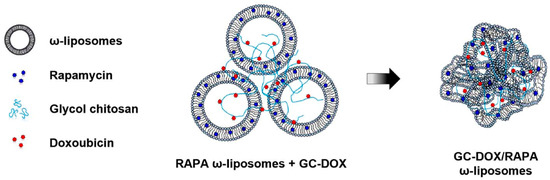
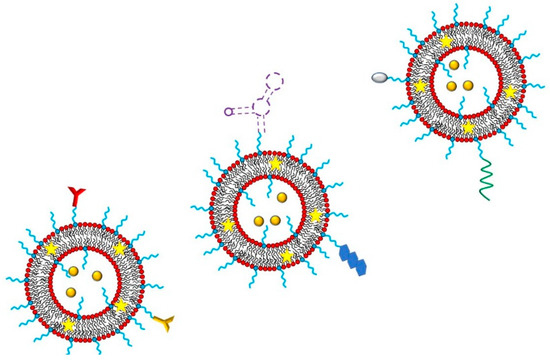
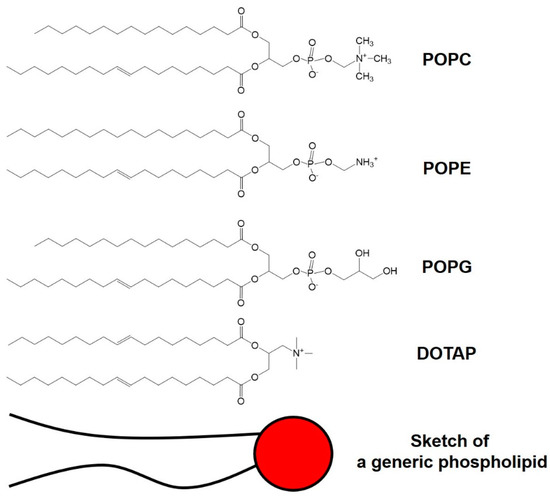
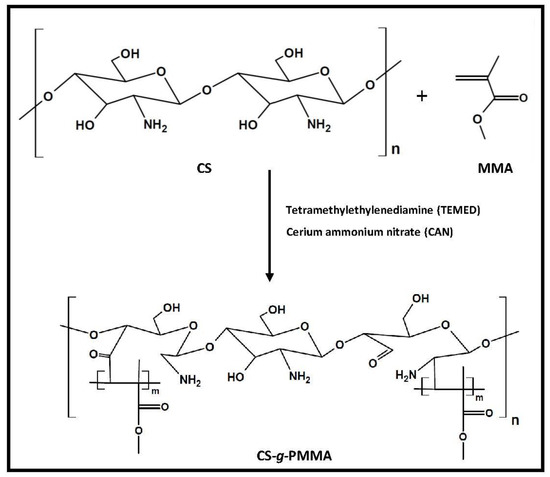
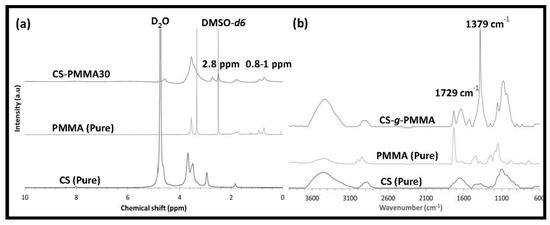
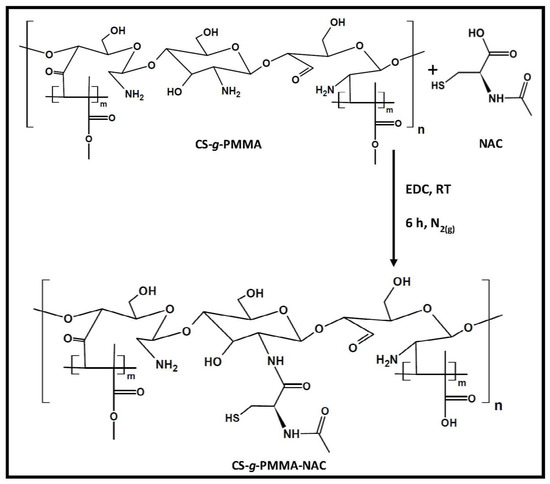
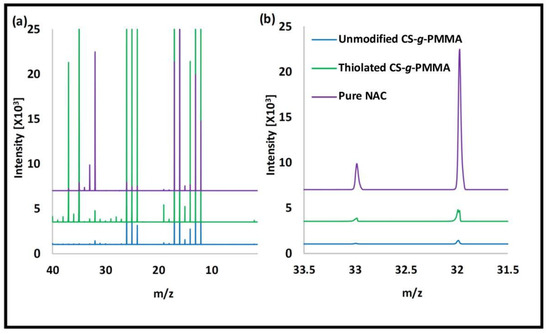
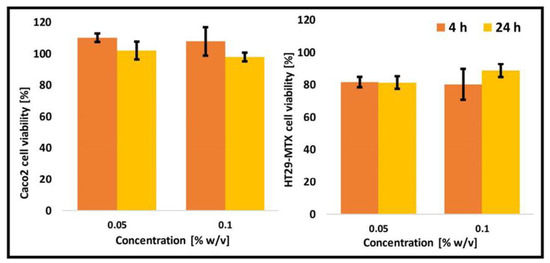
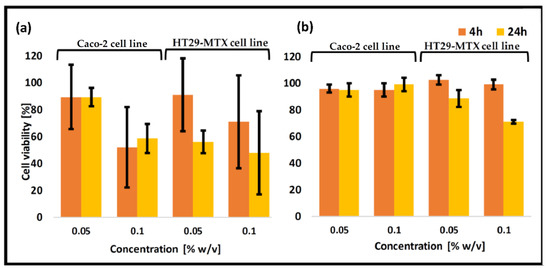
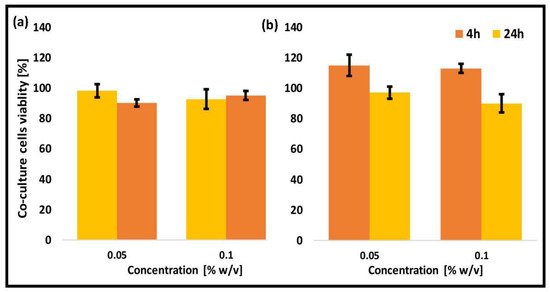
