- 1Division of Allergy, Pulmonary and Critical Care Medicine, Department of Internal Medicine, Hallym University Kangnam Sacred Heart Hospital, Hallym University College of Medicine, Seoul, South Korea
- 2Department of Family Medicine, Healthcare System Gangnam Center, Seoul National University Hospital, Seoul, South Korea
- 3Department of Statistics and Actuarial Science, Soongsil University, Seoul, South Korea
- 4Department of Internal Medicine, Chonnam National University Hwasun Hospital, Chonnam National University Medical School, Hwasun, South Korea
- 5Department of Endocrinology and Metabolism, Kyung Hee University School of Medicine, Seoul, South Korea
- 6Division of Pulmonary Medicine and Allergy, Department of Internal Medicine, Hanyang University College of Medicine, Seoul, South Korea
- 7Department of Family Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, South Korea
- 8Department of Digital Health, Samsung Advanced Institute for Health Sciences & Technology, Sungkyunkwan University, Seoul, South Korea
Although both diabetes mellitus (DM) and underweight are associated with increased risk of tuberculosis (TB), there are limited data evaluating TB risk while considering two factors simultaneously—body mass index (BMI) and DM. A retrospective cohort study was performed with 10,087,903 participants of the Korean National Health Screening Program in 2009. The cohort was followed up to the date of TB incidence, death, or until December 31, 2018. We compared the incidence and risk of TB according to BMI category and DM. During the 7.3-year follow-up duration, the incidence of TB was 0.92 per 1,000 person-years in the normal weight without DM, 2.26 in the normal weight with DM, 1.80 in the underweight without DM, and 5.35 in the underweight with DM. Compared to the normal weight without DM, the normal weight with DM, the underweight without DM, and the underweight with DM showed a 1.51-fold (95% CI, 1.46–1.57), a 2.21-fold (95% CI, 2.14–2.28), and a 3.24-fold (95% CI, 2.95–3.56) increased risk of TB, respectively. However, compared to the normal weight without DM, the severely obese without DM and those with DM showed a 0.37 (95% CI, 0.36–0.38) and a 0.42 (95% CI, 0.36–0.48)-fold decreased risk of TB, respectively. There was no significant joint effect of BMI and DM on the risk of incident TB in the overall population; a synergistic effect of underweight and DM was evident in participants <65 years of age, current smokers, and heavy drinkers. In conclusion, being underweight or DM individually increases the risk of incident TB. Based on our study results, a focused screening of incident TB in patients with DM may be beneficial.
Introduction
Worldwide, tuberculosis (TB) is a communicable disease that is a major cause of ill health and the leading cause of death from a single infectious agent (1, 2). TB is curable and preventable—the number of people developing infections and disease (and thus the number of deaths) can also be reduced through multispectral action to address TB determinants (1).
Whereas, obesity is known to increase the risk of various types of infections (3), being underweight predisposes patients to the development of TB (4). This suggests that the impact of body weight on TB risk is opposite to the impact of body weight on other infections. A previous systematic review of six cohort studies also showed an inverse relationship between body mass index (BMI) and TB development, and the incidence of TB was lower in the overweight and obese populations than in those with normal weight (4).
The inverse association between BMI and TB interestingly presents a paradox with regard to diabetes mellitus (DM) (5). Obesity is a major determinant of DM (6, 7), and DM is a well-known risk factor for TB (3, 8, 9). A systematic review comparing 13 studies examining the association between DM and TB found that diabetic patients had about a three-fold increased risk of developing TB when compared to those without DM (8). Accordingly, we may conclude that the obese population should have an increased risk of TB because they are also more likely to have DM; however, this is not consistent with available epidemiological data (5, 10).
