Abstract
Excessive nitrogen promotes the formation of nonproductive tillers in rice, which decreases nitrogen use efficiency (NUE). Developing high-NUE rice cultivars through balancing nitrogen uptake and the formation of productive tillers remains a long-standing challenge, yet how these two processes are coordinated in rice remains elusive. Here we identify the transcription factor OsGATA8 as a key coordinator of nitrogen uptake and tiller formation in rice. OsGATA8 negatively regulates nitrogen uptake by repressing transcription of the ammonium transporter gene OsAMT3.2. Meanwhile, it promotes tiller formation by repressing the transcription of OsTCP19, a negative modulator of tillering. We identify OsGATA8-H as a high-NUE haplotype with enhanced nitrogen uptake and a higher proportion of productive tillers. The geographical distribution of OsGATA8-H and its frequency change in historical accessions suggest its adaption to the fertile soil. Overall, this study provides molecular and evolutionary insights into the regulation of NUE and facilitates the breeding of rice cultivars with higher NUE.
Similar content being viewed by others
Main
Nitrogen is an essential macronutrient vital for plant growth and development. Insufficient nitrogen fertilizer in the soil can severely restrict crop growth, while overapplication of nitrogen fertilizers negatively impacts the environment1,2. To increase crop productivity in a sustainable fashion, there is increasing interest in breeding cultivars with high nitrogen use efficiency (NUE). As a major determinant of crop yield, plant NUE is an inherently complex trait governed by multiple intertwined biological processes, including nitrogen uptake, transport, assimilation and remobilization3,4,5. The identification of key genetic components involved in NUE regulation holds great promise for crop improvement.
Rice serves as a major staple crop for over half of the world’s population. Rice yield is largely influenced by the number of effective panicles per unit of land area6. The introduction of the semi-dwarf gene sd1 into modern rice cultivars during the first ‘Green Revolution’ and the application of synthetic nitrogen fertilizers since the 1960s greatly improved lodging resistance and increased yield in modern rice cultivars6,7. Nevertheless, the large amount of nitrogen fertilizers applied during rice production increases the carbon footprint associated with fertilizer production and may accelerate environmental degradation due to the run-off of excessive fertilizers into waterways1,2. In addition, excessive nitrogen promotes the formation of tillers that fail to bear effective panicles, known as nonproductive tillers. Nonproductive tillers channel nutrients away from grain production, while a larger proportion of productive tillers in rice is often associated with higher NUE8,9. Thus, breeding high-NUE rice cultivars with a high proportion of productive tillers is an essential route to sustainable agriculture.
Over the past few decades, extensive efforts have been devoted to dissecting the molecular basis of nitrogen uptake and NUE in rice10,11,12. OsNRT1.1B13 and OsNPF6.1 (ref. 14) were discovered as the major nitrate transporters in rice, whose overexpression leads to enhanced nitrate uptake. The transcription factor nitrogen-mediated tiller growth response 5 (NGR5) promotes tiller formation upon nitrogen perception in the absence of gibberellins (GA) signaling10. The transcription regulator OsTCP19 inhibits tiller formation, whose activity varies in response to nitrogen availability11. Despite these advancements, the molecular mechanisms coordinating nitrogen uptake and productive tiller formation remain unclear, hindering the molecular breeding of rice cultivars with higher NUE and yield.
In this study, through the identification and characterization of the transcription factor OsGATA8, we uncover a mechanistic connection between NUE and productive tiller formation. We show that OsGATA8 coordinates nitrogen uptake and tiller formation in rice through transcriptional regulation of key components involved in these two biological processes. For breeding application, we identify the elite haplotype OsGATA8-H, which confers high nitrogen uptake efficiency (NUpE) while promoting the formation of productive tillers. These results provide insights into how nitrogen uptake and the formation of productive tillers are coordinated in rice and demonstrate a strategy for balancing these two processes to breed rice cultivars with high NUE and yield.
Results
OsGATA8 was identified as a putative negative regulator of NUE
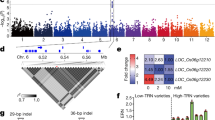
From an agronomical perspective, NUE in rice is defined as grain yield divided by the amount of nitrogen input3,15. NUE is often correlated with plant height and the number of tillers. Increasing nitrogen application promotes stem elongation and tiller formation, but overapplication of nitrogen promotes excessive tiller formation, especially nonproductive tillers (Extended Data Fig. 1 and Supplementary Fig. 1). Thus, the productive tiller number ratio (PTNR; productive tiller number low nitrogen (LN) condition/productive tiller number under high nitrogen (HN) condition) and plant height ratio (PHR; plant height under LN condition/plant height under HN condition) are often used as proxies of NUE16. PTNR is also called effective panicle number ratio in previous studies14,16. Accordingly, NUE is often assessed by measuring the yield per plant (YPP), PHR and PTNR14,16. Rice with high PTNR and PHR tended to have higher NUE compared to rice with low PTNR and PHR under both HN and LN conditions (Supplementary Fig. 2). Previously, we designed a genome-wide association study (GWAS)-based strategy to uncover candidate NUE genes followed by functional validation through genetic complementation tests14,17. Using PTNR and PHR as the proxies of NUE on a core collection of rice germplasm consisting of 117 varieties14, we identified a major quantitative trait locus in linkage disequilibrium block between the coordinates 13,548,357 and 13,572,267 on chromosome 1 (Extended Data Fig. 2a and Supplementary Table 1). In total, 51 SNPs causing missense mutations were identified in the three genes present in this linkage disequilibrium block (Supplementary Table 2). We quantified the expression of these three genes in response to different nitrogen concentrations in rice seedlings using qRT–PCR and found that only Os01g0343300 was significantly induced by high concentrations of nitrogen (1 mM and 5 mM NH4NO3; Extended Data Fig. 2b). Os01g0343300 encodes OsGATA8 (ref. 18), a member of the GATA family transcription factors, which are widespread among eukaryotes and participate in diverse biological processes19. OsGATA8 tends to be expressed at lower levels in rice cultivars with higher PTNR and PHR compared with rice cultivars with lower PTNR and PHR (Extended Data Fig. 2c), indicating a negative correlation between OsGATA8 expression level and NUE. Confocal microscopy using rice protoplasts expressing OsGATA8-GFP revealed that OsGATA8 is localized to the nucleus (Supplementary Fig. 3a). We characterized the tissue-wide expression pattern of OsGATA8 in various rice tissues after the flowering stage using qRT–PCR assays and found that OsGATA8 is highly expressed in roots and tiller buds (Supplementary Fig. 3b), suggesting that OsGATA8 may have a role in these tissues.
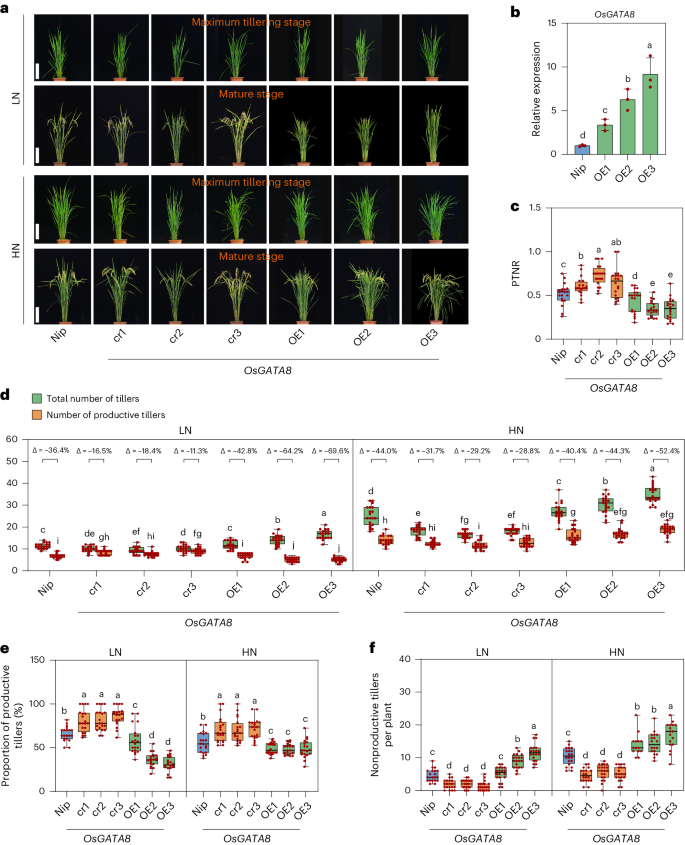
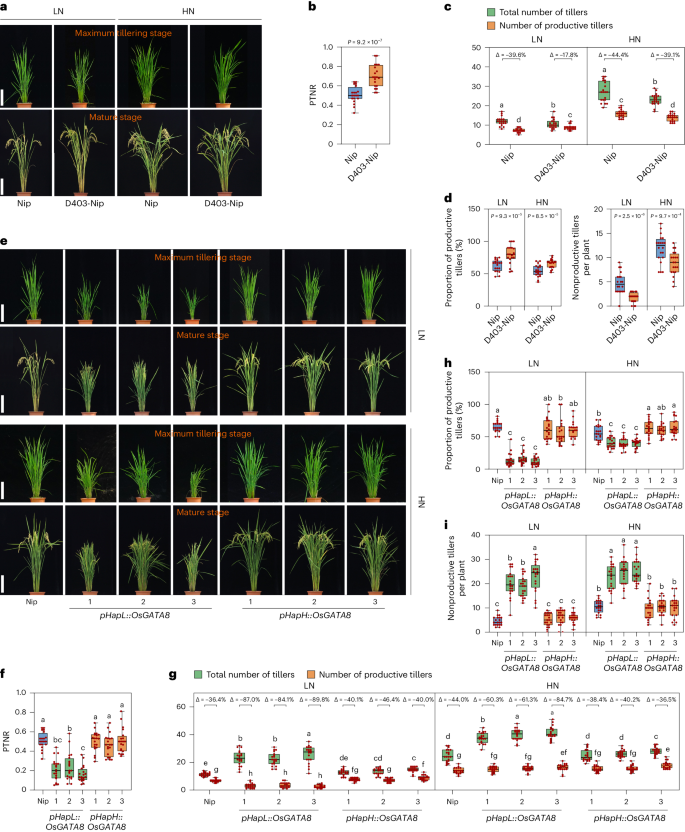
To verify the role of OsGATA8 in NUE, we generated OsGATA8 knockout plants using CRISPR–Cas9 in two japonica rice cultivars Nipponbare (Nip; cr1, cr2 and cr3) and Zhonghua 11 (cr4), as well as OsGATA8 overexpression lines with the constitutive ZmUbi1 promoter in the Nip background (OE1, OE2 and OE3) and evaluated their phenotypes under LN and HN field conditions at the maximum tillering stage and the mature stage (Fig. 1a,b and Supplementary Fig. 3c,d). Compared with the wild-type (WT) plants, both PTNR and the proportion of productive tillers were higher in the OsGATA8 knockout plants but were lower in the OsGATA8 overexpression plants (Fig. 1c–f and Supplementary Fig. 3e–g). These results indicate that OsGATA8 is a negative regulator of PTNR and the proportion of productive tillers.
