruitment criteria of these trials. Alarcon 2001, Garcia 2004, and Sotelo 1988 exclusively included participants with viable cysts; Carpio 2008 and Das 2007 included participants with viable or non‐viable cysts, or both; and the other studies included participants exclusively with non‐viable cysts. Carpio 2008 and Das 2007 included participants with extraparenchymal cysts, whilst the other studies included participants with intraparenchymal cysts only. Baranwal 1998, Gogia 2003, Kalra 2003, Singhi 2000, and Singhi 2004 only included children (< 16 years), whilst Das 2007, Garcia 2004, and Sotelo 1988 only included adults (≥ 16 years). All of the remaining studies included both children and adults. There was substantial heterogeneity (I2 = 70%).
9.

Forest plot of comparison: 1 Albendazole versus placebo or no anthelmintic, outcome: 1.12 Complete radiological clearance of lesions.
Overall, the direction of effect indicated a benefit of albendazole versus placebo or no anthelmintic (RR 1.22, 95% CI 1.07 to 1.39; 13 trials, 1324 participants; Analysis 1.12).
1.12. Analysis.

Comparison 1: Albendazole versus placebo or no anthelmintic, Outcome 12: Complete radiological clearance of lesions
The numerators from the albendazole intervention and placebo comparison arms of Das 2007 differed to a previous version of this review (Abba 2010), as data were extracted at 12 months of follow‐up rather than three.
Reduction of number of lesions
Three included studies reported a reduction of the number of lesions. Sotelo 1988 recruited adult participants (≥ 16 years) with viable, intraparenchymal cysts. Carpio 2008 and Padma 1995 both recruited participants of any age, with viable or non‐viable cysts, or both, irrespective of intraparenchymal/extraparenchymal position anatomically. There was moderate heterogeneity (I2 = 45%). Studies that exclusively recruited participants with a single cyst were not included in this analysis, as these data were more appropriately interpreted as a complete radiological clearance of lesions (Analysis 1.12).
Overall, the direction of effect indicated no benefit or harm of albendazole versus placebo or no anthelmintic (RR 1.33, 95% CI 0.89 to 1.98; 3 trials, 115 participants; Analysis 1.13).
1.13. Analysis.

Comparison 1: Albendazole versus placebo or no anthelmintic, Outcome 13: Reduction of number of lesions
Evolution of cysts
Six included studies reported the evolution of cysts in a manner that could be extracted for meta‐analysis (Figure 10). Baranwal 1998 and Gogia 2003 recruited children (< 16 years) with non‐viable, intraparenchymal cysts only. Chaurasia 2010, Khurana 2012, and Padma 1994 also recruited participants exclusively with non‐viable, intraparenchymal cysts, but of any age. Carpio 2008 recruited participants of any age, with viable or non‐viable cysts, or both, irrespective of intraparenchymal/extraparenchymal position anatomically. There was very low heterogeneity (I2 = 0%).
10.

Forest plot of comparison: 1 Albendazole versus placebo or no anthelmintic, outcome: 1.14 Evolution of cysts.
Overall, the direction of effect indicated a benefit of albendazole versus placebo or no anthelmintic (RR 1.27, 95% CI 1.10 to 1.47; 6 trials, 434 participants; Analysis 1.14).
1.14. Analysis.

Comparison 1: Albendazole versus placebo or no anthelmintic, Outcome 14: Evolution of cysts
Several studies reported evolution of cysts in a manner that could not be extracted for meta‐analysis. de Souza 2009 reported a more detailed sequence of degeneration at lesion‐level, with non‐viable enhancing cysts considered separately at early and late stages (outside of the scope of this review). Garcia 2004 also reported evolution of cysts at lesion‐level: when considering viable cysts, 79/192 (41.1%) of cysts from participants receiving albendazole persisted unchanged, compared to 243/279 (87.1%) of viable cysts from participants who received placebo (RR 2.12, 95% CI 1.59 to 2.81; 471 cysts). Kalra 2003 reported participant‐level partial resolution or calcification of cysts in 37/47 (78.7%) of participants who received albendazole and 26/46 (56.5%) of participants who did not receive albendazole (odds ratio 3.1, 95% CI 1.2 to 7.9; 93 participants). These data could not be extracted, as "partial resolution" was not defined. All participants were children (< 16 years) with one or two cysts on enrolment. Singhi 2000 reported an "improvement in lesion" (disappearance or reduction in size) at participant‐level in 82/90 (91.1%) of participants with a single cyst who received albendazole and had a follow‐up CT after three months, compared to 73/86 (84.9%) of participants with a single cyst who did not receive albendazole. It should also be noted that these 176 participants were selected for this analysis because they had a single non‐viable cysts and also received a follow‐up CT; 380 participants with a single cyst were originally enrolled in the trial. Finally, Das 2007 reported participant‐level data on "partial resolution" of cysts at three months, six months, 12 months, and then yearly to five years. It appears that their data were not cumulative, therefore it is likely that each follow‐up point reports the change since the last follow‐up, with CT scans every six months in participants with residual lesions. The denominator is therefore difficult to confirm. At 12 months, 65/148 (43.9%) of participants who received albendazole were reported to have partial resolution of their cysts, compared to 60/150 (40%) of participants who received placebo. No statistical analyses were performed on these data, due to the denominator uncertainty.
Radiological resolution/development of oedema
One included study reported data regarding the resolution and development of oedema (Padma 1995). In this small study, 12/16 (75%) participants who received albendazole had improvement of oedema on CT compared to 11/13 (84.6%) participants who received placebo at three months (Analysis 1.15), with no significant difference between groups. In the same population there was one instance of worsening oedema in both the albendazole and placebo arms (Analysis 1.16). Based on the report of this study, the administration of corticosteroids is unclear.
1.15. Analysis.

Comparison 1: Albendazole versus placebo or no anthelmintic, Outcome 15: Radiological resolution of oedema
1.16. Analysis.

Comparison 1: Albendazole versus placebo or no anthelmintic, Outcome 16: Radiological development of oedema
Radiological resolution/development of raised intracranial pressure
These outcomes were not addressed by any of the included studies.
Subgroup analyses of note: radiological findings at follow‐up
Complete radiological clearance of lesions
Stratification by cyst viability: evidence of benefit of albendazole versus placebo or no anthelmintic persisted for participants with viable cysts (RR 2.52, 95% CI 1.65 to 3.85; 3 trials, 207 participants; I2 = 0%) and participants with non‐viable cysts (RR 1.22, 95% CI 1.05 to 1.41; 8 trials, 748 participants; I2 = 62%), with lower heterogeneity in the evidence for participants with viable cysts. (Analysis 1.17)
1.17. Analysis.

Comparison 1: Albendazole versus placebo or no anthelmintic, Outcome 17: Complete radiological clearance of lesions subgroup analysis: viability of cysts
Outcomes of interest: adverse events associated with treatment (side effects) at follow‐up
Frequency and nature of adverse events
Seven included studies explicitly mentioned the frequency and nature of adverse events. The frequency of adverse events varied depending on how they were reported, and could not be incorporated into a meta‐analysis due to the nature of this reporting and the varying definitions between trials.
Chaurasia 2010 and Khurana 2012 each reported very few events, each with only a single episode of benzodiazepine‐associated rash that resolved after cessation of the offending medication. No adverse events or side effects attributable to albendazole administration were reported. Neither study administered corticosteroids to participants of either group.
Carpio 2008, Garcia 2004, and Kalra 2003 each reported common adverse events and side effects, observed in all participants. Carpio 2008 reported seizures, headache, and gastrointestinal complications (nausea, abdominal pain, or vomiting) in the first month of follow‐up comparing the rates between participants receiving albendazole or placebo (P = 0.64, 0.95 and 0.38 respectively). Three participants in the placebo group had intracranial hypertension within the first eight days of treatment. Garcia 2004 reported seizure, headache, paraesthenia, paresis, dizziness, abdominal pain, nausea, diarrhoea, and rash as separate side effects in participants who received albendazole or placebo. Only abdominal pain differed between the two groups, favouring no anthelmintic (P = 0.006). For all other comparisons, P ≥ 0.2. Three additional adverse events were reported, all in the placebo group and all likely unrelated to neurocysticercosis (seizures during hyperemesis of pregnancy, arteriovenous malformation bleed, and gastric malignancy). Kalra 2003 reported headache, vomiting, and visual disturbance in the first 15 days of treatment, with no significant difference between the albendazole and no‐anthelmintic arms of the trial. Data were not provided. All of these studies had a corticosteroid regimen that varied between participants receiving albendazole and those who received placebo or no anthelmintic, so it is difficult to determine whether corticosteroids improved or worsened the frequency or nature of adverse events.
Alarcon 2001 and Sotelo 1988 only presented data for participants who received an anthelmintic. Alarcon 2001 reported that 8/27 (29.6%) of participants receiving albendazole for three days and 6/27 (22.2%) of participants receiving albendazole for eight days reported a headache. Data from the 29 participants who did not receive albendazole are not discussed. Of the participants who received albendazole, 14/54 (25.9%) reported vomiting or dizziness. Each albendazole duration group reported one participant that suffered from a seizure and one participant that suffered from a focal motor deficit. Sotelo 1988 pooled data from the 10 participants in the albendazole arm and 10 participants in the praziquantel arm of the trial: 14/20 (70%) suffered from headache, 9/20 (45%) nausea, 6/20 (30%) vomiting, 4/20 (20%) hyperthermia, 3/20 (15%) seizure, 2/20 (10%) somnolence, 1/20 (5%) diplopia, and 1/20 (5%) transient hemiparesis.
Adverse events requiring withdrawal of anthelmintics
None of the included studies reported an adverse event in any of their participants requiring withdrawal of treatment.
Comparison: praziquantel versus placebo or no anthelmintic
Two studies evaluated praziquantel as an intervention, comparing its use to no anthelmintic. The data from each study were either from very small populations or not presented in an extractable form, and therefore have not been included in further meta‐analysis.
Outcome of interest: seizure status at follow‐up
Foyaca‐Sibat 2001 presented data for 143 participants with neurocysticercosis and recurrent seizures, who were recruited from a population where most participants also had HIV, pulmonary tuberculosis, and/or urinary schistosomiasis. Of the participants for which data were presented, 71 received praziquantel, prednisolone, and phenytoin, and 72 received phenytoin alone. Results were presented as statistical analysis on an ordinal scale of seizure response in four categories, devised by the institution in which the study took place and not extractable, therefore these data were not considered for further analysis. Based on the graphs presented, most participants who received praziquantel had either resolution of seizures or reduced seizure duration and frequency. Most participants who received phenytoin alone had no change in their symptoms or reduced seizure frequency alone.