Better understanding of the complex interplay between BMI and DM on the risk of TB has critical implications for global health. However, there is a lack of research focusing on the interaction between these two risk factors—DM and BMI—for TB development, although DM and BMI may endocrinologically interact with each other. We found two Asian studies that examined the joint effect of DM and BMI on incident TB (5, 10). However, they did not use the Asian-Pacific classifications of BMI, which limits interpretation of the studies, because the BMI cut-off points for defining overweight and obesity should be lower for Asians (11). Additionally, one study defined DM by self-reporting, which might result in under-reporting or misclassification (10). They also had limitations due to small study populations and numbers of cases [e.g., 491 incident TB cases among 167,392 participants (5)] and did not fully adjust for potential confounding factors such as socioeconomic status, including income status (5). Considering there is still uncertainty in the complex interaction between BMI and DM regarding the risk of TB, a confirmatory study on the issue is warranted.
Therefore, in the present study, using a nationwide population-based data in Korea, we aimed to investigate the contributions of BMI and DM to future TB risk while taking into consideration the potential interactions between BMI and DM.
Materials and Methods
Study Population and Design
We conducted a retrospective cohort study using the Korean National Health Insurance Service (NHIS) database. South Korea has a single-payer universal health system, and the NHIS maintains claims data on all reimbursed inpatient and outpatient visits, procedures, and prescriptions (12). Additionally, the NHIS database includes data from annual or biennial health screening exams provided free of charge by the Ministry of Health and Welfare. Approximately 72% of all eligible persons underwent screening from 2011 to 2014 (13).
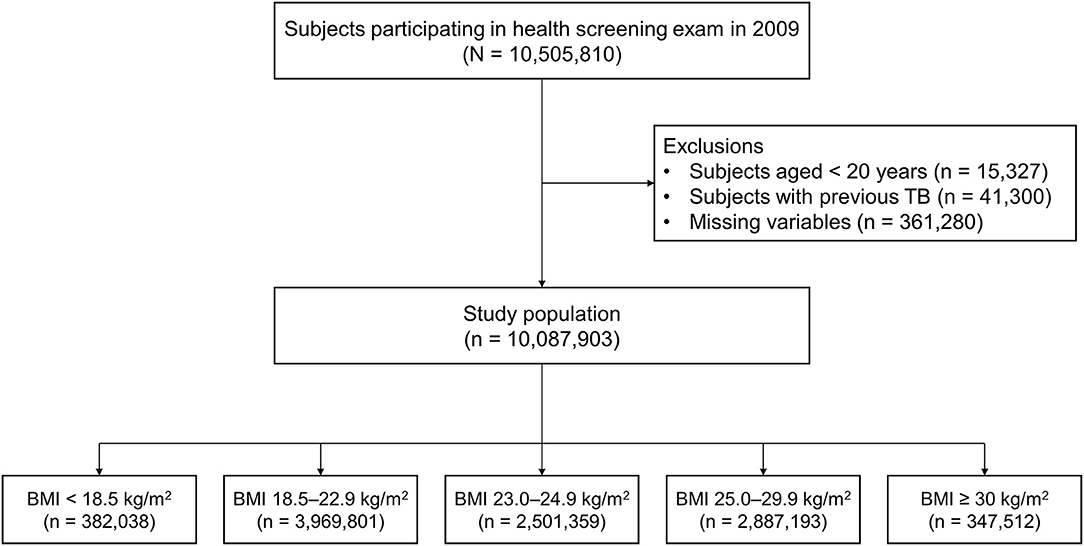
This study initially included 10,505,810 subjects who participated in the health screening exam between January 1, 2009, and December 31, 2009. We excluded participants who were aged less than 20 years (n = 15,327), those previously diagnosed with TB before the enrollment (n = 41,300), and those who had missing data (n = 361,280). Finally, a total of 10,087,903 participants were included in this study (Figure 1). The cohort was followed from baseline to the date of incident TB, death, or until the end of the study period (December 31, 2018), whichever came first.
The institutional review board of Hallym University Kangnam Sacred Heart Hospital approved the study and waived the requirement for informed consent because the NHIS database was constructed after anonymization (application no. 2020-08-015).
Exposure: BMI and DM
Height and weight were measured in medical institutes during the health examinations. BMI was calculated as body weight (kg) divided by height (m) squared and was classified into five categories—underweight (<18.5 kg/m2), normal weight (18.5–22.9 kg/m2), overweight (23.0–24.9 kg/m2), obese (25.0–29.9 kg/m2), and severely obese (≥30 kg/m2)—according to the Asia-Pacific BMI criteria established by the Western Pacific Region of the World Health Organization (11).