a, The phenotypes of Nip, the OsGATA8-cr knockout lines and the OsGATA8 overexpression lines at the maximum tillering stage (about 40 days after transplanting) and the mature stage under LN and HN conditions. Scale bars, 20 cm. LN, 75 kg ha−1 net nitrogen; HN, 300 kg ha−1 net nitrogen. b, Relative expression of OsGATA8 in Nip and OsGATA8 overexpression lines. Total RNA was extracted from the root tissue of 2-week-old seedlings. Each analysis was repeated with three independent root tissues of seedlings. Data are presented as mean ± s.d. c, The PTNR of Nip, the OsGATA8-cr knockout lines and the OsGATA8 overexpression line at the mature stage under LN and HN conditions. n = 20 plants. PTNR, productive tiller number under LN condition/productive tiller number under HN condition. d, The total number of tillers and productive tillers of Nip, the OsGATA8-cr knockout lines and the OsGATA8 overexpression lines under LN and HN conditions. ‘Δ’ represents the percentage difference compared with the total number of tillers. n = 20 plants. e,f, The proportion of productive tillers (PT%; e) and the number of nonproductive tillers (f) of Nip, the OsGATA8-cr knockout lines and the OsGATA8 overexpression lines under LN and HN conditions. n = 20 plants. In b–f, different letters indicate significant differences (P < 0.05, one-way ANOVA, Duncan’s new multiple range test); for P values, see source data. In c–f, box plots denote the 25th percentile, the median and the 75th percentile, with minimum to maximum whiskers.
The OsGATA8–OsAMT3.2 module restricts nitrogen uptake
To investigate how OsGATA8 downregulates NUE, we examined the total nitrogen content in the shoots and roots of the knockout line OsGATA8-cr1 and WT seedlings grown under LN and HN conditions. The nitrogen content of the OsGATA8-cr1 line was significantly higher than that of the WT in both shoots and roots under both LN and HN conditions (Extended Data Fig. 3a). Consistently, a higher 15NH4+ influx rate was observed in the OsGATA8-cr1 plants under both HN (2.0 mM) and LN (0.2 mM) conditions compared with WT plants (Extended Data Fig. 3b). On the contrary, we did not observe any effect of knocking out OsGATA8 on 15NO3− influx rate (Extended Data Fig. 3b). These results suggest that OsGATA8 regulates the uptake of ammonium but not nitrate.
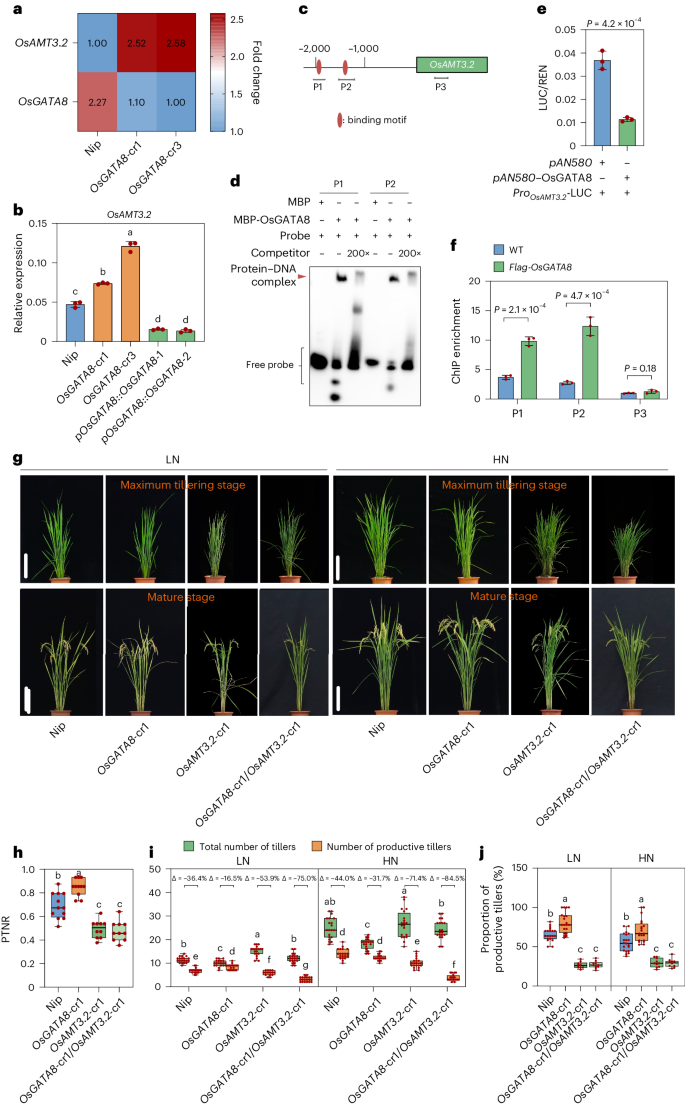
To gain insights into how OsGATA8 affects ammonium uptake in rice, we performed an RNA-sequencing (RNA-seq) analysis on seedlings of WT and the OsGATA8 knockout lines and identified 619 differentially expressed genes (DEGs; Supplementary Table 3), among which 19 genes were annotated to the term ‘transporter activity’ (GO:0005215) in Gene Ontology analysis (Extended Data Fig. 3c). Among them, OsAMT3.2 was the only ammonium transporter gene (Extended Data Fig. 3d). Notably, knocking out OsGATA8 significantly increased the expression of OsAMT3.2 (Fig. 2a). Consistently, qRT–PCR assay verified that the expression of OsAMT3.2 was significantly upregulated in the OsGATA8 knockout lines but was repressed in the pOsGATA8::OsGATA8 transgenic lines (Fig. 2b). Moreover, a DNA affinity purification sequencing (DAP-seq) analysis identified OsAMT3.2 as a putative direct target of OsGATA8 (Supplementary Table 4 and Extended Data Fig. 3e). Furthermore, electrophoretic mobility shift assays (EMSA) and luciferase (LUC) assays in rice protoplasts showed that OsGATA8 directly binds to the promoter of OsAMT3.2 to repress its transcription (Fig. 2c–e and Supplementary Fig. 4). The direct interaction between OsGATA8 and the promoter of OsAMT3.2 was further verified by a chromatin immunoprecipitation-quantitative PCR (ChIP–qPCR) assay using shoot tissue of p35S::Flag-OsGATA8 transgenic plants (Fig. 2c,f). In addition, DAP-seq analysis showed that another ammonium transporter gene, OsAMT1.2, is also a putative target of OsGATA8 (Extended Data Fig. 3e). qRT–PCR, EMSA, LUC and ChIP–qPCR assays showed that OsGATA8 also represses the transcription of OsAMT1.2 by directly binding to its promoter (Supplementary Figs. 4 and 5). These results suggest that OsGATA8 represses ammonium uptake in rice by downregulating the expression of OsAMT3.2 and OsAMT1.2.
a, The relative expression of two DEGs in WT and OsGATA8 knockout lines. The color key (blue to red) indicates gene expression as fold changes (fragments per kilobase of exon model per million mapped fragments (FPKM)). For each gene, the minimum FPKM value was set as 1.00. b, Relative expression of OsAMT3.2 in the OsGATA8 knockout lines and the OsGATA8 overexpression lines driven by native promoter. Total RNA was extracted from the root tissue of 2-week-old seedlings. Values represent mean ± s.d. derived from three independent seedlings. c, Schematic diagram of OsAMT3.2 displaying the promoter and the transcribed region. Horizontal bars indicate the location of the probes used in the EMSA. P1 and P2 of OsAMT3.2 correspond to the predicted OsGATA8 binding motifs, while P3 of OsAMT3.2 is a negative control site without predicted OsGATA8 binding motifs. d, An EMSA testing the binding strength of OsGATA8 to the predicted binding motifs in OsAMT3.2 promoters using probes as shown in c. MBP, maltose-binding protein. The results are representative of three independent experiments. e, LUC assay in rice protoplasts on the effect of OsGATA8 on the transcription of OsAMT3.2. Values represent mean ± s.d. derived from three independent samples of rice protoplasts. f, ChIP–qPCR assay of the interaction between OsGATA8 and the promoter of OsAMT3.2 in the shoot of 35S::Flag-OsGATA8 transgenic plants at the four-leaf stage. Values represent mean ± s.d. derived from three independent samples; P values were calculated with two-tailed Student’s t test. g, Phenotypes of Nip, the OsGATA8 knockout mutant, the OsAMT3.2 knockout mutant and the OsGATA8/OsAMT3.2 double-knockout mutant at the maximum tillering stage (about 40 days after transplanting) and the mature stage under LN and HN conditions. LN, 75 kg ha−1 net nitrogen; HN, 300 kg ha−1 net nitrogen. Scale bars, 20 cm. h, PTNR of the genotypes in g under LN and HN conditions. n = 10 plants. PTNR, productive tiller number under LN condition/productive tiller number under HN condition. i,j, The total number of tillers, productive tillers (i) and proportion of productive tillers (PT%; j) of the genotypes in g under LN and HN conditions. ‘Δ’ represents the percentage difference compared with the total number of tillers. n = 20 plants. In b and h–j, different letters indicate significant differences (P < 0.05, one-way ANOVA, Duncan’s new multiple range test). For P values, see source data. In e and f, significant difference was determined by two-tailed Student’s t test. In h–j, box plots denote the 25th percentile, the median and the 75th percentile, with minimum to maximum whiskers.