Outcomes of interest: radiological findings at follow‐up
Sotelo 1988 was a small study comparing 10 participants who received praziquantel to five participants who did not receive an anthelmintic. Of the 10 participants who received praziquantel, seven had complete radiological clearance of lesions; two had a reduction in the number of lesions; and one had no change in the number of lesions. Of the five participants who received no anthelmintic, five had no change in the number of lesions. Sotelo 1988 also reported data from 10 participants who were randomized to receive albendazole (included in Analysis 1.12 and Analysis 1.13 and discussed previously).
Outcomes of interest: adverse events associated with treatment (side effects) at follow‐up
Some of the potential adverse events or side effects from praziquantel administration, pooled with data from participants receiving albendazole (Sotelo 1988), are discussed above.
Discussion
Summary of main results
Findings for the most reported primary outcome of interest (seizure recurrence), including subgroup analysis by number of cysts, and two secondary outcomes (complete radiological clearance of lesions and evolution of cysts and adverse events), are presented in Table 1.
The results of studies comparing anthelmintic treatment with no anthelmintic were mixed and therefore difficult to interpret; recruitment criteria differed greatly between trials, including the number of cysts per participant, cyst viability, cyst anatomical position, and participant age. There is substantial heterogeneity in most comparisons as a result. Almost all of the included studies assessed albendazole; we found only two studies comparing praziquantel with no active treatment, from which we could not draw any meaningful conclusions on its efficacy.
Overall, albendazole probably makes little or no difference to seizure recurrence (low‐certainty evidence) compared to no anthelmintic. However, when considering the analysis stratified by number of cysts (single/multiple), there is probably a reduction of seizure reduction for participants with a single cyst (moderate‐certainty evidence). For participants with multiple cysts, we are uncertain as to whether albendazole reduces seizure recurrence, as the evidence is of very low certainty for this group. Studies contributing to the single cyst subgroup analysis only included participants with non‐viable intraparenchymal cysts, and most only recruited children, whereas studies contributing to the multiple cysts subgroup included participants with viable and non‐viable cysts, both intraparenchymal and extraparenchymal.
With regard to radiological findings, albendazole probably improves complete radiological clearance of lesions and evolution of cysts, with moderate‐certainty evidence. These findings were conserved with stratification by cyst viability.
Seizure recurrence
For seizure recurrence, most studies individually trended towards a benefit of albendazole, though the pooled analysis showed no evidence to support benefit or harm, with low‐certainty evidence. One study in particular, Das 2007, favoured no anthelmintic, and, as the largest study of the analysis, this finding had a large effect on the pooled results. It should be noted that Das 2007 recruited participants of any age, with multiple cysts of any viability or anatomical position (Figure 2), and also had an unclear risk of bias in three categories (random sequence generation, allocation concealment, and blinding). As such, its inclusion in the analysis contributed to an assessment of low‐certainty evidence for this outcome. However, we could not justify its exclusion from the analysis, given that its exclusion did not significantly improve the heterogeneity between studies, and there was no 'Risk of bias' domain that suggested a high risk.
Analysis was stratified by number of cysts, revealing that for participants with a single cyst, there was a probable benefit of albendazole compared to that of no anthelmintic, with moderate‐certainty evidence. For participants with multiple cysts, the pooled evidence was of very low certainty, therefore we are uncertain whether albendazole reduces seizure recurrence for people with multiple cysts, though the trend of the findings was towards a detriment/harm of albendazole versus placebo or no anthelmintic.
It is important to note the low heterogeneity between studies recruiting participants with single cysts (Figure 2), likely because all studies recruited participants with non‐viable, intraparenchymal cysts, and most studies (three out of five) only recruited children. As a result, stratification by cyst viability (viable versus non‐viable) and age (< 16 years versus ≥ 16 years) showed a similar trend to stratification by number of cysts (single versus multiple). Stratification by number of cysts was chosen for analysis as it has the strongest biological plausibility: summative burden of neurocysticerci could impact on the benefit or harm of anthelmintic treatment depending on the number of cysts, given that administration could lead to a co‐ordinated parasite death and degradation, and therefore a detrimental immune response with increased inflammation and oedema, in participants with multiple cysts.
Given that the meta‐analysis of data from participants with a single cyst exclusively includes participants with a non‐viable intraparenchymal cyst, the results should be interpreted with caution. Unfortunately, data for single viable cysts are lacking from the studies identified by this review for comparison. Any data available for viable cysts are complicated by substantial heterogeneity between participants and low‐certainty evidence, and as such are difficult to interpret (Figure 2). The fact that a significant effect of albendazole is observed in a population with a single non‐viable cyst may be considered counterintuitive, given the consensus that these cysts are already non‐viable. Degradation, however, takes months to years (a dying parasite rather than a dead parasite, both of which can appear as a non‐viable cyst), and it is plausible that treatment with anthelmintics could hasten this process.
Complete radiological clearance of lesions
For complete radiological clearance of lesions, there was evidence of a probable benefit of albendazole compared to placebo or no anthelmintic. Data contributing to this finding were all from follow‐up within 12 months of treatment. For data to be included into the analysis for this outcome, all lesions at participant‐level must have resolved completely and not calcified. One study, Das 2007, reported results that suggested a reduction in complete radiological clearance in participants receiving albendazole compared to placebo, though it must be noted that this is one of the few studies that recruited participants with two cysts or more (without an upper limit), and as such included participants with the largest neurocysticerci burden. Despite the heterogeneity and direction of findings introduced by the inclusion of Das 2007, the overall certainty of the evidence contributing to the pooled analysis was moderate.
Evolution of cysts
For evolution of cysts, there was evidence of a probable benefit of albendazole compared to placebo or no anthelmintic. Data contributing to this finding were all from follow‐up within 12 months of treatment. The certainty of the evidence contributing to the pooled analysis was moderate. This outcome intended to capture the progression of cysts from viable to non‐viable and events of calcification in addition to full radiological clearance, rather than full radiological clearance alone. All data were extracted at the participant level, not the lesion level. This limited the number of studies that could contribute to this outcome, as many studies reported lesion‐level evolution, rather than presenting the data in such a way that would permit participant‐level data extraction.
Adverse events
Participants treated with either albendazole or praziquantel appeared to experience more adverse events during treatment than those receiving placebo or no anthelmintic, with the most commonly reported adverse events or side effects being headache, abdominal pain, and nausea/vomiting. Studies reporting adverse events did so to a varying degree, therefore the frequency and nature of adverse events and side effects differed greatly between trials. None of the included studies reported an adverse event that required the withdrawal of treatment.
Overall completeness and applicability of evidence
We identified 16 relevant published studies. Most were published prior to the last update of this Cochrane Review and were included previously (Abba 2010). Three studies were new: two were published after the last update (Chaurasia 2010; Khurana 2012), and one was published prior to it (Foyaca‐Sibat 2001). Two studies had more data available after the publication of further manuscripts since the last update of this review (Carpio 2008; de Souza 2009). Unpublished data were provided for two studies (Alarcon 2001; Carpio 2008), after corresponding authors were contacted to clarify stratification between populations. All of the included studies were small: the largest enrolled 300 participants, and the smallest 25 participants.
This review does not fully represent the geographical burden of neurocysticercosis. All studies were based in Central and South America and South‐East Asia, apart from one study from South Africa (Foyaca‐Sibat 2001), which could only be included in the results narrative, rather than the meta‐analysis, due to its design and reporting methods. Consequently, there were no studies included in the meta‐analysis from Africa or China, where T solium is endemic and neurocysticercosis frequently reported.
The study participants varied, including participants of all ages and those with different numbers and types of lesion. Studies that recruited children tended to include participants with a single, non‐viable intraparenchymal cyst, whilst studies that recruited adults mostly included participants with multiple cysts of varying viability and anatomical position. This is likely a result of the natural history of neurocysticercosis: older participants have had more opportunity for cumulative exposure, leading to multiple cysts at different stages of progression.
Treatment comparisons of the included studies were wide‐ranging, including varying treatment duration (from 3 to 28 days of albendazole), the use of placebo or no anthelmintic treatment, and the concurrent use of corticosteroids for no participants/all participants/only participants receiving an anthelmintic. The true frequency of adverse events during treatment was difficult to ascertain given the different levels of reporting between studies. Headache, abdominal pain, nausea and vomiting were common, however in keeping with the known side effect profile of albendazole in the treatment of other helminth infections in clinical practice.
Of the 16 included studies, 11 reported seizure‐related outcomes, arguably the most important set of outcomes to patients. All but one of the included studies assessed and reported on the radiological presence of cysts and their resolution or evolution. However, this may not directly correlate with the presence or severity of seizures or other symptoms. Radiological outcomes specific to neurocysticercosis are easy to assess, though their utility may be limited clinically.
All of the included studies were small. In addition, considerable heterogeneity was introduced by one relatively large but poorly reported study, with results mostly running in the opposite direction to those reported in other studies (Das 2007). This is perhaps explained by the study's recruitment criteria (multiple cysts with mixed viability and anatomical positions), but could also be due to unclear risk of bias for random sequence generation, allocation concealment, and blinding. This study was also one of the few trials that administered corticosteroids in the albendazole treatment arm but not in the placebo arm. As a result, the certainty of the evidence to which this study contributed was downgraded on GRADE assessment.
Quality of the evidence
The risk of bias varied between studies; there appeared to be no correlation between risk of bias and either the trial size or how recently the study was conducted. Five studies had a low risk of bias across all indicators, six had an unclear risk of bias in one to three indicators, and five had a high risk of bias in one to two indicators, along with zero to three indicators at unclear risk of bias.
Risk of bias was one of the key GRADE assessment considerations, along with inconsistency, which resulted in the downgrading of evidence to low certainty for the outcome seizure recurrence before stratification. After stratifying by number of cysts, risk of bias and inconsistency continued to be of concern for participants with multiple cysts, along with indirectness. Together these considerations resulted in an assessment of very low‐certainty evidence. For participants with a single cyst, imprecision was the only GRADE consideration of concern, resulting in an assessment of moderate‐certainty evidence. Risk of bias was not a consideration of concern for the evidence available for this population.
For the outcome complete radiological clearance of lesions, inconsistency was the only GRADE consideration of concern, resulting in an assessment of moderate‐certainty evidence. For the outcome evolution of cysts, imprecision was the only consideration of concern, resulting in an assessment of moderate‐certainty evidence.
Potential biases in the review process
Extraction of data exclusively at the participant level for meta‐analysis in this review may have introduced selective outcome reporting, but we believe this method of extraction is preferable to including separate extraction and analyses at lesional‐level. A large proportion of studies contributing to our findings recruited individuals with a single cyst. Studies that pooled lesional data could not be included in meta‐analysis given our methods, but have been included in the narrative of the results.