The presence of DM was defined using International Classification of Diseases, 10th Revision (ICD-10) diagnosis codes E11–E14 plus a prescription history of hypoglycemic agents (14). Regarding DM control status, the well-controlled status was defined as fasting blood glucose <130 mg/dl and poorly controlled status was defined as fasting blood glucose ≥ 130 mg/dl (15).
Outcome: Tuberculosis
The primary outcome of this study was incident TB, which was defined using the rare intractable disease (RID) registration codes for TB (V206, V246, and V000). All physicians should report to the public health center when they diagnose TB or suspected TB cases. The notification data include the patients' personal information, examination results, treatments, and treatment outcomes. These patients are also registered in the RID program (16). Hence, these clinical settings allowed us to identify every incident TB in the whole Korean population.
Covariates
Information on participants' lifestyles was obtained from the health screening program self-questionnaire. Smoking status was classified into never, former, and current smoker categories. Those who consumed over 30 g of alcohol per day were defined as heavy drinkers. Regular physical activity was defined as when individuals performed strenuous exercise for at least 20 min three times per week or moderate exercise for at least 30 min five times per week (14).
Regarding comorbidities, hypertension, and dyslipidemia were defined using the ICD-10 diagnosis codes I10–I15 and E78, respectively (17).
Statistical Analysis
Analysis of variance and a chi-squared test for continuous and categorical variables, respectively, were performed to examine the differences among BMI categories. The incidence rate of TB was calculated by dividing the number of incident cases by the total follow-up duration (1,000 person-years). Cox proportional hazards regression analyses were used to evaluate the association of BMI with the incidence of TB, without considering the time-varying nature of BMI. The multivariable model was fully adjusted for age, sex, smoking status, alcohol consumption (heavy drinker or not), regular physical activity, income (lowest 20% or not), hypertension, and dyslipidemia. The analyses were stratified by the presence of DM, along with sex and age group (<65 or ≥65 years). The joint effects of DM and BMI on TB development were assessed, and the effects were also assessed in subgroups that were stratified according to age, sex, smoking status, and alcohol consumption (18). Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). All tests were two-sided, and P-values < 0.05 were considered statistically significant.
Results
Baseline Characteristics
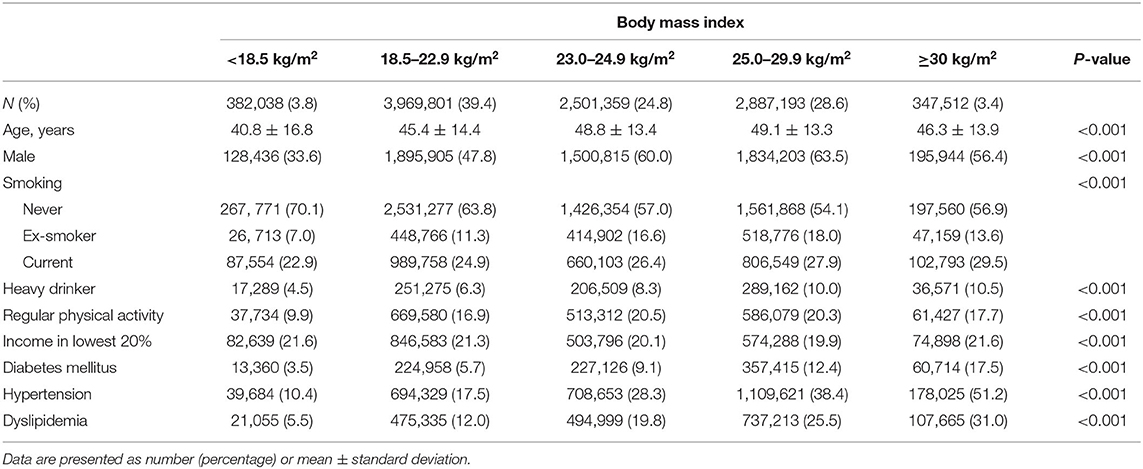
Table 1 depicts the baseline characteristics of the study population according to BMI category. The distribution of the total study cohort was as follows: 3.8% were underweight (<18.5 kg/m2), 39.4% were normal weight (18.5–22.9 kg/m2), 24.8% were overweight (23.0–24.9 kg/m2), 28.6% were obese (25.0–29.9 kg/m2), and 3.4% were severely obese (≥30 kg/m2). Underweight participants were more likely to be younger (mean ± standard deviation, 40.8 ± 16.8 years) and never smokers (70.1%) compared with those in other BMI categories. Additionally, underweight participants were less likely to be males (33.6%), heavy drinkers (4.5%), perform regular physical activity (9.9%), and have comorbidities including DM (3.5%), hypertension (10.4%), and dyslipidemia (5.5%) compared with those in other BMI categories.