Ammonium transporters in rice are important membrane proteins involved in the uptake, transport and allocation of ammonium in plants20. Phylogenetic analysis of the nine ammonium transporters in rice shows that they are mainly divided into the following two subfamilies: AMT1 and AMT2. OsAMT3.2 and OsAMT1.2 belong to the AMT2 and AMT1 subfamilies, respectively21 (Supplementary Fig. 6). It was previously reported that knocking out or overexpressing OsAMT1.2 alone did not affect ammonium uptake in rice, nor did it incur any phenotypic change22,23. Thus, we focused on testing the role of OsAMT3.2 in nitrogen uptake in rice. Notably, OsAMT3.2 and OsGATA8 display similar tissue-wide and nitrogen-responsive expression patterns (Extended Data Fig. 4a,b), suggesting a plausible OsGATA8–OsAMT3.2 molecular module in the regulation of nitrogen uptake. To test the role of OsAMT3.2 in regulating NUE, we constructed two OsAMT3.2 knockout lines (cr1 and cr2) with different mutational sites and two OsAMT3.2 overexpression lines (OE1 and OE2; Extended Data Fig. 4c,d). We measured nitrogen content and 15NH4+ influx rates in seedlings of the OsAMT3.2 knockout and overexpression lines and found that OsAMT3.2 positively regulates ammonium uptake in rice (Extended Data Fig. 4e,f). Consistently, the number of productive tillers, biomass and yield decreased in the OsAMT3.2-cr1 and OsAMT3.2-cr2 lines, but increased in the OsAMT3.2-OE1 and OsAMT3.2-OE2 lines, compared with the WT (Supplementary Fig. 7a–e). NUpE and nitrogen utilization efficiency (NUtE) are two major factors determining the overall NUE3. Therefore, we quantified NUE, NUpE and NUtE in rice plants at the mature stage under LN and HN conditions and found that NUE, NUpE and NUtE all decreased in the OsAMT3.2-cr1 and OsAMT3.2-cr2 lines, but increased in the OsAMT3.2-OE1 and OsAMT3.2-OE2 lines, compared with the WT (Supplementary Fig. 7f–h), thus verifying that OsAMT3.2 promotes high NUE.
To genetically test whether OsAMT3.2 functions downstream of OsGATA8, we generated the OsGATA8-cr1/OsAMT3.2-cr1 double mutant by crossing OsGATA8-cr1 with OsAMT3.2-cr1. The homozygous OsGATA8-cr1/OsAMT3.2-cr1 line showed decreased PTNR, reduced number of productive tillers and reduced proportion of productive tillers, compared with the OsGATA8-cr1 line (Fig. 2g–j), suggesting that OsAMT3.2 functions downstream of OsGATA8. These results indicate that OsGATA8 negatively regulates nitrogen uptake by repressing OsAMT3.2 expression.
The OsGATA8–OsTCP19 module promotes tillering
An intriguing observation with the OsGATA8 overexpression lines was its excessive tiller production (Fig. 1d and Extended Data Figs. 1b and 5a–c). This is unexpected as reduced nitrogen uptake in the OsGATA8 overexpression lines due to reduced expression of OsAMT3.2 (Fig. 2b) would presumably reduce tiller formation. We thus hypothesized that OsGATA8 may affect tiller development by regulating additional targets besides OsAMT3.2. We thus examined the expression levels of five candidate genes that have been reported to be involved in rice tillering formation and nitrogen response, including TB1 (ref. 24), DLT11, OsNGR5 (ref. 10), OsTCP19 (ref. 11) and OsMADS57 (ref. 25) in the OsGATA8-cr1 and pOsGATA8::OsGATA8 overexpression plants. We found that the expressions of OsTB1 and OsTCP19 negatively correlated with the expression level of OsGATA8 (Supplementary Fig. 8). Based on the results of DAP-seq, OsGATA8 primarily recognizes the TTCCKAATTTT (K represents T or G or A) motif (Supplementary Fig. 9), which exists only in the promoter of OsTCP19 among the five candidate genes examined (Fig. 3a), which suggests that OsTCP19 is a direct target for transcription regulation by OsGATA8. In situ hybridization assay showed that OsGATA8 and OsTCP19 were both expressed in the shoot apical meristem (Supplementary Fig. 10). EMSA and LUC assay in the rice protoplasts showed that OsGATA8 directly binds to the promoter of OsTCP19 and represses its transcription (Fig. 3b,c). The direct association between OsGATA8 and the promoter of OsTCP19 was further validated by a ChIP–qPCR assay using young tillers of the p35S::Flag-OsGATA8 transgenic plants at the four-leaf stage (Fig. 3d).
a, Schematic diagram of OsTCP19 with the promoter and transcribed region. Horizontal bars indicate the location of the probes used in the EMSA. P1 corresponds to the predicted OsGATA8 binding motif, while P2 is a negative control in the coding region without the predicted OsGATA8 binding motif. b, An EMSA testing the binding strength of OsGATA8 to the predicted binding motifs in the OsTCP19 promoter using probes as shown in a. The results are representative of three independent experiments. An asterisk represents a nonspecific signal. c, LUC assays in rice protoplasts on the effect of OsGATA8 on the transcription of OsTCP19. Values represent mean ± s.d. derived from three independent samples of rice protoplasts. d, ChIP–qPCR assay of the interaction between OsGATA8 and the promoters of OsTCP19 in the shoot of p35S::Flag-OsGATA8 transgenic plants at the four-leaf stage. Values represent mean ± s.d. derived from three independent samples. e, Phenotypes of Nip, the OsGATA8 knockout mutant, the OsTCP19 knockout mutant and the OsGATA8/OsTCP19 double-knockout mutant at the maximum tillering stage (about 40 days after transplanting) and the mature stage under LN and HN conditions. Scale bars, 20 cm. LN, 75 kg ha−1 net nitrogen; HN, 300 kg ha−1 net nitrogen. f, PTNR of the genotypes in e under LN and HN conditions. n = 20 plants. PTNR, productive tiller number under LN condition/productive tiller number under HN condition. g,h, The total number of tillers, productive tillers (g) and proportion of productive tillers (PT%; h) of the genotypes in e under LN and HN conditions. ‘Δ’ represents the percentage difference compared with the total number of tillers. n = 20 plants. In c and d, significant difference was determined by two-tailed Student’s t test; in f–h, different letters indicate significant differences (P < 0.05, one-way ANOVA, Duncan’s new multiple range test). For P values, see source data. Box plots denote the 25th percentile, the median and the 75th percentile, with minimum to maximum whiskers.
To determine the genetic relationship between OsGATA8 and OsTCP19, we generated OsGATA8-cr/OsTCP19-cr double-knockout lines (Fig. 3e and Supplementary Fig. 11a,b). Consistent with the putative role of OsGATA8 as a transcriptional repressor of OsTCP19, the OsGATA8-cr/OsTCP19-cr double-mutant lines in backgrounds of Nip and ZH11 exhibited decreased PTNR, increased nonproductive tillers and decreased proportion of productive tillers compared with OsGATA8-cr under both LN and HN conditions (Fig. 3e–h and Supplementary Fig. 11). These results suggest that OsGATA8 promotes the formation of nonproductive tillers by directly repressing the expression of OsTCP19.
Natural variation in the OsGATA8 promoter influences NUE
To locate the causal genetic variations in OsGATA8 that affect NUE, we analyzed the sequence of OsGATA8 in 117 rice varieties. Twenty-one insertions and deletions (InDels) and 108 SNPs were detected in the promoter of OsGATA8, but no variation was found in the coding region of OsGATA8 (Supplementary Tables 5 and 6). We resequenced the promoter of OsGATA8 and conducted an association analysis with the variants we identified. Association analysis revealed that a 245-bp deletion (chr1: 13,569,676) is significantly associated with PTNR (P = 1.78 × 10−5; Supplementary Fig. 12 and Supplementary Tables 5, 7 and 8). Given the association of the 245-bp deletion in the promoter of OsGATA8 with higher NUE, we classified the 117 rice cultivars into the following two groups based on the presence or absence of the 245-bp deletion: group 1 with HapH (OsGATA8-H exhibiting high NUE, 14 varieties) and group 2 with HapL (OsGATA8-L displaying low NUE, 103 varieties; Supplementary Fig. 12).
To test whether the presence or absence of the 245-bp sequence affects the transcription activity of the OsGATA8 promoter, we generated constructs carrying promoter variants with sequence features from OsGATA8-H or OsGATA8-L and performed an LUC reporter assay in rice protoplasts. Our results confirmed that only the 245-bp deletion (888 bp upstream of the start codon) affects the activity of the OsGATA8 promoter (Extended Data Fig. 6a,b). To verify the effect of the 245-bp deletion in planta, we generated a genome-edited mutant line (D403) carrying a 403-bp deletion in the OsGATA8 promoter spanning the 245-bp deletion in the backgrounds of Nip (Fig. 4a and Supplementary Fig. 13) and a current cultivar Ningjing 4 (N4; Supplementary Figs. 13 and 14a). In addition, we also obtained three other genome-edited mutant lines in the Nip background. These mutant lines carry 4-bp (line D4-Nip), 11-bp (line D11-Nip) and 20-bp (line D20-Nip) of deletions outside the 245-bp deletion region, respectively (Supplementary Fig. 13). We quantified the expression of OsGATA8 in the seedlings of these four lines and found that only the D403 line showed a significant decrease in OsGATA8 expression (Extended Data Fig. 6c and Supplementary Figs. 13 and 14b). Consistently, line D403 showed higher PTNR and proportion of productive tillers compared with the WT (Fig. 4b–d and Supplementary Fig. 14c–f). To verify that the reduced OsGATA8 expression in the D403 line is caused by the deletion of the 245-bp region, but not the flanking sequences, we performed LUC assays using segments of the OsGATA8 promoter. We found that the flanking sequences (115-bp upstream and 43-bp downstream) of the 245-bp deletion did not significantly affect the promoter activity of OsGATA8 (Extended Data Fig. 6a,b). These results suggest that the OsGATA8 allele with the 245-bp deletion in its promoter is a hypomorphic allele with reduced expression.