The results after stratification by single or multiple cysts for the analysis of seizure recurrence could be explained by a number of variables (cyst viability, cyst anatomical position), as studies contributing to the data for single cysts exclusively recruited participants with non‐viable intraparenchymal cysts. As a result, the observed low heterogeneity was likely due to multiple factors contributing to the comparison of more homogenous populations. We have presented the results and forest plots for all subgroup stratifications for clarity, leading with number of cysts given the highest biological plausibility.
Finally, consideration of seizures being equal could have biased our results. Some studies showed significance in general seizure improvement that was not present in individuals with focal seizures. These findings are included in the narrative of our results. However, most studies reported seizure outcomes without defining the type of seizure; even if our methods were to have included stratification by general/focal seizures it would not have changed our results.
Agreements and disagreements with other studies or reviews
Our results vary slightly from other recent meta‐analyses and reviews assessing albendazole compared to placebo or no anthelmintic, but the conclusions reached are similar.
A meta‐analysis in children (Mazumdar 2007; four trials, 400 participants) with neurocysticercosis revealed a higher remission of seizures in those treated with albendazole compared to controls (RR 1.26, 95% CI 1.09 to 1.46). This agrees with our findings, and the four studies were included in our search results. Three of these studies contribute to our analysis of five trials that recruited participants with a single non‐viable intraparenchymal cyst. The authors of this review also reviewed 10 observational studies with varying results. The overall recommendation of this review was support for the use of albendazole for the treatment of children with one or two cysts secondary to neurocysticercosis.
Del Brutto 2006 reported a meta‐analysis of 11 randomized studies of participants with neurocysticercosis located in or adjacent to the cerebral parenchyma. Anthelmintic treatment was associated with complete radiological clearance of viable cysts (44% versus 19%; P = 0.025) at follow‐up. Studies on non‐viable cysts showed a trend towards lesion resolution favouring anthelmintic drugs (72% versus 63%; P = 0.38), but this was not significant unless an outlying trial was excluded (69% versus 55%; P = 0.006). In participants with non‐viable cysts, risk for seizure recurrence was lower after anthelmintic treatment (14% versus 37%; P < 0.001). The single trial evaluating the frequency of seizures in participants with viable cysts showed a 67% reduction in the rate of generalized seizures with treatment (P = 0.006). All studies identified by Del Brutto 2006 were included in our literature search; nine contributed to our review (Alarcon 2001; Baranwal 1998; Garcia 2004; Gogia 2003; Kalra 2003; Padma 1994; Padma 1995; Singhi 2004; Sotelo 1988), and two were excluded because there was no supportive treatment arm for comparison (Alarcon 1989; Carpio 1995).
White 2018 provides updated guidelines for the treatment of neurocysticercosis by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Treatment is recommended for viable cysts, with monotherapy (albendazole) for one to two cysts and dual therapy (albendazole and praziquantel) for more than two cysts. Data from Garcia 2004, included in this review, recruited participants exclusively with viable cysts and showed a significant reduction in the numbers of generalized seizures amongst participants. These findings, along with the data from Carpio 2008, are the main evidence used in White 2018 for viable cyst recommendations. Our findings do not provide sufficient evidence to support or dispute this; data for single viable cysts are lacking, and pooled analyses for multiple cysts have substantial heterogeneity. However, given the trend observed that albendazole may be detrimental with regard to seizure recurrence in participants with multiple cysts (Analysis 1.2), including participants with viable cysts, advocating anthelmintic treatment as standard for patients with multiple cysts should be done with caution. White 2018 advises that corticosteroids be used whenever anthelmintics are used. This could, in theory, mitigate the detrimental risk of anthelmintics in patients with multiple cysts, and our review was not designed to consider the impact of corticosteroids on anthelmintic administration specifically in the meta‐analysis. However, a trend towards harm in participants with multiple cysts receiving albendazole was observed (more seizure recurrence), despite the fact that Das 2007, the largest study contributing to the meta‐analysis for participants with multiple cysts, administered corticosteroids to participants receiving albendazole.
The updated IDSA/ASTMH guidelines recommend albendazole treatment for single enhancing (non‐viable) cysts, which is supported by the results of this review, and no treatment for calcified lesions alone. Extraparenchymal cysts should be treated on a case‐by‐case basis, with the focus of therapy centred around neuroendoscopy, cerebrospinal fluid diversion, or other surgical managements depending on the anatomical nature of the cyst. Given the heterogeneity introduced by the inclusion of Das 2007, a large study that included both intraparenchymal and extraparenchymal cysts, it could be that the inclusion of such complex patients impacted on the results of this trial.
Authors' conclusions
Implications for practice.
For participants presenting with seizures, the evidence suggests a benefit of albendazole for those with a single cyst, with a reduction in seizure recurrence. The evidence supporting this is of moderate certainty and is exclusively from studies that recruited participants with non‐viable intraparenchymal cysts, with a large proportion of participants being children (< 16 years). Evidence of benefit or harm of albendazole treatment for participants with more than one cyst is lacking, due in part to few studies reporting on such populations, but also to a large trial with very few exclusion criteria to recruitment, leading to substantial heterogeneity when included in analysis. That being said, the observed trend in participants with multiple cysts was that of potential harm from albendazole treatment, with more seizure recurrence compared to placebo or no anthelmintic, although the certainty of this evidence is very low.
This review suggests that there is a benefit of albendazole treatment with regard to complete radiological clearance of lesions secondary to neurocysticercosis as well as the evolution of cysts, regardless of viability or participant age. Treatment for calcified lesions is not recommended. It should also be noted that radiological abnormalities do not necessarily correlate with clinical symptoms.
In summary, this review supports the use of albendazole treatment for patients with a single cyst. For patients with multiple cysts, there is no strong evidence either way, but our results suggest that there may be a risk of albendazole causing more harm, even with corticosteroid adjunctive therapy (there was no clear difference in albendazole efficacy between studies that did or did not co‐administer corticosteroids to all participants). The current recommended duration of treatment (14 days ‐ White 2018) is longer than the treatment used in many of the studies included in this review, and requires further consideration, especially as many patients in the countries in which T solium is endemic are required to self‐fund their health care.
Implications for research.
Our initial search identified multiple studies, particularly those published more recently, which were designed to compare different anthelmintic drug regimens and/or the addition of corticosteroid adjunctive therapy to anthelmintic treatment, even though robust evidence for the benefit of anthelmintic treatment versus no anthelmintics is lacking. These studies were excluded from our review, as per our protocol; our focus was on the benefit of anthelmintics compared to no anthelmintic. As a result, there have been very few additional randomized controlled trials included in this review compared to the previous version (Abba 2010). Many of the additional manuscripts available from our updated search reported new analyses from studies conducted prior to 2010.
The certainty of evidence supporting the use of anthelmintics for people with neurocysticercosis is moderate at best. The literature would benefit from further randomized controlled trials (particularly trials that recruit either participants with a single viable cyst or participants with multiple cysts) to address this question, central to the management of neurocysticercosis. In particular, there is a need for larger studies with data reported at the participant level (rather than the lesion level). The presentation of data in future studies should aim to allow for the stratification of participants by cyst number, cyst viability, and cyst anatomical position in order to support future systematic review and meta‐analyses and permit an exploration of the nature of heterogeneity. All of these suggestions aim to improve the certainty of evidence available to support clinical decision‐making; at present, none of the evidence found in this review could be considered of high certainty.
What's new
| Date | Event | Description |
|---|---|---|
| 27 May 2021 | New citation required and conclusions have changed | This review updates the Cochrane Review 'Anthelmintics for people with neurocysticercosis' (Abba 2010). A new team of authors worked on this review update. The criteria for inclusion of trials has changed. |
| 27 May 2021 | New search has been performed | The review author team revised the protocol, including updated definitions within the Methods (intervention/outcomes). This was approved by the Cochrane Infectious Diseases Group editorial team on 16 March 2018 (see Appendix 2). |
History
Protocol first published: Issue 4, 2006 Review first published: Issue 2, 1996
| Date | Event | Description |
|---|---|---|
| 15 February 2010 | Amended | Corrected search dates in abstract |
| 1 September 2009 | New citation required and conclusions have changed | This review replaced an earlier Cochrane Review 'Drugs for treating neurocysticercosis (tapeworm infection of the brain)' (Salinas 1999), which was withdrawn from the Cochrane Library in 2005 due to the availability of new trial evidence. A new team of authors worked on this review. The criteria for inclusion of trials has changed. |
| 5 May 2009 | New search has been performed | Search updated. |
Acknowledgements
The Academic Editor is Professor Mical Paul.
This review replaces the Abba 2010 Cochrane Review, which previously replaced the original Cochrane Review on this topic (Salinas 1999). We are grateful to Rodrigo Salinas and colleagues for the opportunity to update this review. We acknowledge that much of this protocol is based upon the original Salinas 1999 Cochrane Review and subsequent Abba 2010 Cochrane Review update.
The editorial base of the Cochrane Infectious Diseases Group is funded by UK aid from the UK government for the benefit of low‐ and middle‐income countries (project number 300342‐104). The views expressed do not necessarily reflect the UK government’s official policies.
Appendices
Appendix 1. Search methods: detailed search strategies
| Cochrane Central Register of Controlled Trials Issue 10 of 12, October 2020 |
|
| 1 | 'MeSH descriptor: [Taenia solium] explode all trees' |
| 2 | (Taenia solium):ti, ab, kw |
| 3 | Neurocysticercosis:ti, ab,kw |
| 4 | 'MeSH descriptor: [Neurocysticercosis] explode all trees' |
| 5 | "brain and cysticercosis" |
| 6 | cerebral cysticercosis |
| 7 | #1 or #2 or #3 or #4 or #5 or #6 |
| 8 | Albendazole:ti, ab, kw |
| 9 | Praziquantel:ti, ab, kw |
| 10 | 'MeSH descriptor: [albendazole] explode all trees' |
| 11 | 'MeSH descriptor: [praziquantel] explode all trees' |
| 12 | 'MeSH descriptor: [Anticestodal agents] explode all trees' |
| 13 | antihelminthic* OR anti‐helminthic* OR anthelmintic*:ti, ab, kw |
| 14 | #8 or #9 or #10 or #11 or #12 or #13 |
| 15 | #7 and #14 |
Pubmed
| Search | Query |
| #3 | Search taenia solium Field: Title/Abstract |
| #5 | Search "Taenia solium"[Mesh] Field: Title/Abstract |
| #6 | Search "Neurocysticercosis"[Mesh] Field: Title/Abstract |
| #7 | Search neurocysticerc* Field: Title/Abstract |
| #8 | Search "brain cysticerc*" or "cerebral cysticerc*" Field: Title/Abstract |
| #9 | Search ((((#8) OR #7) OR #6) OR #5) OR #3 Field: Title/Abstract |
| #10 | Search albendazole Field: Title/Abstract |
| #12 | Search "Albendazole"[Mesh] Field: Title/Abstract |
| #13 | Search praziquantel Field: Title/Abstract |
| #16 | Search "Praziquantel"[Mesh] Field: Title/Abstract |
| #17 | Search "Anticestodal Agents"[Mesh] Field: Title/Abstract |
| #18 | Search antihelminthic* OR anti‐helminthic* OR anthelmintic* Field: Title/Abstract |
| #19 | Search (((((#18) OR #17) OR #16) OR #13) OR #12) OR #10 Field: Title/Abstract |
| #20 | Search (#19) AND #9 Field: Title/Abstract |
| #22 | Search "Randomized Controlled Trial" [Publication Type] OR "Controlled Clinical Trial" [Publication Type] Field: Title/Abstract |
| #23 | Search randomized or placebo Field: Title/Abstract |
| #25 | Search randomly or trial or groups Field: Title/Abstract |
| #28 | Search "drug therapy" [Subheading] Field: Title/Abstract |
| #29 | Search (((#28) OR #25) OR #23) OR #22 Field: Title/Abstract |
| #30 | Search (animals[MeSH Terms]) NOT humans[MeSH Terms] Field: Title/Abstract |
| #31 | Search (#29) NOT #30 Field: Title/Abstract |
| #32 | Search (#31) AND #20 Field: Title/Abstract |
| #33 | Search (#31) AND #20 Filters: Humans; Field: Title/Abstract |
Database: Embase 1947‐Present, updated daily
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 taenia solium.mp. or Taenia solium/
2 neurocysticercosis/
3 neurocysticerc*.mp.