BMI Alters the Risk of Incident TB
During the 7.3-year follow-up duration, the incidence of TB was 0.92 per 1,000 person-years in the normal weight without DM, 2.26 in the normal weight with DM, 1.80 in the underweight without DM, and 5.35 in the underweight with DM.
In participants with DM, compared with the normal weight, the underweight showed an increased risk of incident TB [adjusted hazard ratio (aHR), 2.13; 95% confidence interval (CI), 1.93–2.35]; however, overweight (aHR, 0.58; 95% CI, 0.55–0.61), obese (aHR, 0.44; 95% CI, 0.41–0.46), and severely obese (aHR, 0.29; 95% CI, 0.25–0.33) participants showed decreased risks of incident TB.
A similar trend was also observed in participants without DM. Compared with the normal weight, the underweight showed an increased risk of incident TB (aHR, 2.21; 95% CI, 2.14–2.28); however, overweight (aHR, 0.54; 95% CI, 0.53–0.55), obese (aHR, 0.36; 95% CI, 0.35–0.37), and severely obese (aHR, 0.28; 95% CI, 0.26–0.31) participants showed decreased risks of incident TB (within group HR column in Table 2).
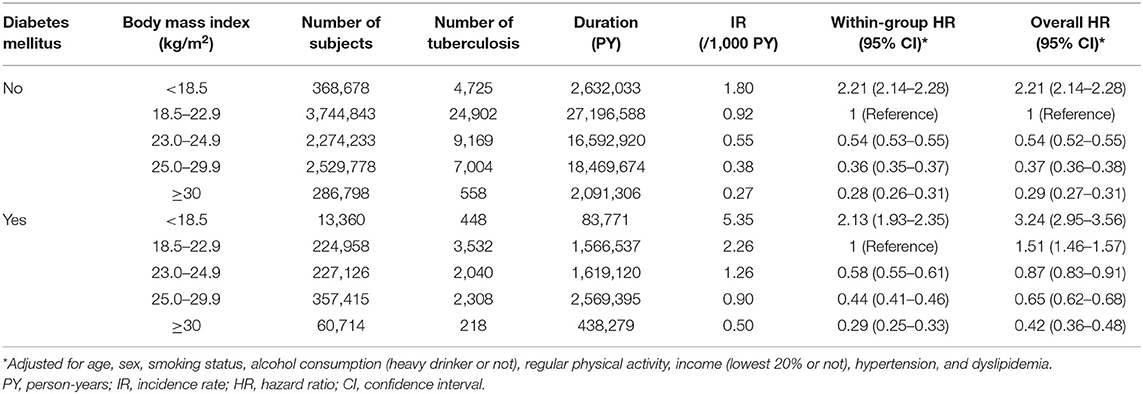
Table 2. Hazard ratios and 95% confidence intervals for incident tuberculosis by diabetes mellitus and body mass index.