a, The phenotypes of Nip and the D403-Nip line at the maximum tillering stage and mature stage compared with Nip under LN and HN conditions. LN, 75 kg ha−1 net nitrogen; HN, 300 kg ha−1 net nitrogen. Scale bars, 20 cm. b, The PTNR of Nip and the D403-Nip line at the maximum tillering stage and mature stage under LN and HN conditions. n = 20 plants. c, The number of total number of tillers and productive tillers of Nip and the D403-Nip line under LN and HN conditions. ‘Δ’ represents the percentage difference compared with the total number of tillers. n = 20 plants. d, The proportion of productive tillers (PT%; left) and the number of nonproductive tillers (right) of Nip and the D403-Nip lines under LN and HN conditions. n = 20 plants. e, The phenotypes of Nip and six OsGATA8 transgenic lines with the HapL and HapH promoters (pHapL::OsGATA8 and pHapH::OsGATA8) at the maximum tillering stage (about 40 days after transplanting) and the mature stage under LN and HN conditions. Scale bars, 20 cm. f, PTNR of the genotypes in e. n = 20 plants. PTNR, productive tiller number under LN condition/productive tiller number under HN condition. g, The number of total number of tillers and productive tillers in the genotypes in e under LN and HN conditions. ‘Δ’ represents the percentage difference compared with the total number of tillers. n = 20 plants. h,i, The proportion of productive tillers (PT%; h) and nonproductive tillers (i) of the genotypes in e under LN and HN conditions. n = 20 plants; values represent mean ± s.d. In b and d, P values were calculated with two-tailed Student’s t test; in c and f–i, different letters indicate significant differences (P < 0.05, one-way ANOVA, Duncan’s new multiple range test). For P values, see source data. In b–d and f–i, box plots denote the 25th percentile, the median and the 75th percentile, with minimum to maximum whiskers.
To identify the superior allele of OsGATA8 associated with higher NUE, we compared the averaged relative expression of OsGATA8 and PTNR in the HapL and HapH varieties. Transcriptome assays showed that the averaged relative expression of OsGATA8 in the HapH varieties was significantly lower than that in the HapL varieties under HN conditions (Supplementary Fig. 15a), while phenotypic data revealed that the PTNR of the HapH varieties was significantly higher than that of the HapL varieties (Supplementary Fig. 15b), suggesting that HapH is the elite haplotype conferring higher NUE. To further validate this notion, we generated OsGATA8 transgenic lines with the OsGATA8 coding sequence from Nip driven by a 1.5-kb upstream sequence derived from the two OsGATA8 haplotypes (pHapH or pHapL, three independent lines for each; Fig. 4e and Supplementary Fig. 15c). Consistent with the results from the haplotype analysis, the pHapH::OsGATA8 transgenic plants exhibited significantly higher PTNR and the proportion of productive tillers than the pHapL::OsGATA8 transgenic plants, under both LN and HN conditions (Fig. 4f–i). These results verify that OsGATA8-H is an elite haplotype with higher NUE.
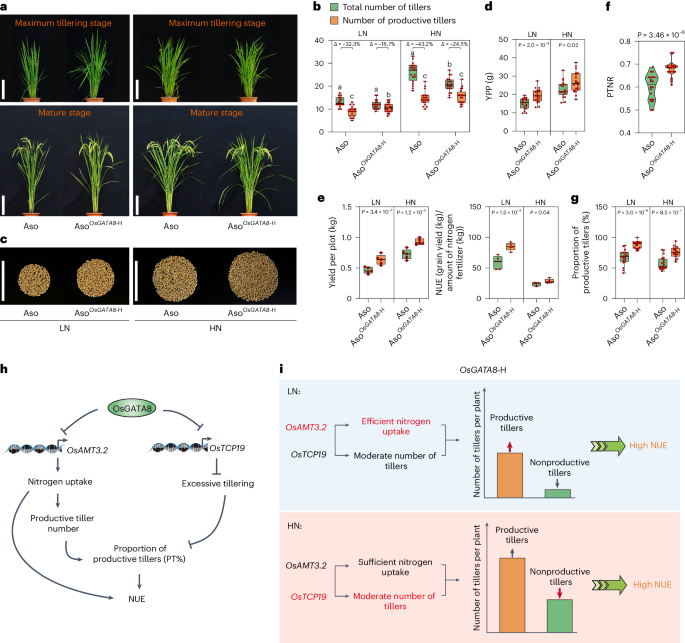
To further investigate the function of OsGATA8-H in regulating rice tillering formation and nitrogen uptake, we generated a near-isogenic line (NIL) of the japonica variety ‘Asominori’ (Aso) with OsGATA8-H from the indica variety ‘IR24’ through marker-assisted selection (Fig. 5a and Supplementary Fig. 16a). We measured the total tiller numbers and productive tiller numbers in OsGATA8 transgenic plants and AsoOsGATA8-H from seedling to maturing stages and found that the total tiller numbers were lowered in the AsoOsGATA8-H plants at the maximum tillering stage under HN condition (Fig. 5a,b and Extended Data Figs. 5 and 7a). We quantified the expression of OsGATA8 in rice tiller buds and roots from seedling to maturing stage in the Aso and AsoOsGATA8-H lines and found that the expression of OsGATA8 increased since the seedling stage, peaked at the maximum tillering stage and decreased thereafter. Consistent with the lower total tiller numbers in the AsoOsGATA8-H plants, the expressions of OsGATA8 were generally lowered in the AsoOsGATA8-H plants and specifically repressed in the young tillers that can be transformed into the productive tillers (Extended Data Fig. 7b–d). We also found that the expression of OsAMT3.2 was repressed by OsGATA8 in roots under both LN and HN conditions (Supplementary Fig. 17a), and OsTCP19 was only repressed by OsGATA8 in tiller buds under HN conditions (Supplementary Fig. 17b,c). In addition, we found that the expression of OsAMT3.2 and OsTCP19 was dynamically regulated by OsGATA8 during rice growth and development (Supplementary Fig. 17a,b). Thus, OsGATA8-H increased proportion of productive tillers predominantly via upregulating OsAMT3.2 to uptake ammonium under LN, whereas downregulating OsTCP19 to reduce nonproductive tillers under HN where the availability of nitrogen is not a rate-limiting factor (Supplementary Fig. 17). We thus speculate that this temporal expression pattern of OsGATA8 is conducible for its dual role in promoting nitrogen supply to the developing tillers during vegetative growth while reducing nonproductive tillers at the onset of reproductive growth26.
a, Phenotypes of Aso and AsoOsGATA8-H under LN and HN conditions at the maximum tillering and mature stages. LN, 75 kg ha−1 net nitrogen; HN, 300 kg ha−1 net nitrogen. Scale bars, 20 cm. b, The number of total number of tillers and productive tillers of the genotypes in a under LN and HN conditions. ‘Δ’ represents the percentage difference compared with the total number of tillers. n = 20 plants. c, Grains from one Aso plant and one AsoOsGATA8-H plant under LN and HN conditions. Scale bars, 10 cm. d, Comparison of the YPP between Aso and AsoOsGATA8-H under LN and HN conditions. n = 20 plants. e, The yield per plot (10 plants × 4 rows) and NUE of Aso and AsoOsGATA8-H under LN and HN conditions (n = 5 plots). NUE = grain yield (kg)/amount of nitrogen fertilizer (kg). f,g, PTNR (f) and proportion of productive tillers (PT%, g) of the genotypes in a. n = 20 plants. PTNR, productive tiller number under LN condition/productive tiller number under HN condition. h, A proposed model of OsGATA8 regulating rice NUE by balancing nitrogen uptake and tillering growth in rice. i, A proposed model of the role of OsGATA8 in coordinating nitrogen uptake and tiller development. Under LN conditions, OsGATA8-H confers high proportion of productive tillers (PT%) predominantly via enhanced ammonium uptake through upregulated OsAMT3.2 (highlighted in red). Under HN conditions, OsGATA8-H confers a high PT% predominantly via maintaining a moderate number of tillers through upregulated OsTCP19 (highlighted in red). In b, different letters indicate significant differences (P < 0.05, one-way ANOVA, Duncan’s new multiple range test); for P values, see source data; in d–g, P values were calculated with two-tailed Student’s t test. In b, d, e and g, box plots denote the 25th percentile, the median and the 75th percentile, with minimum to maximum whiskers; in f, the bars in the violin plots represent the 25th percentile, median and 75th percentile.
To further elucidate how OsGATA8 regulates NUE in rice during vegetative growth and reproductive growth, we quantified the biomass, nitrogen content and yield in the OsGATA8 NIL line (AsoOsGATA8-H) at the seedling, maximum tillering and mature stages and calculated the NUE accordingly (Fig. 5c–e and Supplementary Fig. 18). We found that at the seedling and maximum tillering stages, AsoOsGATA8-H plants displayed increased biomass, nitrogen content and NUE under LN condition, but no differences were observed in biomass and NUE compared with Aso under HN condition (Supplementary Fig. 18a,b). At the mature stage, AsoOsGATA8-H plants exhibited increased yield, biomass nitrogen content and NUE under both LN and HN conditions (Fig. 5c–e and Supplementary Fig. 18c). These results suggest that OsGATA8 has a dual role in regulating NUE. On one hand, OsGATA8 downregulates nitrogen uptake by repressing the expression of OsAMT3.2 (Fig. 2). On the other hand, OsGATA8 promotes tiller formation by downregulating the expression of OsTCP19, which encodes an inhibitor of tillering in rice (Fig. 3). As a consequence of N-induced expression of OsGATA8, tillering is promoted while nitrogen uptake is downregulated. This leads to nonproductive tillers and a decrease in yield and NUE27.
