Open AccessArticle
Analysis of Osteosarcoma Cell Lines and Patient Tissue Using a 3D In Vivo Tumor Model—Possible Effects of Punicalagin
by
Anna Rebecca Dorn, Sara Neff, Sophia Hupp, Melissa Engelhardt, Eric Pion, Ulrich Lenze, Carolin Knebel, Anna Duprée, Simone Schewe, Markus Weber, Christian Wulbrand, Axel Hillmann, Florian Weber, Phillip Clarke, Philipp Kainz, Thiha Aung and Silke Haerteis
Viewed by 1393
Abstract
Osteosarcomas are the most common primary malignant bone tumors and mostly affect children, adolescents, and young adults. Despite current treatment options such as surgery and polychemotherapy, the survival of patients with metastatic disease remains poor. In recent studies, punicalagin has reduced the cell
[...] Read more.
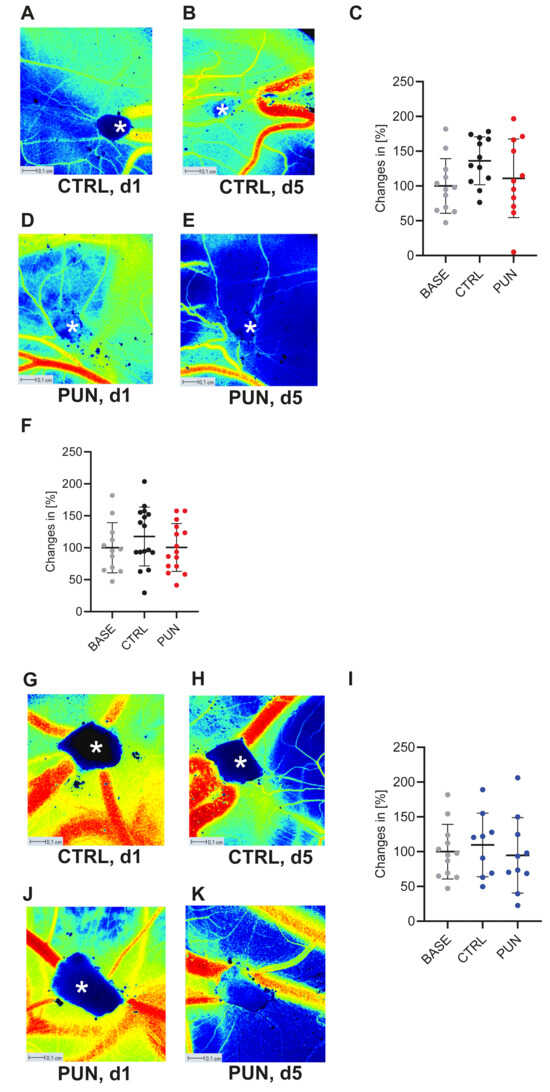
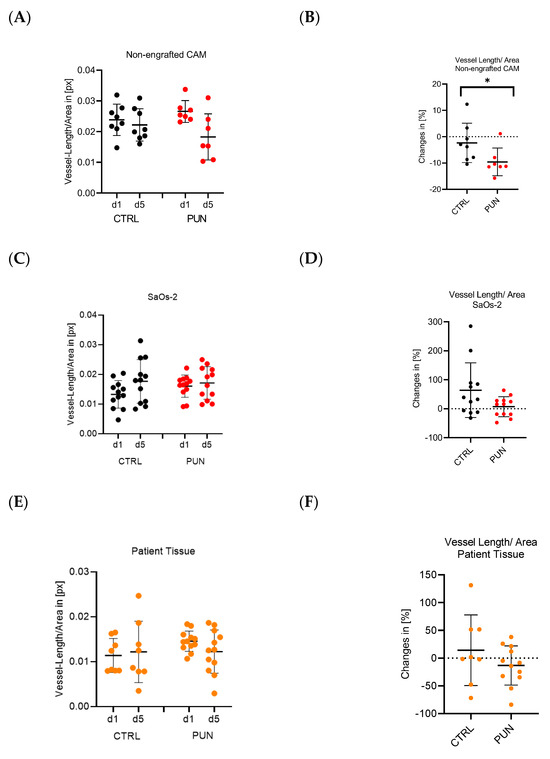
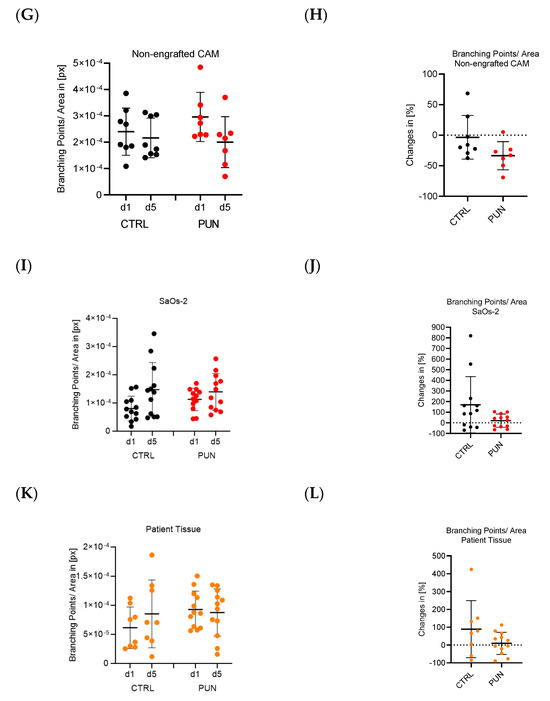
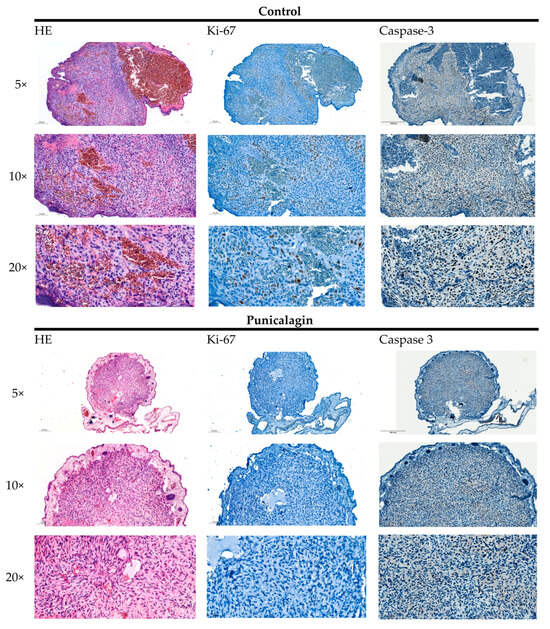
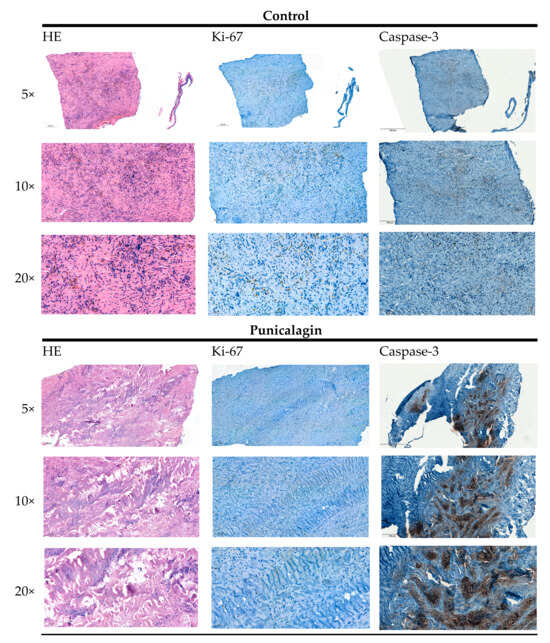
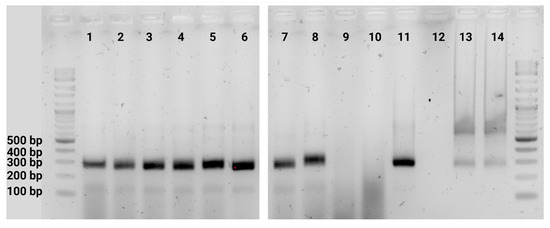
Osteosarcomas are the most common primary malignant bone tumors and mostly affect children, adolescents, and young adults. Despite current treatment options such as surgery and polychemotherapy, the survival of patients with metastatic disease remains poor. In recent studies, punicalagin has reduced the cell viability, angiogenesis, and invasion in cell culture trials. The aim of this study was to examine the effects of punicalagin on osteosarcomas in a 3D in vivo tumor model. Human osteosarcoma biopsies and SaOs-2 and MG-63 cells, were grown in a 3D in vivo chorioallantoic membrane (CAM) model. After a cultivation period of up to 72 h, the tumors received daily treatment with punicalagin for 4 days. Weight measurements of the CAM tumors were performed, and laser speckle contrast imaging (LSCI) and a deep learning-based image analysis software (CAM Assay Application v.3.1.0) were used to measure angiogenesis. HE, Ki-67, and Caspase-3 staining was performed after explantation. The osteosarcoma cell lines SaOs-2 and MG-63 and osteosarcoma patient tissue displayed satisfactory growth patterns on the CAM. Treatment with punicalagin decreased tumor weight, proliferation, and tumor-induced angiogenesis, and the tumor tissue showed pro-apoptotic characteristics. These results provide a robust foundation for the implementation of further studies and show that punicalagin offers a promising supplementary treatment option for osteosarcoma patients. The 3D in vivo tumor model represents a beneficial model for the testing of anti-cancer therapies.
Full article
►▼
Show Figures