4 ("brain cysticerc*" or "cerebral cysticerc*").mp.
5 1 or 2 or 3 or 4
6 albendazole.mp. or albendazole/
7 praziquantel.mp. or praziquantel/
8 anthelmintic agent/ or anthelminths.mp.
9 (antihelminthic* or anti‐helminthic* or anthelmintic*).mp.
10 6 or 7 or 8 or 9
11 5 and 10
12 randomized controlled trial/ or controlled clinical trial/
13 (randomized or randomised or placebo or double‐blind* or single‐blind*).ti. or (randomized or randomised or placebo or double‐blind* or single‐blind*).ab.
14 12 or 13
15 11 and 14
LILACS
tw:(neurocysticercosis) AND ( db:("LILACS") AND type_of_study:("clinical_trials"))
Clinicaltrials.gov:
Neurocysticercosis , Taeniasis, Cysticercosis
WHO ICTRP: Neurocysticerc*
Appendix 2. Prespecified changes for review update 2021
| Protocol section | Revised protocol |
| Background and research question |
|
| Inclusion criteria |
|
| Methods |
|
| This table was approved by the Cochrane Infectious Diseases Group editorial team on 14 September 2018. | |
Data and analyses
Comparison 1. Albendazole versus placebo or no anthelmintic.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Seizure recurrence | 10 | 1054 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.78, 1.14] |
| 1.2 Seizure recurrence subgroup analysis: number of cysts | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.2.1 Participants with a single lesion | 5 | 396 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.40, 0.91] |
| 1.2.2 Participants with multiple (> 1) lesions | 2 | 321 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.05 [1.28, 3.31] |
| 1.3 Seizure recurrence subgroup analysis: viability of cysts | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.3.1 Participants with viable cysts | 2 | 174 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.62, 1.33] |
| 1.3.2 Participants with non‐viable cysts | 6 | 507 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.38, 0.78] |
| 1.4 Seizure recurrence subgroup analysis: position of cysts | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.4.1 Participants with intraparenchymal cysts | 8 | 681 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.51, 0.86] |
| 1.5 Seizure recurrence subgroup analysis: age of participants | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.5.1 Children (under 16 years old) | 4 | 335 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.30, 0.69] |
| 1.5.2 Adults (16 years old or older) | 2 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [1.06, 1.98] |
| 1.6 Seizure recurrence subgroup analysis: corticosteroid use | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.6.1 Participants receiving corticosteroids | 3 | 208 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.61, 1.13] |
| 1.6.2 Participants not receiving corticosteroids | 3 | 230 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.50, 1.88] |
| 1.7 Death (any cause) | 2 | 470 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.24, 2.85] |
| 1.8 Hospital admission (any cause) | 1 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.50 [1.52, 4.11] |
| 1.9 Headache | 1 | 161 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.91, 1.06] |
| 1.10 Signs of focal neurological deficit | 1 | 161 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.65, 1.23] |
| 1.11 Surgical intervention | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.07, 38.94] |
| 1.12 Complete radiological clearance of lesions | 13 | 1324 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [1.07, 1.39] |
| 1.13 Reduction of number of lesions | 3 | 115 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.89, 1.98] |
| 1.14 Evolution of cysts | 6 | 434 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [1.10, 1.47] |
| 1.15 Radiological resolution of oedema | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.61, 1.28] |
| 1.16 Radiological development of oedema | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.06, 11.77] |
| 1.17 Complete radiological clearance of lesions subgroup analysis: viability of cysts | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.17.1 Participants with viable cysts | 3 | 207 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.52 [1.65, 3.85] |
| 1.17.2 Participants with non‐viable cysts | 8 | 748 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [1.05, 1.41] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Alarcon 2001.
| Study characteristics | ||
| Methods | Type of study: randomized controlled trial Duration: participants followed up for 12 months (primary outcome) and 24 months (secondary outcome), recruited between January 1989 and December 1996 |
|
| Participants | Number: 95 enrolled, data available for 83 after exclusions/loss to follow‐up (36 male, 47 female) Inclusion criteria: males and females of all ages, neurological signs and symptoms for between 1 week and 3 years, 1 to 6 non‐enhancing parenchymal cysts on CT Exclusion criteria: ring‐like or nodular enhanced cysts on CT, perilesional oedema, subarachnoid or intraventricular cysts, hydrocephalus, previous treatment with albendazole or praziquantel, pregnant women, and clinical evidence intracranial hypertension Types of lesion: viable cysts Position of lesions: intraparenchymal Number of lesions: 1 to 6 |
|
| Interventions | Group 1. Albendazole: 15 mg/kg daily for 3 days Group 2. Albendazole: 15 mg/kg daily for 8 days Group 3. No albendazole All groups: antiepileptics (symptomatic only) |
|
| Outcomes | Included in meta‐analysis: seizure recurrence (≥ 24 months), surgical intervention (24 months), complete radiological clearance of lesions (12 months) Included in narrative only: frequency and nature of adverse events |
|
| Notes | Location: Ecuador Source of funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated, but trial described as "randomized". |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No placebo used, and no blinding described. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Evaluation of the number of cysts on CT at baseline and at follow‐up was performed by a single neuroradiologist (GD) blinded to treatment allocation." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 95 participants randomized: 8 excluded before starting treatment, 4 lost to follow‐up, 83 (87%) included in the analysis. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence of other bias |
Baranwal 1998.
| Study characteristics | ||
| Methods | Type of study: randomized controlled trial Duration: participants followed up for 15 months, dates not stated |
|
| Participants | Number: 72 enrolled, data available for 63 after exclusions/loss to follow‐up (34 male, 29 female) Inclusion criteria: children aged 2 to 12 years, focal seizures for < 3 months, "single small enhancing computerised tomographic lesion" on CT < 20 mm Exclusion criteria: neurological deficit, suspected tuberculosis Types of lesion: non‐viable cyst Position of lesions: intraparenchymal Number of lesions: 1 |
|
| Interventions | Group 1. Albendazole: 15 mg/kg daily in 2 divided doses for 28 days Group 2. Placebo: identical dextrose capsules for 28 days All groups: prednisolone (all), antiepileptics (symptomatic only) |
|
| Outcomes | Included in meta‐analysis: seizure recurrence (15 months), complete radiological clearance of lesions (3 months), evolution of cysts (3 months) Included in narrative only: frequency and nature of adverse events |
|
| Notes | Location: India Source of funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Numbered in a random sequence using a random number table |
| Allocation concealment (selection bias) | Low risk | Quote: "Envelopes containing albendazole or placebo capsules for the full course of therapy were prepared in advance and numbered in a random sequence..." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "All persons involved in the study, i.e. patient, clinical investigator and neuroradiologist were blind to this random allocation. Results were decoded after completion of 6 months of study to identify the groups." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All persons involved in the study, i.e. patient, clinical investigator and neuroradiologist were blind to this random allocation. Results were decoded after completion of 6 months of study to identify the groups." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 72 participants randomized: 9 lost to follow‐up, 63 (88%) included in the analysis. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence of other bias |
Carpio 2008.
| Study characteristics | ||
| Methods | Type of study: randomized controlled trial Duration: participants followed up for 24 months, recruited between February 2001 and February 2003 |
|
| Participants | Number: 178 enrolled, data available for 178 before exclusions/loss to follow‐up (97 male, 77 female, 4 missing sex data) Inclusion criteria: males and females of all ages, new onset symptoms associated with neurocysticercosis for < 2 months, active and/or transitional neurocysticercosis cysts on axial CT or MRI of the brain Exclusion criteria: only calcified cysts, pregnancy, papilloedema, active tuberculosis, syphilis, ocular cysticercosis, active gastric ulcers, or any progressive or life‐threatening disorder Types of lesion: viable or non‐viable cysts, or both Position of lesions: intraparenchymal or extraparenchymal, or both Number of lesions: ≥ 1 |
|
| Interventions | Group 1. Albendazole: 400 mg twice daily for 8 days (if ≥ 50 kg) or 15 mg/kg daily (if < 50 kg, including children) Group 2. Placebo: identical tablets for 8 days All groups: prednisolone (all), antiepileptics (symptomatic only) |
|
| Outcomes | Included in meta‐analysis: seizure recurrence (24 months), death (any cause) (24 months), headache (12, 24 months), signs of focal neurological deficit (12, 24 months), complete radiological clearance of lesions (12 months), reduction of number of lesions (12 months), evolution of cysts (12 months) Included in narrative only: frequency of seizures, frequency and nature of adverse events |
|
| Notes | Location: Ecuador Source of funding: NINDS grant R01‐NS39403. Glaxo/SKB and Acromax Co supplied the active drug and placebo. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were allocated to treatment group according to a stratified block randomisation scheme. Two strata were considered: centre (sex centres) and location of the cyst (parenchymal vs extraparenchymal). Permuted blocks of size 4 and 6 were used to balance the treatment allocation within each stratum." |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomisation lists were kept in electronic form on a computer accessible only to the statistician..." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "This paper reports the results of a double blind, randomised, placebo controlled trial..." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All other research staff were blinded to the treatment arm." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 178 participants randomized: 8 did not receive their allocation; 15 dropped out of the trial after treatment; 161 (90%) included in the analysis (including 7 that died before the end of follow‐up). |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence of other bias |
Chaurasia 2010.