OsGATA8-H has been artificially selected in fertile regions
To examine whether OsGATA8 has been under artificial selection during the domestication of rice, we calculated the nucleotide diversity within a 30-kb region covering OsGATA8 in the rice 3K population28 and found a selective sweep signal between Oryza rufipogon and indica, indicating that OsGATA8-H was artificially selected in rice during the domestication process of rice (Supplementary Fig. 19a). Next, we analyzed the selection process of OsGATA8-H over the breeding history of Asian cultivated rice. We first developed a 245-bp InDel marker in the OsGATA8 promoter and genotyped OsGATA8 in 146 wild rice varieties from different regions of Asia. We identified OsGATA8-H in most of the surveyed wild rice varieties distributed in southern China, Myanmar and Sri Lanka (Supplementary Fig. 19b and Supplementary Table 9). Haplotype analysis of OsGATA8 in the 446 wild rice29 varieties revealed that the OsGATA8-H originated from two haplotypes in wild rice, Hap (O.rufip)-5 and Hap (O.rufip)-9 (Supplementary Fig. 19c). We also investigated the distribution of OsGATA8-H in cultivated rice varieties and found that OsGATA8-H exists in only 17% of the indica and tropical japonica varieties in the rice 3 K population (Extended Data Fig. 8a), while it is completely absent in the aus varieties (Extended Data Fig. 8b and Supplementary Table 10). We further analyzed 135 indica rice varieties from the 1950s to the 2000s and found that the frequency of the presence of OsGATA8-H in varieties before 1960 was relatively low, which is consistent with the frequency of this haplotype in the 146 wild rice varieties, but was significantly increased since that time (Extended Data Fig. 8c and Supplementary Table 11). This observation correlates with the sharply increased popularization and large-scale use of industrially synthesized chemical fertilizers in agricultural production since the 1960s (Extended Data Fig. 8d and Supplementary Table 12). Additionally, we analyzed the frequencies of OsGATA8 haplotypes in the rice 3 K population from countries with different amounts of soil nitrogen in Asia. We found that the frequency of OsGATA8-H positively correlates with the regional soil nitrogen content (Extended Data Fig. 8e and Supplementary Table 13). These observations together suggest that OsGATA8-H may have been under artificial selection for adaptation to fertile soil and HN conditions.
Breeding potential of OsGATA8-H/OsTCP19-H
Excessive nitrogen fertilizer reduces the proportion of productive tillers and NUE in rice. To investigate whether OsGATA8-H has the breeding potential to improve the proportion of productive tiller and NUE in rice, we examined the proportion of productive tiller, PTNR and yield using the OsGATA8-H/L varieties under LN and HN conditions (Supplementary Figs. 20 and 21). Our results revealed that the increased application of nitrogen results in a dramatic increase in the formation of nonproductive tillers, and artificial selection toward OsGATA8-H led to decreased nonproductive tiller formation under HN. The AsoOsGATA8-H line not only exhibited higher PTNR (Fig. 5f), increased proportion of productive tillers (Fig. 5g), panicle length, grain number per panicle (Extended Data Fig. 9a,b) and grain YPP (Fig. 5c,d), but also showed increased yield per plot and NUE at the mature stages (Fig. 5e and Extended Data Fig. 9a). Consistently, we observed increased OsAMT3.2 expression in the roots of the AsoOsGATA8-H line and higher nitrogen concentrations in the shoots of the AsoOsGATA8-H line, which is consistent with the expected effect of reduced OsGATA8 expression (Supplementary Figs. 18 and 22). These results further demonstrate that OsGATA8 negatively regulates yield and NUE.
Previous studies identified OsTCP19 as a modulator of tillering in response to nitrogen and found that the elite haplotype OsTCP19-H (harboring a 29-bp InDel in its promoter) is prevalent in wild rice, but has been largely lost in modern cultivars. Moreover, excessive nitrogen leads to an increased number of nonproductive tillers in OsTCP19-H cultivars11. Here we showed that OsGATA8-H is associated with reduced tiller formation and efficient ammonium uptake. These observations prompted us to test the potential of achieving high NUE under both HN and LN conditions by combining the two elite haplotypes via cross-breeding. We constructed two NILs in the indica rice 9311 background, one with OsGATA8-H from wild rice (O. rufipogon) and the other carrying both OsGATA8-H and OsTCP19-H, an elite haplotype with high NUE11 (Supplementary Fig. 16b,c and Extended Data Fig. 10a). Compared with the WT 9311, both the 9311OsGATA8-H and 9311OsGATA8-H/OsTCP19-H lines exhibit increased PTNR, productive tiller numbers and proportion of productive tillers (Extended Data Fig. 10b–d). An increase in YPP and NUE was also observed for 9311OsGATA8-H compared with 9311 (Extended Data Fig. 10e). Notably, 9311OsGATA8-H/OsTCP19-H exhibited even higher NUE and grain yield than 9311OsGATA8-H (Extended Data Fig. 10e). These results demonstrate that OsGATA8-H and OsTCP19-H represent a superior haplotype combination for high NUE and yield.
Discussion
Excessive nitrogen input promotes the formation of nonproductive tillers, which fail to accumulate photo-assimilated products30. Yet how this process is regulated remains poorly understood. In this study, we identified a new transcription factor OsGATA8 as a coordinator of NUE and tiller formation. OsGATA8 negatively regulates nitrogen uptake by repressing transcription of the ammonium transporter gene OsAMT3.2 and promotes tiller formation by repressing transcription of OsTCP19, a negative modulator of tillering. Thus, our results establish an intrinsic link between nitrogen uptake and the development of productive tillers.
Moreover, we identify OsGATA8-H as an elite haplotype with reduced expression, which confers enhanced nitrogen uptake, an increased proportion of productive tillers and higher NUE under both high and LN conditions (Fig. 5h,i). Under LN conditions, the relatively higher expression of the ammonium transporter gene OsAMT3.2 leads to increased ammonium uptake, allowing an increased supply of nitrogen to rice tillers to promote their development into effective panicles (Figs. 2 and 5h). Meanwhile, the relatively higher expression of OsTCP19 prevents excessive tiller formation under HN conditions (Figs. 3 and 5h). Therefore, OsGATA8-H may promote NUE and yield in rice under a broad range of nitrogen conditions given its dual role in the transcriptional regulation of OsAMT3.2 and OsTCP19 (Fig. 5i).
Previous studies showed that OsTCP19 has a role in geographical adaptation to fertile soil in rice and that its elite haplotype, OsTCP19-H, is found mainly in aus rice varieties from regions with LN11. Here we found that OsGATA8-H is mainly present in indica and tropical japonica rice cultivars but completely absent in the aus varieties and is associated with high soil nitrogen content (Extended Data Fig. 8b,e). NUE and yield can be improved under both HN and LN conditions by combining OsGATA8-H and OsTCP19-H (Extended Data Fig. 10e). As varieties harboring both OsGATA8-H and OsTCP19-H are extremely rare, accounting for only ~7% of the varieties in the rice 3K population (Extended Data Fig. 10f), creation of OsGATA8 promoter alleles that functionally resemble OsGATA8-H via genome editing31,32 in rice cultivars carrying OsTCP19-H offers an expedite way of generating rice germplasm with optimized NUE in diverse rice genetic backgrounds.
Methods
Plant materials and growth conditions
The seeds of the 117 accessions were collected, stored and supplied by the State Key Laboratory of Crop Genetics & Germplasm Enhancement and Utilization, Jiangsu Collaborative Innovation Center for Modern Crop Production, Nanjing Agricultural University, China. Germination, transplantation and cultivation of the 117 cultivars were performed concurrently in the same fields (HN or LN). All 117 rice cultivars are capable of developing productive tillers with normal grains for harvest in Nanjing, China (31°139′ N, 119°22′ E, 30 m above sea level). All materials were planted in the field at the experimental farm of the Nanjing Agricultural University, Nanjing, China (31°139′ N, 119°22′ E, 30 m above sea level). For the field experiments, the accessions were grown in a completely randomized block design with four replicates. The field experiments were carried out as a randomized block design with two nitrogen levels (300 kg ha−1 net nitrogen and 75 kg ha−1 net nitrogen) in two blocks. All the SNP data and phenotype data are shown previously14. Phosphate and potassium fertilizers were both applied at 100 kg ha−1. There were 20 cm and 17 cm between rows and individuals, respectively. Rice seedlings were cultured in International Rice Research Institute nutrient solution33. All transgenic materials were obtained through Agrobacterium tumefaciens-mediated transformation as previously described34. Daytime conditions in the greenhouse were 30 °C for 14 h; nighttime conditions were 28 °C and dark for 10 h.
GWAS
We investigated plant height and productive tiller number at the mature stage under LN and HN conditions. We calculated the ratio of plant height and productive tiller number under LN/HN (PHR and PTNR) and used them as proxies of NUE to carry out GWAS. We carried out GWAS and prioritization of the candidate genes as described in our previously published papers14,17 with minor modifications.
Rice genome editing by CRISPR–Cas9
Single-guide RNAs were designed with CRISPR-P (v2.0)35 (http://crispr.hzau.edu.cn/CRISPR2/). Constructs for the genome editing of rice plants were generated using a CRISPR plasmid toolbox as described before36. For single-target edits, a pair of oligonucleotides bearing the spacer sequence were annealed and ligated into a BsaI-digested binary vector backbone carrying the rice codon-optimized SpCas9 driven by the maize ubiquitin promoter and the single-guide RNA scaffold driven by the OsU6 promoter. For two-target edits, a pair of oligonucleotides bearing one of the two spacer sequences were annealed and ligated into BsmBI-digested intermediate vector backbones pYPQ131c or pYPQ132c, respectively. A Goldengate reaction with BsaI was performed on the completed pYPQ131c-sgRNA1, pYPQ132c-sgRNA2 and pYPQ142 to generate an intermediate plasmid bearing two guide RNA-encoding genes. Finally, a multiway LR reaction was performed with the completed double-guide RNA plasmid pYPQ142-sgRNA1 + 2, the Cas9 plasmid pYPQ167 and the binary vector backbone pCam1300 to generate the final construct using the LR Clonase II Plus Kit (Invitrogen). Agrobacterium-based rice transformation was performed as described above to obtain individual transformation events. Homozygous, Cas9-free mutants were obtained through genetic segregation and genotyping by PCR–Sanger sequencing.
Observation of material phenotype
The number of tillers in rice was surveyed at 7-day intervals, starting 14 days after transplanting of rice seedlings. The total tiller number was scored at the maximum tillering stage of rice (about 40 days after transplanting), and the productive tiller number was scored at the mature stage of rice. The proportion of productive tillers is calculated by dividing the number of productive tillers at the mature stage by the number of total number of tillers at the maximum tillering stage. The number of nonproductive tillers is calculated by subtracting the number of productive tillers at the mature stage from the total number of tillers at the maximum tillering stage (Extended Data Fig. 1a). The productive tillers referred to panicles with more than five full grains. Normally, the nitrogen uptake and usage during the grain-filling stage of rice determine the formation of rice grains and productive tillers. PTNR was evaluated by calculating the relative PTNR in plants grown under HN and LN conditions.