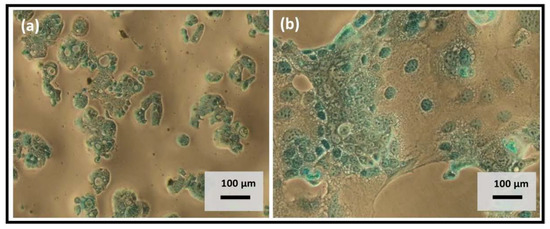
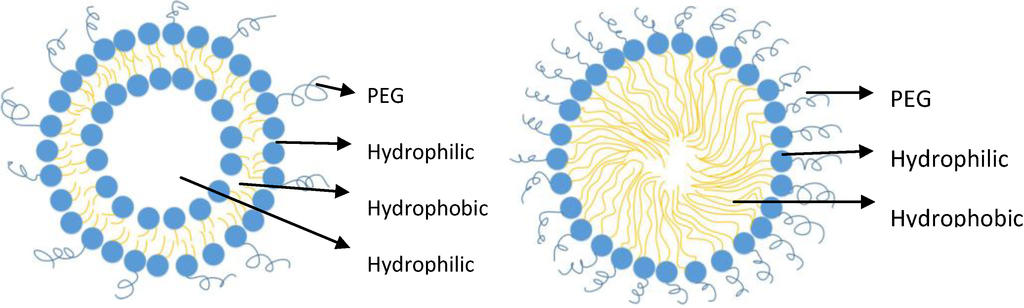
The enteric system residing notorious
Salmonella typhimurium (
S. typhi) is an intracellular, food-borne, and zoonotic pathogen causing typhoid fever. Typhoid fever is one of the leading causes of mortality and morbidity in developing and underdeveloped countries. It also increased the prevalence
[...] Read more.