| Study characteristics | ||
| Methods | Type of study: randomized controlled trial Duration: participants followed up for 6 months, recruited between November 2007 and October 2008 |
|
| Participants | Number: 67 enrolled, data available for 67 with no exclusions/loss to follow‐up (43 male, 24 female) Inclusion criteria: males and females of all ages, new‐onset seizures < 2 weeks, solitary contrast enhancing lesion on CT < 20 mm in diameter Exclusion criteria: neurological deficit (other than minimal), evidence of raised intracranial pressure, cerebral oedema on CT, already received anthelmintic or steroid, systemic disease (e.g. pulmonary tuberculosis, renal failure, or symptomatic secondary epilepsies) Types of lesion: non‐viable cyst Position of lesions: intraparenchymal Number of lesions: 1 |
|
| Interventions | Group 1. Albendazole: 15 mg/kg daily in 2 divided doses for 3 days Group 2. Placebo: identical tablets for 3 days All groups: antiepileptics (symptomatic only) |
|
| Outcomes | Included in meta‐analysis: seizure recurrence (6 months), complete radiological clearance of lesions (6 months), evolution of cysts (6 months) Included in narrative only: frequency and nature of adverse events |
|
| Notes | Location: India Source of funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was done after inclusion, with the help of a random number table." |
| Allocation concealment (selection bias) | Unclear risk | Quote: "...were randomly allocated to receive either..." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "...we evaluated a 'three day course' of albendazole in a prospective randomized double‐blind trial." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All CT scans were examined by a radiologist who was blinded to the treatment arms." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 67 participants randomized: 67 (100%) included in the analysis. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence of other bias |
Das 2007.
| Study characteristics | ||
| Methods | Type of study: randomized controlled trial Duration: participants followed up for 5 years; recruitment dates not described, but the trial was conducted between January 1997 and January 2005 |
|
| Participants | Number: 300 enrolled, data available for 300 before exclusions/loss to follow‐up (178 male, 122 female) Inclusion criteria: males and females (age not stated but all recruited ≥ 18 years), recent‐onset seizures, residents of Burdwan District (West Bengal) in Eastern India, CT and MRI results with at least 2 lesions strongly suggestive of neurocysticercosis with at least 1 in the vesicular stage, and antibodies against cysticercosis detected by ELISA on at least 3 occasions Exclusion criteria: history of primary seizure disorder, family history of seizure, pre‐existing focal neurological deficit, any metabolic or hereditary disease Types of lesion: unclear, though likely non‐viable cysts with or without viable cysts Position of lesions: mixed intra/extraparenchymal Number of lesions: ≥ 2 |
|
| Interventions | Group 1. Albendazole 15 mg/kg daily plus dexamethasone 2 mg orally at 8‐hour intervals for 14 days (dexamethasone tapered "over time") Group 2. Placebo: 14 days All groups: antiepileptics (all) |
|
| Outcomes | Included in meta‐analysis: seizure recurrence (3 months), death (all cause) (12 months), hospital admission (all cause) (3 months), complete radiological clearance of lesions (12, 60 months) Included in narrative only: headache, evolution of cysts |
|
| Notes | Location: India Source of funding: Burdwan Medical College and Hospital |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were randomly allocated into two groups." |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described for participants, investigators, or medical staff, though placebo used |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described for participants, investigators, or medical staff, though placebo used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 300 participants randomized: 2 died in the first 3 months, 298 (99%) included in the analysis. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence of other bias |
de Souza 2009.
| Study characteristics | ||
| Methods | Type of study: randomized controlled trial Duration: participants followed up formally for 24 months, enrolled between May 2002 and October 2003, with telephone follow‐up in October 2006 |
|
| Participants | Number: 123 enrolled, data available for 103 after exclusions/loss to follow‐up (male/female data not available) Inclusion criteria: males and females of all ages, new‐onset focal or generalized seizures, MRI‐confirmed parenchymal solitary cysticercal lesion Exclusion criteria: history of epilepsy, albendazole or praziquantel received previously, evidence of other lesions on CT/MRI, significant neurological deficits, raised intracranial pressure, seizures refractory to acute treatment Types of lesion: non‐viable cyst Position of lesions: intraparenchymal Number of lesions: 1 |
|
| Interventions | Group 1. Albendazole 15 mg/kg daily in 2 divided doses for 28 days Group 2. No albendazole All groups: antiepileptics (all) |
|
| Outcomes | Included in meta‐analysis: complete radiological clearance of lesions (24 months) Included in narrative only: seizure recurrence, time to seizure remission, evolution of cysts, radiological resolution/development of oedema |
|
| Notes | Location: India Source of funding: Indian Council for Medical Research |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The patients were randomised to two groups by means of a random number table consisting of 200 numbers..." |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No placebo used, and no blinding described. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quotes: "The physicians who reviewed patient details at these follow‐up visits were blinded to the two treatment groups...", "...two independent trained physicians who were blinded to treatment allocation analysed the MRIs." Note that no placebo was used. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 123 participants randomized: 20 lost to follow‐up within the first 12 months, 103 (84%) included in the analysis. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence of other bias |
Foyaca‐Sibat 2001.
| Study characteristics | ||
| Methods | Type of study: randomized controlled trial Duration: participants followed up for 1 month, enrolled over a 2‐year period, dates not stated |
|
| Participants | Number: 163 enrolled, data available for 143 after exclusions but before loss to follow‐up (65 male, 68 female) Inclusion criteria: uncontrolled epilepsy probably due to neurocysticercosis, active/chronic forms of neurocysticercosis after CT, without signs of raised intracranial pressure Exclusion criteria: previous history of neurological disease apart from epilepsy, metabolic disorders, cerebrovascular disease, meningoencephalitis, head injury, immunomodulatory agents received in the previous 6 months, alternative cause for intracranial calcification, suspicion of tuberculoma/pyogenic brain abscess/mycotic granuloma, primary or metastatic brain tumour, uncontrollable tonic‐clonic generalized motor seizures despite regular phenytoin 300 mg at night Types of lesion: viable or non‐viable cysts, or both Position of lesions: not stated Number of lesions: not stated |
|
| Interventions | Group 1. Praziquantel 100 mg/kg daily in 4 divided doses for 1 day with prednisolone 40 mg daily for 5 days Group 2. No praziquantel or prednisolone All groups: phenytoin 400 mg once at night for 5 days (all) |
|
| Outcomes | Included in meta‐analysis: none Included in narrative only: seizure recurrence, frequency of seizures |
|
| Notes | Location: South Africa Source of funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...by block‐randomization procedure." |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The study was designed as a double blind, randomized trial..." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The study was designed as a double blind, randomized trial..." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 163 participants randomized: 34 lost to follow‐up, 129 (79%) included in the analysis. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence of other bias |
Garcia 2004.
| Study characteristics | ||
| Methods | Type of study: randomized controlled trial Duration: participants followed up for 30 months, enrolled between January 1997 and March 1999 |
|
| Participants | Number: 120 enrolled, data available for 120 before exclusions/loss to follow‐up (61 male, 59 female) Inclusion criteria: adult patients, viable parenchymal cysts on CT, serological confirmation of Taenia solium infection by ELISA, history of 1 or more spontaneous seizures within the previous 6 months but less than 10 years in duration Exclusion criteria: pregnancy, primary generalized seizures, > 20 cysts on CT, evidence of other diseases not attributable to cysticercosis on CT, moderate or severe intracranial hypertension, status epilepticus, focal neurological deficits, unstable vital signs, impending risk of death Types of lesion: viable cysts Position of lesions: intraparenchymal Number of lesions: 1 to 20 |
|
| Interventions | Group 1. Albendazole 400 mg twice daily and dexamethasone 2 mg 3 times a day for 10 days Group 2. Placebos: 2 placebos of similar appearance for 10 days All groups: antiepileptics (all) |
|
| Outcomes | Included in meta‐analysis: seizure recurrence (30 months), complete radiological clearance of lesions (6 months) Included in narrative only: frequency of seizures, evolution of cysts, nature and frequency of adverse events |
|
| Notes | Location: Peru Source of funding: US Food and Drug Administration (FD‐R‐001107‐03) and the National Institute of Allergy and Infectious Diseases, National Institutes of Health (U19‐A145431) and the Tropical Medicine Department of SmithKline Beecham |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Random assignment of the patients to one of the two study groups was performed...in blocks of six according to a pre‐established list taken from a random‐numbers table." |
| Allocation concealment (selection bias) | Low risk | Quote: "in Lima, by a biostatistician not otherwise involved in the study." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind, placebo‐controlled trial" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind, placebo‐controlled trial" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 120 randomized: 4 did not receive allocated treatment, 2 excluded, 14 lost to follow‐up, 116 (97%) included in the analysis (those that received allocated treatment). |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence of other bias |
Gogia 2003.
| Study characteristics | ||
| Methods | Type of study: randomized controlled trial Duration: participants followed up for 6 months, enrolled between March 2000 and July 2000 |
|
| Participants | Number: 72 enrolled, data available for 72 with no exclusions/loss to follow‐up (38 male, 34 female) Inclusion criteria: children (age not stated, but all participants 1.5 to 12 years), ring‐enhancing lesions in brain parenchyma on CT, seizures without a history of epilepsy Exclusion criteria: evidence of tuberculosis in the body, known epilepsy on antiepileptic prophylaxis, chronic central nervous system disorders (e.g. congenital malformation, hydrocephalus, cerebral palsy), neurodegenerative disorders, disc/nodular/calcific lesions on CT Types of lesion: non‐viable cysts Position of lesions: intraparenchymal Number of lesions: ≥ 1 |
|
| Interventions | Group 1. Albendazole 15 mg/kg daily in 2 divided doses for 28 days Group 2. Placebo for 28 days All groups: prednisolone 2 mg/kg daily for the 3 days preceding albendazole/placebo (all) and phenytoin 5 mg/kg daily (all) |
|
| Outcomes | Included in meta‐analysis: seizure recurrence (6 months), complete radiological clearance of lesions (6 months), evolution of cysts (6 months) Included in narrative only: none |
|
| Notes | Location: India Source of funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The enrolled children were randomized using a random number table." |
| Allocation concealment (selection bias) | Low risk | Quotes: "...coded as drug A or drug B...", "The drugs were dispensed in coded envelopes." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The investigators and the patients were thus blinded to which drug was being given to which patient. The radiologist responsible for reading the X‐rays was also blinded to the drug therapy." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The investigators and the patients were thus blinded to which drug was being given to which patient. The radiologist responsible for reading the X‐rays was also blinded to the drug therapy." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 72 randomized: 72 (100%) included in analysis. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence of other bias |
Kalra 2003.