Real-time PCR and RNA-seq
Total RNA was isolated from tissues of rice seedlings, leaves, tiller buds or roots using a plant RNA purification kit (Invitrogen). Real-time PCR was conducted with I-Cycle (Bio-Rad). The reaction system contained 200 ng complementary DNAs, 0.5 μl of 10 mmol l−1 gene-specific primers and 20 μl of real-time PCR SYBR Mix (2X SYBR Green Pro Taq HS Premix II; Accurate Biotechnology, AG11702). OsACTIN1 was used as the internal control. All of the quantitative real-time primers and primers involved in this paper are listed in Supplementary Table 14. At least three independent biological replicates were collected for each experiment. Seedlings of Nip and OsGATA8-cr lines were grown for 2 weeks in a basic nutrient solution containing 1.44 mM nitrogen. The seedlings of 175 rice varieties from the rice 3K population were grown in a basic nutrient solution containing 1.44-mM nitrogen for 2 weeks and treated with nitrogen-free nutrient solution for 1 h. Seedling tissues weighing 2 g were collected for subsequent RNA-seq. Sequencing and data analysis were conducted by Shenzhen BGI using Illumina HiSeq 2000 Plus.
Transient transactivation assay in rice protoplasts
Rice protoplasts were prepared from 2-week-old seedlings as previously described37. The vector pGreenII 0800-LUC was used to analyze the activity of the different promoters. The 1500-bp upstream start codons of OsAMT1.2 and OsAMT3.2 were cloned into vector pGreen II0800-LUC to generate reporters, and the full-length coding sequence of OsGATA8 was inserted into vector pAN580 to generate the effector. Empty vectors of pGreen II0800-LUC and Pan580 were used as controls. After 16 h of penetration at 28 °C in the dark, the protoplast protein was extracted, and firefly LUC activity and Renilla (REN) LUC activity were measured using the dual-LUC reporter assay system (Promega, E1910). The ratio between LUC and REN activities was measured three times.
Phylogenetic analysis
The amino acid sequences of ammonium transporters (AMTs) in rice and their homologs in Arabidopsis thaliana were aligned by MEGA7 software. Phylogenetic trees were constructed with the aligned protein sequences using MEGA7 software with the neighbor-joining method. The accession numbers and databases of sequences for constructing these phylogenetic trees can be found in the Michigan State University (MSU) Rice Genome Annotation Project (http://rice.plantbiology.msu.edu/) and the National Center for Biotechnology Information database (https://www.ncbi.nlm.nih.gov/).
15N accumulation assay
Rice seedlings were grown in International Rice Research Institute nutrient solution (1.44 mM NH4NO3) for 3 weeks and were changed once a day. Uniform seedlings were chosen for further treatments. Then, the seedlings were pretreated with 2 mM (NH4)2SO4 and 2 mM KNO3 for 1 week and transferred to a nitrogen-free solution for starvation treatment for 4 days, then transferred to 0.1 mM CaSO4 for 1 min and treated with 0.2 mM and 2 mM (15NH4)2SO4 and Ca(15NO3)2 for 10 min, respectively. Finally, the roots of the seedlings were collected after being washed with 0.1 mM CaSO4 solution and deionized water. The samples were dried at 70 °C for 7 days and then detected the 15N content using an isotope ratio mass spectrometer system (model Flash 2000 HT; DELTAV Advantage; Thermo Fisher Scientific).
Nitrogen content determination and NUE calculation
After drying, the aboveground parts of the plants were ground into a uniform powder at the maximum tillering and mature stages. One gram of homogeneous powder was weighed, and the nitrogen content of the plant was determined using the micro Kjeldahl method. NUpE was calculated by dividing the total nitrogen in a shoot by the amount of nitrogen fertilizer. NUtE was calculated by dividing the dry shoot biomass or grain yield by the total nitrogen in the shoot (NUE = NUpE × NUtE3).
EMSA
The full-length coding sequence of OsGATA8 was inserted into vector pMAL-c4x and transformed into competent Escherichia coli BL21 (DE3) cells. E. coli DE3 containing pMAL-OsGATA8 was added to one-thousandth of isopropyl-β-d-thioglycolopy-ranoside at a concentration of 0.1 M and incubated at 16 °C for 20 h. Purification of recombinant protein using maltose magnetic beads. The LightShiftTM Chemiluminescent EMSA Kit (Thermo Fisher Scientific) was used for performing the EMSA. All primers used for probes and competitors are listed in Supplementary Table 14. Detailed experimental steps of EMSA were described previously12.
ChIP assays
ChIP assays were performed as described previously37. In brief, 4 g of transgenic rice seedlings of p35S::Flag-OsGATA8 were collected and cross-linked with 1% formaldehyde under a vacuum for 10 min. Glycine was added to the sample to a final concentration of 125 mM for quenching cross-linking, and the samples were ground into powder in liquid nitrogen. Chromatin was separated and ultrasonically fragmented. Anti-Flag antibodies were used for immunoprecipitation. DNA was purified and used for real-time PCR. The relevant primers are shown in Supplementary Table 14.
Subcellular localization
The full-length coding sequences of OsGATA8 and OsTCP19 were inserted into the vector pAN580 (primers listed in Supplementary Table 14) to generate the OsGATA8-GFP and OsTCP19-mCherry constructs, respectively. The OsGATA8-GFP and OsTCP19-mCherry plasmids were cotransformed into rice protoplasts, and fluorescence signals were detected using a laser confocal scanning microscope (Leica TCS SP8) at 16 h after transformation.
Fluorescence in situ hybridization
RNA probes with fluorescence-labeled OsGATA8 and OsTCP19 were synthesized (primers listed in Supplementary Table 14). Rice seedlings grown for 10 days were selected, and the shoot base was sampled for longitudinal sectioning to obtain rice tiller bud slices. The experiment was performed at Shanghai Rochenpharma Biotechnology. Experimental operation was performed as described in http://www.rochenpharma.com/.
DAP-seq
The full-length coding sequences of OsGATA8 were inserted into the vector Halo (provided by Genedenovo Biotechnology) to obtain the expression vector OsGATA8-Halo. DNA was extracted from the leaves of rice seedlings grown for 1 week and used to construct a cDNA library. The experimental process and data analysis were performed at Guangzhou Genedenovo Biotechnology.
Nucleotide diversity estimation
Sequence data of OsGATA8 and the 30-kb flanking regions that cover OsGATA8 were obtained from the 3,000 Rice Genomes Project of 2,832 varieties and the rice HapMap3 of 376 O. rufipogon accessions. The primers used to verify the distribution of OsGATA8-H in wild rice are shown in Supplementary Table 14. The DNA of 146 wild rice and sequence data of 135 indica cultivars were provided by the Chinese Academy of Agricultural Sciences. To construct the haplotype network of OsGATA8, we also obtained the SNPs from the 3,000 Rice Genomes Project of 2,832 varieties and the rice HapMap3 of 376 O. rufipogon accessions.
Statistics and reproducibility
Numbers (n) of samples or replicates are indicated in the figure legends (Figs. 1–5, Extended Data Figs. 1–10, and Supplementary Figs. 3, 5–8, 11, 14, 15, 17, 18, 21 and 22) and Methods. For bar charts, all values are presented as mean ± s.d. For box plots, box plots denote the 25th percentile, the median and the 75th percentile, with minimum to maximum whiskers. For violin plots, the bars represent the 25th percentile, median and 75th percentile. For pairwise comparisons, significance analysis was calculated by two-tailed Student’s t test using Excel 2010, and the exact P values are displayed. For multiple-group comparisons, significance analysis was calculated by one-way analysis of variance (ANOVA; P < 0.05) followed by Duncan’s new multiple range test as indicated in figure legends (Figs. 1b–f, 2b,h,i, 3f–h, 4c,f–i and 5b; Extended Data Figs. 4a,c,e,f, 6b,c, 9b and 10b–e; and Supplementary Figs. 3f–h, 5a, 7b–h, 8, 11c–g, 14d, 15c, 17c and 22) using SPSS version 18.0 (IBMA) and indicated with different letters.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Data supporting the findings of this work are available within the paper and its Supplementary Information. A reporting summary for this article is available as Supplementary Information. All datasets have been deposited to public databases. All genetic materials in the current study are available from the corresponding author. Sequence data of rice varieties can be found in our previous study10 (https://doi.org/10.1038/s41467-019-13187-1). Sequence data from this study can be found in the MSU database (http://rice.plantbiology.msu.edu/) under the following accessions: OsGATA8 (LOC_Os01g24070), OsTCP19 (LOC_Os06g12230), OsAMT3.2 (LOC_Os03g62200) and OsAMT1.2 (LOC_Os02g40730). Sequence for constructing the phylogenetic tree of AMTs (Supplementary Fig. 6) can be found in the Arabidopsis Information Resource database (https://www.arabidopsis.org/) or the MSU database (http://rice.plantbiology.msu.edu/). The DAP-seq and RNA-seq data have been uploaded to the website National Center for Biotechnology Information Sequence Read Archive database (DAP-seq data: SRR28790977, SRR28790978; RNA-seq data: SRR28799732, SRR28799733, SRR28799734). Source data are provided with this paper.
Code availability
All software used in the study are publicly available on the Internet as described in Methods and Reporting summary.
References
Chen, X. et al. Producing more grain with lower environmental costs. Nature 514, 486–489 (2014).
Lu, Y. L. et al. Addressing China’s grand challenge of achieving food security while ensuring environmental sustainability. Sci. Adv. 1, e1400039 (2015).
Xu, G. H., Fan, X. R. & Miller, A. J. Plant nitrogen assimilation and use efficiency. Annu. Rev. Plant Biol. 63, 153–182 (2012).
Fan, X. et al. Plant nitrate transporters: from gene function to application. J. Exp. Bot. 68, 2463–2475 (2017).
Krapp, A. Plant nitrogen assimilation and its regulation: a complex puzzle with missing pieces. Curr. Opin. Plant Biol. 25, 115–122 (2015).
Liao, Z. et al. SLR1 inhibits MOC1 degradation to coordinate tiller number and plant height in rice. Nat. Commun. 10, 2738 (2019).
Bhardwaj, A. K. et al. Switching to nanonutrients for sustaining agroecosystems and environment: the challenges and benefits in moving up from ionic to particle feeding. J. Nanobiotechnology 20, 19 (2022).
Zhang, X. et al. Managing nitrogen for sustainable development. Nature 528, 51–59 (2015).