The enteric system residing notorious
Salmonella typhimurium (
S. typhi) is an intracellular, food-borne, and zoonotic pathogen causing typhoid fever. Typhoid fever is one of the leading causes of mortality and morbidity in developing and underdeveloped countries. It also increased the prevalence of multidrug resistance globally. Currently, available anti-bacterial modalities are unable to penetrate into the intracellular compartments effectively for eradicating
S. typhi infection. Therefore, in this study, we developed nanostructured lipid-based carriers in the form of a self-nanoemulsifying drug delivery system (SNEDDS) for targeted delivery of ciprofloxacin (CIP) into the
S. typhi intracellular reservoirs. Capryol 90, Tween 80, and Span 20 were finalized as suitable oil, surfactant, and co-surfactant, respectively, according to the pseudoternary phase diagram emulsifying region. Targeting capability and mucopenetration of the SNEDDS was attributed to the inclusion of amidated pluronic (NH
2-F
127). Developed NH
2-F
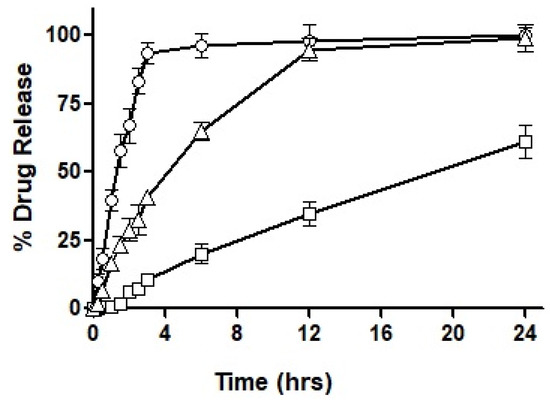
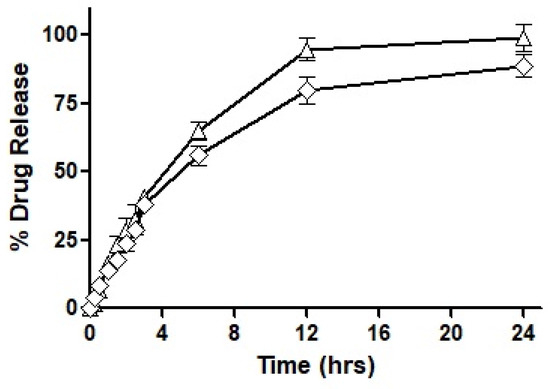
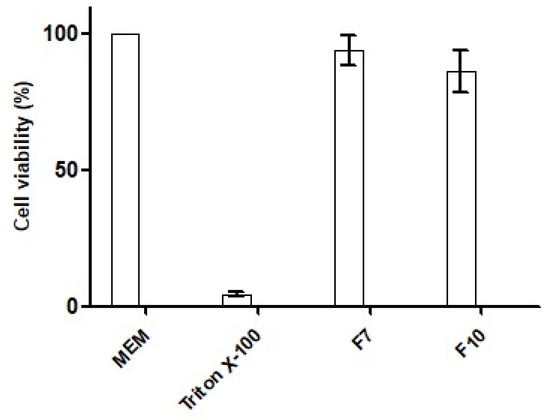
127 SNEDDS were characterized via physicochemical, in vitro, ex vivo, and in vivo evaluation parameters. The size of the SNEDDS was found to be 250 nm, having positively charged zeta potential. In vitro dissolution of SNEDDS showed 80% sustained release of CIP in 72 h with maximum entrapment efficiency up to 90% as well as good hemocompatibility by showing less than 0.2% hemolysis and 90% biocompatibility. The survival rate of
S. typhi in macrophages (RAW 264.7) was minimal, i.e., only 2% in the case of NH
2-F
127 SNEDDS. Macrophage uptake assay via nanostructures confirmed the maximum cellular uptake as evidenced by the highest fluorescence. Biofilm dispersion assay showed rapid eradication of developed resistant biofilms on the gall bladder. In vivo pharmacokinetics showed improved bioavailability by showing an increased area under the curve (AUC) value. Taken together, NH
2-F
127-SNEDDS can be utilized as an alternative and efficient delivery system for the sustained release of therapeutic amounts of CIP for the treatment of
S. typhi.
Full article