| Study characteristics | ||
| Methods | Type of study: randomized controlled trial Duration: participants followed up for 6 months, enrolment dates not stated |
|
| Participants | Number: 123 enrolled, data available for 123 before exclusions/loss to follow‐up (65 male, 58 female) Inclusion criteria: children aged 1 to 14 years, seizures plus 1 or 2 ring‐enhancing lesions < 20 mm, excluding intraventricular cysts Exclusion criteria: evidence of tuberculosis on chest radiograph, positive Mantoux test, history of contact with a person with tuberculosis, intraocular or intraventricular cysts, disc or calcified lesions, multiple lesions (> 2), or hydrocephalus Types of lesion: non‐viable cysts Position of lesions: intraparenchymal Number of lesions: 1 to 2 |
|
| Interventions | Group 1. Albendazole 15 mg/kg daily for 28 days with dexamethasone 0.15 mg/kg for 5 days Group 2. No dexamethasone or albendazole All groups: antiepileptics (all) |
|
| Outcomes | Included in meta‐analysis: seizure recurrence (6 months), complete radiological clearance of lesions (6 months) Included in narrative only: evolution of cysts, frequency and nature of adverse events |
|
| Notes | Location: India Source of funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A simple randomization scheme was used for allocation of patients to the treated or control groups." |
| Allocation concealment (selection bias) | Low risk | Quote: "Random assignment code was concealed up to the time of allocation in sealed envelopes labeled (sic) with a unique patient number." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quotes: "...lack of blinding of the treating physician or patient...", "...open trial..." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quotes: "...lack of blinding of the treating physician or patient...", "...open trial..." (assessed as high risk given contribution to data on seizure recurrence outcome) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 123 participants randomized: 30 lost to follow‐up, 93 (76%) included in the analysis. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence of other bias |
Khurana 2012.
| Study characteristics | ||
| Methods | Type of study: randomized controlled trial Duration: participants followed up for 6 months, enrolment dates not stated |
|
| Participants | Number: 105 enrolled, data available for 105 with no exclusions/loss to follow‐up (75 male, 30 female) Inclusion criteria: all ages, new‐onset seizures < 2 weeks, single enhancing lesions < 20 mm on CT Exclusion criteria: evidence of raised intracranial pressure, neurological deficits, evidence of systemic illness (e.g. tuberculosis, renal failure), abnormality on CT other than solitary cysticercus granuloma, well‐defined epileptic syndromes and symptomatic secondary epilepsies, prior albendazole treatment Types of lesion: non‐viable cyst Position of lesions: intraparenchymal Number of lesions: 1 |
|
| Interventions | Group 1. Albendazole 15 mg/kg daily for 3 days Group 2. Albendazole 15 mg/kg daily for 15 days Group 3. No albendazole All groups: antiepileptics (all) |
|
| Outcomes | Included in meta‐analysis: seizure recurrence (6 months), complete radiological clearance of lesions (6 months), evolution of cysts (6 months) Included in narrative only: frequency and nature of adverse events |
|
| Notes | Location: India Source of funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Included patients were randomly assigned to three groups of 35 each, using a computer‐generated table of random numbers." |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No placebo used, and no blinding described ("open label randomized trial"). |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No placebo used, and no blinding described ("open label randomized trial"). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 105 participants randomized: 105 (100%) included in the analysis. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence of other bias |
Padma 1994.
| Study characteristics | ||
| Methods | Type of study: randomized controlled trial Duration: participants followed up for 3 months, dates not stated |
|
| Participants | Number: 75 enrolled, data available for 75 with no exclusions/loss to follow‐up (52 male, 23 female) Inclusion criteria: all ages, epilepsy, single small enhancing lesion on CT Exclusion criteria: neurological deficit on examination Types of lesion: non‐viable Position of lesions: intraparenchymal Number of lesions: 1 |
|
| Interventions | Group 1. Albendazole 15 mg/kg daily for 7 days Group 2. Placebo for 7 days All groups: no additional management |
|
| Outcomes | Included in meta‐analysis: evolution of cysts (3 months) Included in narrative only: none |
|
| Notes | Location: India Source of funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly allocated to placebo or albendazole therapy." |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "...we carried out a doubleblind, randomized, placebo‐controlled study..." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quotes: "CTs were assessed by a neuroradiologist (N.K.M.) who was not aware of the treatment received by the patient...", "...we carried out a doubleblind, randomized, placebo‐controlled study..." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 75 participants randomized: 75 (100%) included in the analysis. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence of other bias |
Padma 1995.
| Study characteristics | ||
| Methods | Type of study: randomized controlled trial Duration: participants followed up for 3 months, dates not stated |
|
| Participants | Number: 29 enrolled, data available for 29 with no exclusions/loss to follow‐up (22 male, 7 female) Inclusion criteria: all ages, multiple cystic lesions suggestive of cysticercosis on CT Exclusion criteria: calcified lesions only Types of lesion: viable or non‐viable cysts, or both Position of lesions: not stated, presumed mixed intra/extraparenchymal Number of lesions: ≥ 2 |
|
| Interventions | Group 1. Albendazole 15 mg/kg per day in 2 divided doses for 7 days Group 2. Placebo: tablets similar in appearance for 7 days All groups: antiepileptics (participants with history of seizures) |
|
| Outcomes | Included in meta‐analysis: reduction of number of lesions (3 months), radiological resolution/development of oedema (3 months) Included in narrative only: none |
|
| Notes | Location: India Source of funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "We undertook a randomized, double‐blind, placebo‐controlled study..." |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quotes: "...double‐blind, placebo‐controlled study...", "Placebo tablets of similar appearance...", "The CT scans were assessed...by a neuroradiologist who was not aware of the treatment given." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quotes: "...double‐blind, placebo‐controlled study...", "Placebo tablets of similar appearance...", "The CT scans were assessed...by a neuroradiologist who was not aware of the treatment given." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 29 randomized: 29 (100%) included in the analysis. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence of other bias |
Singhi 2000.
| Study characteristics | ||
| Methods | Type of study: randomized controlled trial Duration: participants followed up for 3 to 6 months, recruited 1994 to 1998 |
|
| Participants | Number: 380 participants likely enrolled (description is not clear), data available for 176 after exclusions/loss to follow‐up (of original 500 patient population: 272 male, 228 female) Inclusion criteria: children aged up to 14 years, a single small ring‐enhancing lesion in brain parenchyma on CT/MRI Exclusion criteria: none stated Types of lesion: non‐viable cyst Position of lesions: intraparenchymal Number of lesions: 1 |
|
| Interventions | Group 1. Albendazole 15 mg/kg daily for 28 days (addition of prednisolone unclear) Group 2. No albendazole All groups: antiepileptics (participants with seizures) |
|
| Outcomes | Included in meta‐analysis: complete radiological clearance of lesions (3 to 6 months) Included in narrative only: evolution of cysts |
|
| Notes | Location: India Source of funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quotes: "Children...were chosen at random to receive either albendazole therapy or no specific anticysticercal therapy...", "...randomly allocated children..." |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No placebo used, and no blinding described. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No placebo used, and no blinding described. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 380 participants randomized: 210 followed up (55%), and 176 (46%) included in the analysis. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence of other bias |
Singhi 2004.
| Study characteristics | ||
| Methods | Type of study: randomized controlled trial Duration: participants followed up for 18 months, recruitment dates not stated |
|
| Participants | Number: 133 participants enrolled, data available for 110 after exclusions/loss to follow‐up (66 male, 44 female) Inclusion criteria: children aged up to 14 years, seizure history < 3 months duration, a single small enhancing lesion on CT/MRI Exclusion criteria: neurological deficit Types of lesion: non‐viable cyst Position of lesions: intraparenchymal Number of lesions: 1 |
|
| Interventions | Group 1. Albendazole 15 mg/kg daily for 28 days Group 2: Albendazole 15 mg/kg daily for 28 days plus prednisolone Group 2. No albendazole plus prednisolone All groups: antiepileptics (all) |
|
| Outcomes | Included in meta‐analysis: seizure recurrence (72 weeks), complete radiological clearance of lesions (6 months) Included in narrative only: seizure recurrence after withdrawal of antiepileptics |
|
| Notes | Location: India Source of funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The children were randomly assigned...using random number tables." |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No placebo used, and no blinding described. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All CT scans were assessed by a neuroradiologist (N.K.), who was blinded to treatment assignment and to clinical outcome." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 133 participants randomized: 23 lost to follow‐up (study arm not described), 110 (83%) included in the analysis. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence of other bias |
Sotelo 1988.
| Study characteristics | ||
| Methods | Type of study: randomized controlled trial Duration: participants were followed up for 3 to 4 months, dates not stated |
|
| Participants | Number: 25 enrolled, data available for 25 with no exclusions/loss to follow‐up (16 male, 9 female) Inclusion criteria: adults, parenchymal brain cysticercosis Exclusion criteria: none stated Types of lesion: viable cysts Postition of lesions: intraparenchymal Number of lesions: ≥ 1 |
|
| Interventions | Group 1. Albendazole 15 mg/kg daily for 1 month Group 2. Praziquantel 50 mg/kg daily for 14 days Group 3. No albendazole or praziquantel All groups: antiepileptics (participants already receiving antiepileptics) |
|
| Outcomes | Included in meta‐analysis: complete radiological clearance of lesions (3 to 4 months), reduction of number of lesions (3 to 4 months) Included in narrative only: frequency and nature of adverse events |
|
| Notes | Location: Mexico Source of funding: partially supported by Subsecretaría de Regulación Sanitaria y Desarrollo, Secretaria de Salud de México, México City |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly allocated in three groups..." |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No placebo used, and no blinding described. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No placebo used, and no blinding described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 25 participants enrolled; 25 (100%) included in the analysis. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence of other bias |
CT: computed tomography
ELISA: enzyme‐linked immunosorbent assay
MRI: magnetic resonance imaging
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alarcon 1989 | Not a randomized controlled trial for intervention arms versus control: outcome of control arm determined from comparisons between a CT scan at the beginning of the trial and images taken years before. This trial was included in previous versions of this review. |
| Botero 1993 | No control: albendazole treatment arm only |
| Bustos 2006 | No control: albendazole treatment arm only |
| Carpio 1995 | Quasi‐randomized trial: 1 assignment sequence used |
| Chen 1984 | Article unavailable: full‐text of all articles referencing this trial were accessed, and it does not appear to be relevant to this version of the review |
| Cruz 1995 | No supportive treatment control: 3 albendazole durations compared. This trial was included in previous versions of this review. |
| Del Brutto 1997 | No control: albendazole treatment arm only. |
| Del Brutto 1999 | No supportive treatment control: albendazole and praziquantel compared. This trial was included in previous versions of this review. |
| Escobedo 1987 | No control: albendazole treatment arm only |
| Foyaca‐Sibat 2016 | No comparison of interest: praziquantel, prednisone, and sodium valproate compared to sodium valproate alone |
| Garcia 1997 | No supportive treatment control: 2 albendazole durations compared. This trial was included in previous versions of this review. |
| Gongora‐Rivera 2006 | No supportive treatment control: 2 albendazole doses compared. This trial was included in previous versions of this review. |
| Kaur 2009 | No supportive treatment control: albendazole plus praziquantel compared to albendazole plus placebo. This trial was included in previous versions of this review. |
| Ma 1984 | Article unavailable: full‐text of all articles referencing this trial were accessed, and it does not appear to be relevant to this version of the review |
| Pretell 2000 | Likely a quasi‐randomized trial: patients were "openly assigned" with "initial group" receiving praziquantel and "remaining patients" not receiving praziquantel. Authors contacted for clarification without reply. |
| Singhi 2003 | No supportive treatment control: 2 albendazole durations compared. This trial was included in previous versions of this review. |
| Sotelo 1990 | No supportive treatment control: 2 albendazole durations compared to 2 praziquantel durations. This trial was included in previous versions of this review. |
| Thussu 2008 | Quasi‐randomized trial: alternative allocation between albendazole and control arms |
CT: computed tomography
Differences between protocol and review
We added an additional analysis stratification: single and multiple (> 1) cysts. We considered this to be an important subgroup analysis with high biological plausibility, which developed from the included studies as they were considered for this review update.