Wang, W. et al. Expression of the nitrate transporter gene OsNRT1.1A/OsNPF6.3 confers high yield and early maturation in rice. Plant Cell 30, 638–651 (2018).
Wu, K. et al. Enhanced sustainable green revolution yield via nitrogen-responsive chromatin modulation in rice. Science 367, eaaz2046 (2020).
Liu, Y. Q. et al. Genomic basis of geographical adaptation to soil nitrogen in rice. Nature 590, 600–605 (2021).
Yu, J. et al. Enhanced OsNLP4-OsNiR cascade confers nitrogen use efficiency by promoting tiller number in rice. Plant Biotechnol. J. 19, 167–176 (2021).
Hu, B. et al. Variation in NRT1.1B contributes to nitrate-use divergence between rice subspecies. Nat. Genet. 47, 834–838 (2015).
Tang, W. et al. Genome-wide associated study identifies NAC42-activated nitrate transporter conferring high nitrogen use efficiency in rice. Nat. Commun. 10, 5279 (2019).
Liu, Q. et al. Improving crop nitrogen use efficiency toward sustainable green revolution. Annu. Rev. Plant Biol. 73, 523–551 (2022).
Sun, H. et al. Heterotrimeric G proteins regulate nitrogen-use efficiency in rice. Nat. Genet. 46, 652–656 (2014).
Yu, J. et al. Genome-wide association studies identify OsWRKY53 as a key regulator of salt tolerance in rice. Nat. Commun. 14, 3550 (2023).
Nutan, K. K., Singla-Pareek, S. L. & Pareek, A. The Saltol QTL-localized transcription factor OsGATA8 plays an important role in stress tolerance and seed development in Arabidopsis and rice. J. Exp. Bot. 71, 684–698 (2020).
Schwechheimer, C., Schroder, P. M. & Blaby-Haas, C. E. Plant GATA factors: their biology, phylogeny, and phylogenomics. Annu. Rev. Plant Biol. 73, 123–148 (2022).
Xie, Y. M. et al. Plastid-localized amino acid metabolism coordinates rice ammonium tolerance and nitrogen use efficiency. Nat. Plants 9, 1514–1529 (2023).
Xuan, W., Beeckman, T. & Xu, G. H. Plant nitrogen nutrition: sensing and signaling. Curr. Opin. Plant Biol. 39, 57–65 (2017).
Konishi, N. & Ma, J. F. Three polarly localized ammonium transporter 1 members are cooperatively responsible for ammonium uptake in rice under low ammonium condition. New Phytol. 232, 1778–1792 (2021).
Lee, S. et al. Concurrent activation of OsAMT1;2 and OsGOGAT1 in rice leads to enhanced nitrogen use efficiency under nitrogen limitation. Plant J. 103, 7–20 (2020).
Chen, L. et al. OsMADS57 together with OsTB1 coordinates transcription of its target OsWRKY94 and D14 to switch its organogenesis to defense for cold adaptation in rice. New Phytol. 218, 219–231 (2018).
Huang, S. et al. A transcription factor, OsMADS57, regulates long-distance nitrate transport and root elongation. Plant Physiol. 180, 882–895 (2019).
Ren, M. et al. Genome-wide association study of the genetic basis of effective tiller number in rice. Rice 14, 56 (2021).
Yang, H. K., Xiao, Y., Zhang, X., Huang, X. L. & Fan, G. Q. Maize straw mulching with uniconazole application increases the tillering capacity and grain yield of dryland winter wheat (Triticum aestivum L.). Field Crops Res. 284, 108573 (2022).
Wang, W. et al. Genomic variation in 3,010 diverse accessions of Asian cultivated rice. Nature 557, 43–49 (2018).
Huang, X. et al. A map of rice genome variation reveals the origin of cultivated rice. Nature 490, 497–501 (2012).
Kebrom, T. H. et al. Inhibition of tiller bud outgrowth in the tin mutant of wheat is associated with precocious internode development. Plant Physiol. 160, 308–318 (2012).
Tang, X. & Zhang, Y. Beyond knockouts: fine-tuning regulation of gene expression in plants with CRISPR–Cas-based promoter editing. New Phytol. 239, 868–874 (2023).
Shi, L. et al. Promoter editing for the genetic improvement of crops. J. Exp. Bot. 74, 4349–4366 (2023).
Zhang, S. et al. Nitrogen mediates flowering time and nitrogen use efficiency via floral regulators in rice. Curr. Biol. 31, 671–683 (2020).
Hiei, Y., Ohta, S., Komari, T. & Kumashiro, T. Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J. 6, 271–282 (1994).
Liu, H. et al. CRISPR-P 2.0: an improved CRISPR–Cas9 tool for genome editing in plants. Mol. Plant 10, 530–532 (2017).
Lowder, L. G. et al. A CRISPR/Cas9 toolbox for multiplexed plant genome editing and transcriptional regulation. Plant Physiol. 169, 971–985 (2015).
Duan, E. et al. OsSHI1 regulates plant architecture through modulating the transcriptional activity of IPA1 in rice. Plant Cell 31, 1026–1042 (2019).
R Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2017).
Acknowledgements
The National Key Research and Development Project (2021YFF1000400 and 2022YFD1201702 awarded to Chunming Wang), National Natural Science Foundation of China (32272030 awarded to Chunming Wang), Scientific Innovation 2030 Project (2022ZD0401703 awarded to Chunming Wang), the Natural Science Foundation of Jiangsu Province (BK20210384 awarded to X.D. and BK20230982 awarded to G.C.) and China Postdoctoral Science Foundation (grant 2023M741753 awarded to G.C.) supported this study. We thank C. Zhu (Department of Immunology, The University of Texas Southwestern Medical Center, USA) for technical support in statistical analysis. The funding agencies had no role in the study design, data collection and analysis, decision to publish or paper preparation.
Author information
Authors and Affiliations
Contributions
J.W. and Chunming Wang directed the project. Chunming Wang, X.D., W.W. and W.X. designed the experiments. W.W. and G.C. performed most of the experiments. Chunming Wang, X.D., W.X., P.C.R. and H.W. wrote the paper and finalized the paper. Z.L., W.C., W. Tang, J.Y., S.W., X.Z., X.J., X.L., Y.W., Chunyuan Wang, X.C. and W.Z. participated in the experiments. H.W. and W. Terzaghi revised the paper. All the co-authors approved the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Genetics thanks Takeshi Izawa, Nicolaus von Wiren and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Overapplication of nitrogen fertilizers reduces the proportion of productive tillers in rice.
(a) An illustration of tiller development in rice. Tillers that produce effective panicles become productive tillers, while the rest become nonproductive tillers. The proportion of productive tillers (PT%) is defined as the ratio between the number of productive tillers and the total number of tillers at the maximum tillering stage. (b) The development of tillers and panicles in the japonica rice cultivar Nip grown under low nitrogen (75 kg/ha net nitrogen) and high nitrogen (300 kg/ha net nitrogen) conditions. (c,d) Tillers counts of Nipponbare rice grown under low nitrogen (c; 75 kg/ha net nitrogen) and high nitrogen (d; 300 kg/ha net nitrogen) conditions at various time points after transplanting. n = 10 plants. PT% is displayed.
Extended Data Fig. 2 Identification of OsGATA8 on chromosome 1.
(a) Local Manhattan plot (top) and linkage disequilibrium (LD) heatmap (bottom) surrounding the peak on chromosome 1 using P values of PTNR and PHR. The LD heatmap displays SNPs with P < 1.0 × 10−4. P values were determined under the mixed linear model and implemented in Tassel 5. PTNR, productive tiller number ratio (productive tiller number under LN condition/productive tiller number under HN condition); PHR, plant height ratio (plant height under LN condition/plant height under HN condition). HN, high nitrogen (300 kg/ha net nitrogen); LN, low nitrogen (75 kg/ha net nitrogen). The color key (white to red) represents linkage disequilibrium values (r2). (b) Heatmap of the relative expression of the three candidate genes near the peak in a under three nitrogen supply conditions (0-, 1- and 5-mM NH4NO3) based on qRT–PCR of rice seedlings. The color key (blue to red) represents fold changes of gene expression. (c) The PTNR, PHR and the relative expression of OsGATA8 in 16 varieties with extreme NUE. Values represent mean ± SD derived from three individual plants. The dotted lines represent the average values of all varieties with extreme NUE, respectively. In c, P values were calculated with two-tailed Student’s t test. PTNR, productive tiller number ratio (productive tiller number under LN condition/productive tiller number under HN condition); PHR, plant height ratio (plant height under LN condition/plant height under HN condition).
Extended Data Fig. 3 OsGATA8 regulates nitrogen uptake in rice through the direct transcriptional regulation of nitrogen metabolism-related genes.
(a) The nitrogen content of Nip and OsGATA8-cr1 plants in roots and shoots grown under low nitrogen (LN, 0.2 mM NH4NO3) and high nitrogen (HN, 2 mM NH4NO3) concentrations for four weeks. Values represent mean ± SD derived from five individual plants. (b) 15N accumulation assay in roots of the Nip and OsGATA8-cr1 lines under low ammonium (0.1 mM (15NH4)2SO4), high ammonium (1 mM (15NH4)2SO4), low nitrate (0.1 mM Ca(15NO3)2) and high nitrate (1 mM Ca(15NO3)2) conditions. Values represent mean ± SD derived from root tissues of three individual seedlings. (c) Gene Ontology enrichment analysis of 619 DEGs (differentially expressed genes) in OsGATA8 knockout lines under Nip background. (d) The relative expression of DEGs (differentially expressed genes) in transporter activity process (GO:0005215). The color key (blue to red) represents gene expression (FPKM) as fold changes. The proteins encoded are described on the left. (e) A list of nitrogen metabolism genes that OsGATA8 binds to according to DNA affinity purification sequencing (DAP-seq). Fold enrichment represents the number of reads in the purified sample relative to the input sample. In a and b, displayed P values were calculated with two-tailed Student’s t test.
Extended Data Fig. 4 OsAMT3.2 positively regulates rice ammonium uptake.