Joint Effects of Underweight and DM on the Risk of Incident TB
Table 2 (overall HR column) and Figure 2A reveal the joint effects of underweight and DM on the risk of incident TB. In the entire study population, compared with the normal weight without DM, gradually increased risks of incident TB were observed in the normal weight with DM (aHR, 1.51; 95% CI, 1.46–1.57), the underweight without DM (aHR, 2.21; 95% CI, 2.14–2.28), and the underweight with DM (aHR, 3.24; 95% CI, 2.95–3.56). The aHR for the joint effect of double exposure (underweight plus DM) was 3.24, similar to the expected risk assuming the independent effects of underweight and DM (1.51 * 2.21 = 3.34), which denoted no significant interaction between underweight and DM. When examined within each group by DM (DM and non-DM groups), the risk estimates by BMI status were similar in both groups (Figure 2B). Among participants with DM, DM control status (fasting blood glucose of <130 vs. ≥130 mg/dl) did not have a significant impact on the association between the risk of TB and BMI (Supplementary Table 1).
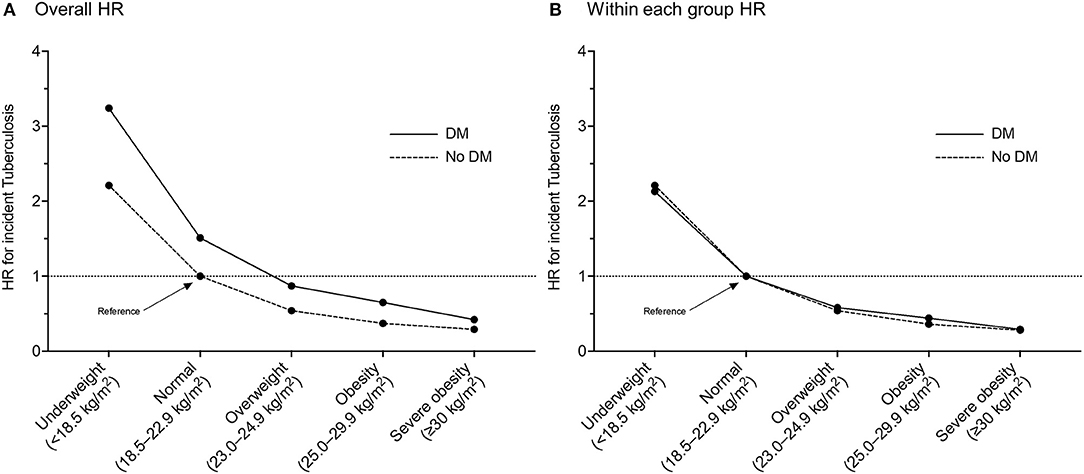
Figure 2. Multivariable hazard ratios of incident tuberculosis in participants with or without diabetes mellitus according to body mass index category: (A) with a reference of normal weight participants without diabetes mellitus; (B) with a reference of normal weight participants within each group by diabetes mellitus. HR, hazard ratio; DM, diabetes mellitus.
Stratified Analyses
Table 3 shows the joint effects of BMI and DM on the risk of incident TB, which is further stratified with sex and age groups. Compared with the normal weight without DM, the male and female underweight with DM showed 3.69-fold (95% CI, 3.31–4.11) and 2.39-fold (95% CI, 1.98–2.89) increased risks of TB, respectively. Likewise, the underweight with DM aged <65 years and those aged ≥ 65 years showed 4.99-fold (95% CI, 4.40–5.67) and 2.17-fold (95% CI, 1.89–2.49) increased risks of TB compared with the normal weight without DM aged <65 and ≥ 65 years, respectively.