Differences between 2010 review and this review update
The review author team revised the protocol, which was approved by the Cochrane Infectious Diseases Group editorial team on 14 September 2018 (see Appendix 2).
The main differences between this review and its previous version, Abba 2010, was the focus on the core question of whether anthelmintics are of benefit or harm in the management of neurocysticercosis compared to no anthelmintic, as there is insufficient evidence in previous review versions to answer this question. Consequently, fewer comparisons were considered in this review update compared to previous versions (e.g. comparison of different anthelmintic treatment durations), with simplified intervention and control populations. Eight studies included in the previous version of this review were therefore not eligible for inclusion (no supportive treatment comparator) in the current review. The reasons for exclusion of these studies are provided in Characteristics of excluded studies. The same subgroup stratifications were considered in this review compared to previous versions, but were not presented for every outcome, unless they changed the message of the overall outcome. We believe this allowed for a more concise review with clearer implications.
Contributions of authors
The selection of studies for inclusion, assessment of methodological quality, and data extraction were undertaken as indicated in the Methods section of the review by EJMM, KA, and LNR. EJMM undertook the analyses, in consultation with KA and LNR. All three review authors contributed to the Discussion and Authors' conclusions section of the review. All review authors read and approved the final review update version.
Sources of support
Internal sources
Liverpool School of Tropical Medicine, UK
Apollo Hospital, Chennai, India
External sources
-
Foreign, Commonwealth and Development Office (FCDO), UK
Project number 300342‐104
Declarations of interest
Edward JM Monk: none known.
Katharine Abba: none known.
Lakshmi N Ranganathan: none known.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Alarcon 2001 {published and unpublished data}
- Alarcon F, Duenas G, Diaz M, Cevallos N, Estrada G. Short course of albendazole therapy for neurocysticercosis: a prospective randomized trial comparing three days, eight days and the control group without albendazole. Revista Ecuatoriana de Neurologia 2001;10:1-2. [Google Scholar]
Baranwal 1998 {published data only}
- Baranwal AK, Singhi PD, Khandelwal N, Singhi SC. Albendazole therapy in children with focal seizures and single small enhancing computerized tomographic lesions: a randomized, placebo controlled, double-blind trial. Pediatric Infectious Disease Journal 1998;17(8):696-700. [DOI] [PubMed] [Google Scholar]
- Baranwal AK, Singhi PD, Khandelwal N, Singhi SC. Morphometry of single small enhancing computed tomographic lesions: outcome and effect of albendazole therapy. Journal of Tropical Pediatrics 2002;48:219-24. [DOI] [PubMed] [Google Scholar]
- Baranwal AK, Singhi PD, Singhi SC, Khandelwal N. Seizure recurrence and single small enhancing computed tomographic lesions: prognostic factors on long-term follow-up. Journal of Child Neurology 2001;6:443-4. [DOI] [PubMed] [Google Scholar]
Carpio 2008 {published and unpublished data}
- Carpio A, Chang M, Zhang H, Romo ML, Jaramillo A, Hauser WA, et al. Exploring the complex associations over time among albendazole treatment, cyst evolution, and seizure outcomes in neurocysticercosis. Epilepsia 2019;60(9):1820-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpio A, Kelvin EA, Bagiella E, Leslie D, Leon P, Andrews H, et al. Effects of albendazole treatment on neurocysticercosis: a randomised controlled trial. Journal of Neurology, Neurosurgery and Psychiatry 2008;79:1050-5. [DOI] [PubMed] [Google Scholar]
- Montgomery MA, Ramos M, Kelvin EA, Carpio A, Jaramillo A, Hauser WA, et al. A longitudinal analysis of albendazole treatment effect on neurocysticercosis cyst evolution using multistate models. Transactions of the Royal Society of Tropical Medicine and Hygiene 2019;1;113(12):781-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romo ML, Wyka K, Carpio A, Leslie D, Andrews H, Bagiella E, et al. The effect of albendazole treatment on seizure outcomes in patients with symptomatic neurocysticercosis. Transactions of the Royal Society of Tropical Medicine and Hygiene 2015;109(11):738-46. [DOI] [PubMed] [Google Scholar]
- Thapa K, Romo ML, Carpio A, Leslie D, Andrews H, Hauser WA, et al. The effect of albendazole treatment on non-seizure outcomes in patients with symptomatic neurocysticercosis. Transactions of the Royal Society of Tropical Medicine and Hygiene 2018;1;112(2):73-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chaurasia 2010 {published data only}
- Chaurasia RN, Garg RK, Agarwall A, Kohli N, Verma R, Singh MK, et al. Three day albendazole therapy in patients with a solitary cysticercus granuloma: a randomized double blind placebo controlled study. Southeast Asian Journal of Tropical Medicine and Public Health 2010;41(3):517-25. [PubMed] [Google Scholar]
Das 2007 {published data only}
- Das K, Mondal GP, Banerjee M, Mukherjee BB, Singh OP. Role of antiparasitic therapy for seizures and resolution of lesions in neurocysticercosis patients: an 8 year randomised study. Journal of Clinical Neuroscience 2007;14(12):1172-7. [DOI] [PubMed] [Google Scholar]
de Souza 2009 {published data only}
- Souza A, Nalini A, Kovoor JM, Yeshraj G, Siddalingaiah HS, Thennarasu K. Natural history of solitary cerebral cysticercosis on serial magnetic resonance imaging and the effect of albendazole therapy on its evolution. Journal of the Neurological Sciences 2010;288(1-2):135-41. [DOI] [PubMed] [Google Scholar]
- Souza A, Nalini A, Kovoor JM, Yeshraj G, Siddalingaiah HS, Thennarasu K. Perilesional gliosis around solitary cerebral parenchymal cysticerci and long-term seizure outcome: a prospective study using serial magnetization transfer imaging. Epilepsia 2011;52(10):1918-27. [DOI] [PubMed] [Google Scholar]
- Souza A, Nalini A, Kovoor JM, Yeshraj G, Siddalingaiah HS, Thennarasu K. Prospective quantitative imaging study by magnetisation transfer for appearance of perilesional gliosis in solitary cerebral cysticercal lesion. Neuroradiology Journal 2010;23(5):574-89. [DOI] [PubMed] [Google Scholar]
- Souza A, Thennarasu K, Yeshraj G, Kovoor JME, Nalini A. Randomized controlled trial of albendazole in new onset epilepsy and MRI confirmed solitary cerebral cysticercal lesion: effect on long-term seizure outcome. Journal of the Neurological Sciences 2009;276:108-14. [DOI] [PubMed] [Google Scholar]
Foyaca‐Sibat 2001 {published data only}
- Foyaca-Sibat H, Ibañez-Valdés L. Clinical trial of praziquantel and prednisone in rural patients with neurocysticercosis presenting with recurrent epileptic attacks. Internet Journal of Neurology 2001;1(2):6 [ePages]. [Google Scholar]
Garcia 2004 {published data only}
- Garcia HH, Pretell EJ, Gilman RH, Martinez SM, Moulton LH, Del Brutto OH, et al. A trial of antiparasitic treatment to reduce the rate of seizures due to cerebral cysticercosis. New England Journal of Medicine 2004;350(3):249-58. [DOI] [PubMed] [Google Scholar]
Gogia 2003 {published data only}
- Gogia S, Talukdar B, Choudhury V, Arora BS. Neurocysticercosis in children: clinical findings and response to albendazole therapy in a randomized, double-blind, placebo controlled trial in newly diagnosed cases. Transactions of the Royal Society of Tropical Medicine and Hygiene 2003;97(4):416-21. [DOI] [PubMed] [Google Scholar]
Kalra 2003 {published data only}
- Kalra V, Dua T, Kumar V. Efficacy of albendazole and short-course dexamethasone treatment in children with 1 or 2 ring-enhancing lesions of neurocysticercosis: a randomised controlled trial. Journal of Pediatrics 2003;143(1):111-4. [DOI] [PubMed] [Google Scholar]
Khurana 2012 {published data only}
- Khurana N, Garg RK, Verma R, Malhotra HS, Singh MK, Shukla R. Three-day versus 15-day course of albendazole therapy in solitary cysticercus granuloma: an open label randomized trial. Journal of the Neurological Sciences 2012;316:36–41. [DOI] [PubMed] [Google Scholar]
Padma 1994 {published data only}
- Padma MV, Behari M, Misra NK, Ahuja GK. Albendazole in single CT ring lesions in epilepsy. Neurology 1994;44:1344-6. [DOI] [PubMed] [Google Scholar]
Padma 1995 {published data only}
- Padma MV, Bahari M, Misra NK, Ahuja GK. Albendazole in neurocysticercosis. National Medical Journal of India 1995;8(6):255-8. [PubMed] [Google Scholar]
Singhi 2000 {published data only}
- Singhi P, Ray M, Singhi S, Khandelwal N. Clinical spectrum of 500 children with neurocysticercosis and response to albendazole therapy. Journal of Child Neurology 2000;15(4):207-13. [DOI] [PubMed] [Google Scholar]
Singhi 2004 {published data only}
- Singhi P, Jain V, Khandelwal N. Corticosteroids versus albendazole for treatment of single small enhancing computed tomographic lesions in children with neurocysticercosis. Journal of Child Neurology 2004;19(5):323-7. [DOI] [PubMed] [Google Scholar]
Sotelo 1988 {published data only}
- Sotelo J, Escobedo F, Penagos P. Albendazole vs praziquantel for therapy for neurocysticercosis. A controlled trial. Archives of Neurology 1988;45(5):532-4. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Alarcon 1989 {published data only}