(a) Relative expression of OsAMT3.2 under different nitrogen conditions (0-, 0.25-, 2.5- and 10-mM NH4+). Values represent mean ± SD derived from root tissues from three individual rice seedlings. (b) Relative expression of OsAMT3.2 in various rice tissues after rice flowering. Values represent mean ± SD derived from three individual plants. (c) Relative expression of OsAMT3.2 in Nipponbare (Nip), OsAMT3.2 knockout and overexpression lines. Values represent mean ± SD derived from root tissues from three individual rice seedlings. (d) The mutation in the OsAMT3.2 CRISPR knockout line in the Nipponbare background. Black bars: the coding region; white bars: the UTRs; red lines: locations of the editing targets. (e,f) 15N accumulation of ammonium in roots and total nitrogen content of shoots in OsAMT3.2 knockout (cr) and OsAMT3.2 overexpression (OE) lines under low ammonium (0.1 mM (15NH4)2SO4) and high ammonium (1.0 mM (15NH4)2SO4) conditions. Values represent mean ± SD derived from three individual rice seedlings. In a and c, total RNA was extracted from root tissue of two-week-old seedlings. In a, c, e and f, different letters indicate significant differences (P < 0.05, one-way ANOVA, Duncan’s new multiple range test), for P values, see source data.
Extended Data Fig. 5 OsGATA8 positively regulates rice tillering formation.
(a) The phenotypes of the OsGATA8-cr knockout lines and OsGATA8 transgenic lines exhibited tillering phenotypes at maximum tillering stage and effective panicle (productive tillers) phenotypes at mature stage under LN and HN conditions. The Nipponbare control has been shown in Extended Data Fig. 1b. Scale bar, 5 cm. LN, low nitrogen (75 kg/ha net nitrogen); HN, high nitrogen (300 kg/ha net nitrogen). Red arrows indicate nonproductive tillers. (b,c) The tillering dynamic changes of OsGATA8 knockout and transgenic lines in a with rice transplanting days under LN and HN conditions. n = 10 plants. Data are presented as mean ± SD. P values were calculated with two-tailed Student’s t test by comparing the values with those of the Nipponbare control (*P < 0.05; **P < 0.01); for P values, see source data.
Extended Data Fig. 6 A 245-bp deletion in the promoter of OsGATA8 reduces the expression of the gene.
(a) Schematic representation of the reporter constructs for the luciferase assay. The black folded lines in P-A, P1-A and P2-A represent a 150-bp deletion relative to the red box. The black folded lines in P-B and P1-B represent a 245-bp deletion relative to the green box. The black folded lines in P-A-D43, P-A-D115 and P-A-D403 represent deletions relative to P-A. (b) Luciferase assays of the promoter activities of P-A, P-B, P1-A, P1-B, P2-A, P2-B, P3-A, P3-B, P-A-D115, P-A-D43 and P-A-D403 using rice protoplasts. Values represent mean ± SD derived from five independent samples of rice protoplasts. (c) Relative expression of four homozygous lines. Values represent mean ± SD derived from three individual rice seedlings. In b and c, different letters indicate significant differences (P < 0.05, one-way ANOVA, Duncan’s new multiple range test); for P values, see source data.
Extended Data Fig. 7 OsGATA8 has a dual role in regulating rice tiller formation during vegetative growth and reproductive growth.
(a) The tillering dynamic changes of Aso and AsoOsGATA8-H lines with rice transplanting days under LN and HN conditions. n = 10 plants. Data are presented as mean ± SD. (b,c) Dynamic changes of OsGATA8 transcription levels in Aso and AsoOsGATA8-H tiller buds and roots with transplanting days. In b, total RNA was extracted from tiller buds of rice on different days after transplanting; in c, total RNA was extracted from roots on different days after transplanting. Values represent mean ± SD derived from root tissues of three individual plants. (d) Expression levels of OsGATA8 in younger and older tillers at various developmental stages. Total RNA was extracted on different days after transplanting from the base of tillers that formed earlier or later. For each plant of a given developmental stage, three tillers that formed earlier and three that formed later were used for RNA extraction. Three plants were sampled for each developmental stage. Values represent mean ± SD derived from the tiller base of three individual plants. In a–d, P values were calculated with two-tailed Student’s t test (*P < 0.05; **P < 0.01), for P values, see source data.
Extended Data Fig. 8 OsGATA8-H is associated with soil nitrogen availability.
(a) The frequency of OsGATA8-H in the rice 3K population. (b) The frequency of OsGATA8-H and OsTCP19-H among different rice subgroups. The red box highlights the distribution difference between OsGATA8-H and OsTCP19-H in Aus and Aro rice. (c) The frequency of OsGATA8-H in wild rice populations and cultivated varieties from different years (1950–2000). (d) The amount of nitrogen fertilizer applied in China from 1961 to 2010. The original data are from the FAOSTAT Database (Food and Agriculture Organization Statistics). (e) Distribution of OsGATA8-H in different regions and subpopulations. The red area represents the soil nitrogen content in the region. The map was made using the ‘maps’ package in R38. Inset, Pearson’s correlation coefficient analysis of the frequency of OsGATA8-H with soil total nitrogen content (g/kg) in 33 countries or regions worldwide. Data on soil nitrogen content in regions or countries are taken from a published article11.
Extended Data Fig. 9 OsGATA8-H significantly improves rice yield and NUE.
(a) The phenotypes of OsGATA8 NIL line at the mature stage under LN and HN conditions. (b) The panicle length and grain number per plant of AsoOsGATA8-H and line at the mature stage under LN and HN conditions (n = 20 plants). In a and b, LN, low nitrogen (75 kg/ha net nitrogen); HN, high nitrogen (300 kg/ha net nitrogen). In b, box plots denote the 25th percentile, the median and the 75th percentile, with minimum to maximum whiskers; different letters indicate significant differences (P < 0.05, one-way ANOVA, Duncan’s new multiple range test); for P values, see source data.
Extended Data Fig. 10 Combining the elite haplotypes OsGATA8-H and OsTCP19-H has breeding potential.
(a) Phenotypes of 9311, 9311OsGATA8-H and 9311OsGATA8-H/OsTCP10-H under LN and HN conditions at the maximum tillering stage and the mature stage (n = 10 plants). Scale bars, 20 cm. (b) PTNR and the number of productive tillers of the genotypes in a under LN and HN conditions (n = 10 plants). PTNR, productive tiller number ratio (productive tiller number under LN condition/productive tiller number under HN condition). (c) The numbers of the total number of tillers and productive tillers of the genotypes in a under LN and HN conditions. ‘Δ’ represents the percentage difference compared with the total number of tillers (n = 10 plants). (d) The proportion of productive tillers (PT%) and nonproductive tillers of the genotypes in a under LN and HN conditions (n = 10 plants). (e) The yield per plant (n = 10 plants) and NUE (n = 3 plots) of the genotypes in a under LN and HN conditions. (f) Frequency of OsGATA8-H/OsTCP19-H in the rice 3 K population. In b–e, different letters indicate significant differences (P < 0.05, one-way ANOVA, Duncan’s new multiple range test); for P values, see source data. LN, low nitrogen (75 kg/ha net nitrogen); HN, high nitrogen (300 kg/ha net nitrogen). In b and e, the bars within violin plots represent 25th percentile, median and 75th percentile. In c and d, box plots denote the 25th percentile, the median and the 75th percentile, with minimum to maximum whiskers.
Supplementary information
Supplementary Information
Supplementary Figs. 1–23.
Supplementary Tables
Supplementary Table 1: Information of SNPs associated with PHR and PTNR in LD block. Supplementary Table 2: Annotation of SNPs in three genes. Supplementary Table 3: RNA-seq analysis of WT (Nip) and OsGATA8 knockout lines seedlings. Supplementary Table 4: Search for OsGATA8 downstream target genes through DNA affinity purification sequencing (DAP-seq). Supplementary Table 5: All variations of OsGATA8 promoter in 117 varieties identified by resequencing based on PCR amplification. The green highlight represents 245 bp deletion and 150 bp insertion, and the red highlight represents the variations completely linked to 245 bp in the associated SNPs of 117 varieties. Supplementary Table 6: Linkage variations of 245 bp deletion in OsGATA8 promoter in 117 varieties identified by RAD-seq. A total of 117 varieties were divided into two haplotypes HapH and HapL through 245 bp linked SNPs. Green highlights represent HapH varieties, and yellow highlights represent HapL varieties. Supplementary Table 7: The PTNR of 117 varieties for association analysis. Supplementary Table 8: The association analysis with the variants identified on the promoter of OsGATA8. P values were determined under the mixed linear model and implemented in Tassel 5. Supplementary Table 9: Allele frequency of OsGATA8-H in wild rice. Supplementary Table 10: Allele frequency of OsGATA8-H and OsTCP19-H in Rice 3K. Supplementary Table 11: OsGATA8-H allele frequency with annual precipitation and soil total nitrogen content. Supplementary Table 12: Agricultural use of nutrient nitrogen, N (total). Supplementary Table 13: OsGATA8-H allele frequency with soil total nitrogen content. Supplementary Table 14: Primers (5ʹ–3ʹ) used in this study.
Supplementary Data 1
Supporting data for Supplementary Fig. 1.
Supplementary Data 2
Supporting data for Supplementary Fig. 2.
Supplementary Data 3
Supporting data for Supplementary Fig. 3.
Supplementary Data 4
Supporting data for Supplementary Fig. 5.
Supplementary Data 5
Supporting data for Supplementary Fig. 7.
Supplementary Data 6
Supporting data for Supplementary Fig. 8.
Supplementary Data 7
Supporting data for Supplementary Fig. 11.
Supplementary Data 8
Supporting data for Supplementary Fig. 14.
Supplementary Data 9
Supporting data for Supplementary Fig. 15.
Supplementary Data 10
Supporting data for Supplementary Fig. 17.
Supplementary Data 11
Supporting data for Supplementary Fig. 18.
Supplementary Data 12
Supporting data for Supplementary Fig. 21.
Supplementary Data 13
Supporting data for Supplementary Fig. 22.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 2
Unprocessed EMSA blots for Fig. 2d.
Source Data Fig. 3
Unprocessed EMSA blots for Fig. 3b.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 9
Statistical source data.
Source Data Extended Data Fig. 10
Statistical source data.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wu, W., Dong, X., Chen, G. et al. The elite haplotype OsGATA8-H coordinates nitrogen uptake and productive tiller formation in rice. Nat Genet (2024). https://doi.org/10.1038/s41588-024-01795-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41588-024-01795-7