- Alarcon F, Escalante L, Duenas G, Montalvo M, Roman M. Neurocysticercosis: short course of treatment with albendazole. Archives of Neurology 1989;46(11):1231-6. [DOI] [PubMed] [Google Scholar]
Botero 1993 {published data only}
- Botero D, Uribe CS, Sanchez JL, Alzate T, Velasquez G, Ocampo NE, et al. Short course albendazole treatment for neurocysticercosis in Columbia. Transactions of the Royal Society of Tropical Medicine and Hygiene 1993;87(5):576-7. [DOI] [PubMed] [Google Scholar]
Bustos 2006 {published data only}
- Bustos JA, Pretell EJ, Llanos-Zavalaga F, Gilman RH, Del Brutto OH, Garcia HH, et al. Efficacy of a 3-day course of albendazole treatment in patients with a single neurocysticercosis cyst. Clinical Neurology and Neurosurgery 2006;108(2):193-4. [DOI] [PubMed] [Google Scholar]
Carpio 1995 {published data only}
- Carpio A, Santillán F, León P, Flores C, Hauser WA. Is the course of neurocysticercosis modified by treatment with antihelminthic agents? Archives of Internal Medicine 1995;155(18):1982-8. [PubMed] [Google Scholar]
Chen 1984 {published data only}
- Chen WK. Effect of praziquantel in the treatment of 111 cases of cerebral cysticercosis cellulosae. Zhonghua Nei Ke Za Zhi [Chinese Journal of Internal Medicine] 1984;23(9):561-3. [PubMed] [Google Scholar]
Cruz 1995 {published data only}
- Cruz I, Cruz ME, Carrasco F, Horton J. Neurocysticercosis: optimal dose treatment with albendazole. Journal of Neurological Sciences 1995;133(1-2):152–4. [DOI] [PubMed] [Google Scholar]
Del Brutto 1997 {published data only}
- Del Brutto OH. Albendazole therapy for subarachnoid cysticerci: clinical and neuroimaging analysis of 17 patients. Journal of Neurology, Neurosurgery and Psychiatry 1997;62(6):659–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Del Brutto 1999 {published data only}
- Del Brutto OH, Campos X, Sanchez J, Mosquera A. Single-day praziquantel versus 1-week albendazole for neurocysticercosis. Neurology 1999;23(52):1079–81. [DOI] [PubMed] [Google Scholar]
Escobedo 1987 {published data only}
- Escobedo F, Penagos P, Rodriguez J, Sotelo J. Albendazole therapy for neurocysticercosis. Archives of Internal Medicine 1987;147(4):738-41. [PubMed] [Google Scholar]
Foyaca‐Sibat 2016 {published data only}
- Foyaca-Sibat H, Ibanez-Valdes LDF. Clinical trial of praziquantel and prednisone in HIV-patients presenting uncontrolled epilepsy due to intraparenchymal neurocysticercosis. Epilepsia 2016;57 Suppl 2:226–33. [Google Scholar]
Garcia 1997 {published data only}
- Garcia H, Gilman R, Catacora M, Verastegui M, Gonzalez AE, Tsang VCW, et al. Serologic evolution of neurocysticercosis patients after antiparasitic therapy. Journal of Infectious Diseases 1997;175:486–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia HH, Gilman RH, Horton J, Martinez M, Herrera G, Altamirano J, et al, Cysticercosis Working Group in Peru. Albendazole therapy for neurocysticercosis: a prospective double-blind trial comparing 7 versus 14 days of treatment. Neurology 1997;48(5):1421–7. [DOI] [PubMed] [Google Scholar]
Gongora‐Rivera 2006 {published data only}
- Gongora-Rivera F, Soto-Hernandez JL, Gonzalez Esquive lD, Cook HJ, Marquez-Caraveo C, Hernandez Davila R, et al. Albendazole trials at 15 or 30 mg/kg/day for subarachnoid and intraventricular cysticercosis. Neurology 2996;66(3):436–8. [DOI] [PubMed] [Google Scholar]
Kaur 2009 {published data only}
- Kaur S, Singhi P, Singhi S, Khandelwal N. Combination therapy with albendazole and praziquantel versus albendazole alone in children with seizures and single lesion neurocysticercosis. Pediatric Infectious Disease Journal 2009;28(5):403–6. [DOI] [PubMed] [Google Scholar]
Ma 1984 {published data only}
- Ma YX. A clinical study on the treatment of cerebral cysticercosis with praziquantel. Zhonghua Yi Xue Za Zhi [Chinese Journal of Internal Medicine] 1984;64(2):79-83. [PubMed] [Google Scholar]
Pretell 2000 {published data only}
- Pretell EJ, Garcia HH, Custodio N, Padilla C, Alvarado M, Gilman RH, et al. Short regimen of praziquantel in the treatment of single brain enhancing lesions. Clinical Neurology and Neurosurgery 2000;102(4):215-8. [DOI] [PubMed] [Google Scholar]
Singhi 2003 {published data only}
- Singhi P, Dayal D, Khandelwal N. One week versus four weeks of albendazole therapy for neurocysticercosis in children: a randomized, placebo controlled double-blind trial. Pediatric Infectious Disease Journal 2003;22(3):268–72. [DOI] [PubMed] [Google Scholar]
Sotelo 1990 {published data only}
- Sotelo J, Del Brutto OH, Penagos P, Escobedo F, Torres B, Rodriguez-Carbajal J, et al. Comparison of therapeutic regimen of anticystercal drugs for parenchymal brain cysticercosis. Journal of Neurology 1990;237:69–72. [DOI] [PubMed] [Google Scholar]
Thussu 2008 {published data only}
- Thussu A, Chattopadhyay A, Sawhney IM, Khandelwal N. Albendazole therapy for single small enhancing CT lesions (SSECTL) in the brain in epilepsy. Journal of Neurology, Neurosurgery and Psychiatry 2008;79(3):272-5. [DOI] [PubMed] [Google Scholar]
Additional references
Boutron 2021
- Boutron I, Page MJ, Higgins JPT, Altman DG, Lundh A, Hróbjartsson A. Chapter 7: Considering bias and conflicts of interest among the included studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Carabin 2011
- Carabin H, Ndimubanze PC, Budke CM, Nguyen H, Qian Y, Cowan LD, et al. Clinical manifestations associated with neurocysticercosis: a systematic review. PLOS Neglected Tropical Diseases 2011;5(5):e1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
Coyle 2012
- Coyle CM, Mahanty S, Zunt JR, Wallin MT, Cantey PT, White AC, et al. Neurocysticercosis: neglected but not forgotten. PLOS Neglected Tropical Diseases 2012;6(5):e1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
Degiorgio 2002
- Degiorgio CM, Houston I, Oviedo S, Sorvillo F. Deaths associated with cysticercosis. Report of three cases and review of the literature. Neurosurgical Focus 2002;12(6):e2. [DOI] [PubMed] [Google Scholar]
Degiorgio 2004
- Degiorgio CM, Medina MT, Duron R, Zee C, Escueta SP. Neurocysticercosis. Epilepsy Currents 2004;4(3):107-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Del Brutto 2006
- Del Brutto OH, Roos KL, Coffey CS, García HH. Meta-analysis: cysticidal drugs for neurocysticercosis: albendazole and praziquantel. Annals of Internal Medicine 2006;145(1):43-51. [DOI] [PubMed] [Google Scholar]
Del Brutto 2017
- Del Brutto OH, Nash TE, White AC, Rajshekhar V, Wilkins PP, Singh G, et al. Revised diagnostic criteria for neurocysticercosis. Journal of Neurological Science 2017;15(372):202-10. [DOI] [PubMed] [Google Scholar]
Garcia 2002
- Garcia HH, Evans CA, Nash TE, Takayanagui OM, White AC Jr, Botero D, et al. Current consensus guidelines for treatment of neurocysticercosis. Clinical Microbiology Reviews 2002;15(4):747-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jüni 2001
- Jüni P, Altman DG, Egger M. Systematic reviews in health care: assessing the quality of controlled clinical trials. BMJ 2001;323(7303):42-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leite 2000
- Leite JP, Terra-Bustamante VC, Fernandes RMF, Santos AL, Chimelli L, Sakomoto AC, et al. Calcified neurocysticercosis lesions and postsurgery seizure control in temporal lobe epilepsy. Neurology 2000;55(10):1485-91. [DOI] [PubMed] [Google Scholar]
Lescano 2009
- Lescano AG, Garcia HH, Gilman RH, Gavidia CM, Tsang VCW, Rodriguez S, et al. Taenia solium cysticercosis hotspots surrounding tapeworm carriers: clustering on human seroprevalence but not on seizures. PLOS Neglected Tropical Diseases 2009;3:e371. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mazumdar 2007
- Mazumdar M, Pandharipande P, Poduri A. Does albendazole affect seizure remission and computed tomography response in children with neurocysticercosis? A systematic review and meta-analysis. Journal of Child Neurology 2007;22(2):135-42. [DOI] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Roman 2000
- Roman G, Sotelo J, Del Brutto O, Flissier A, Dumas M, Wadia N, et al. A proposal to declare neurocysticercosis an international reportable disease. Bulletin of the World Health Organization 2000;78(3):399-406. [PMC free article] [PubMed] [Google Scholar]
Ryan 2016
- Ryan R, Hill S. How to GRADE the quality of the evidence version 3.0. Cochrane Consumers and Communication Group. Cochrane 2016. Available at cccrg.cochrane.org/author-resources.
Savioli 2010
- Savioli LS, Daumerie D. First WHO report on neglected tropical diseases: working to overcome the global impact of neglected tropical diseases. 1st edition. Vol. 1. Geneva: World Health Organization, 2010. [Google Scholar]
Schünemann 2021
- Schünemann HJ, Higgins JPT, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
White 2018
- White AC, Coyle CM, Rajshekhar V, Singh G, Hauser WA, Mohanty A, et al. Diagnosis and treatment of neurocysticercosis: 2017. Clinical Practice Guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clinical Infectious Diseases 2018;66(8):e49–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Abba 2010
- Abba K, Ramaratnam S, Ranganathan LN. Anthelmintics for people with neurocysticercosis. Cochrane Database of Systematic Reviews 2010, Issue 3. Art. No: CD000215. [DOI: 10.1002/14651858.CD000215.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Salinas 1999
- Salinas R, Prasad K. Drugs for treating neurocysticercosis (tapeworm infection of the brain). Cochrane Database of Systematic Reviews 1999, Issue 4. Art. No: CD000215. [DOI: 10.1002/14651858.CD000215.pub2] [DOI] [PubMed] [Google Scholar]