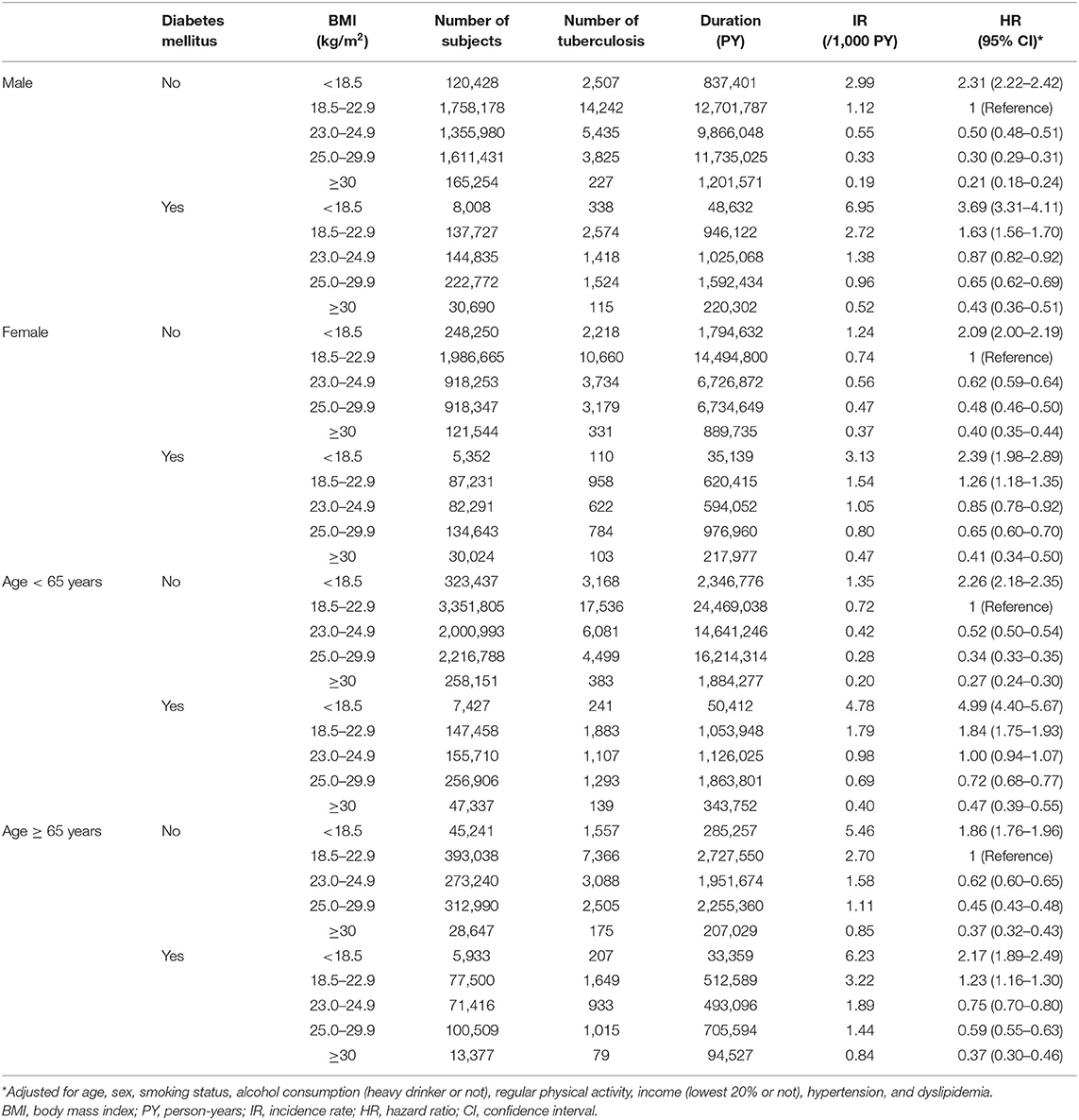
Table 3. Hazard ratios and 95% confidence intervals for incident tuberculosis according to diabetes mellitus state and body mass index plus stratified by sex and age group.
Table 4 shows the joint effects of BMI and DM on the risk of incident TB, which was further stratified by smoking status and alcohol consumption. Compared with the normal weight without DM, the non-current (never and ex-) and current smoker underweight with DM showed 2.92-fold (95% CI, 2.58–3.31) and 3.83-fold (95% CI, 3.31–4.43) increased risks of TB, respectively. Additionally, the underweight with DM who were non-heavy drinkers and those who were heavy drinkers showed 3.08-fold (95% CI, 2.78–3.41) and 4.38-fold (95% CI, 3.46–5.54) increased risks of TB compared with the normal weight without DM who were non-heavy drinkers and heavy drinkers, respectively.
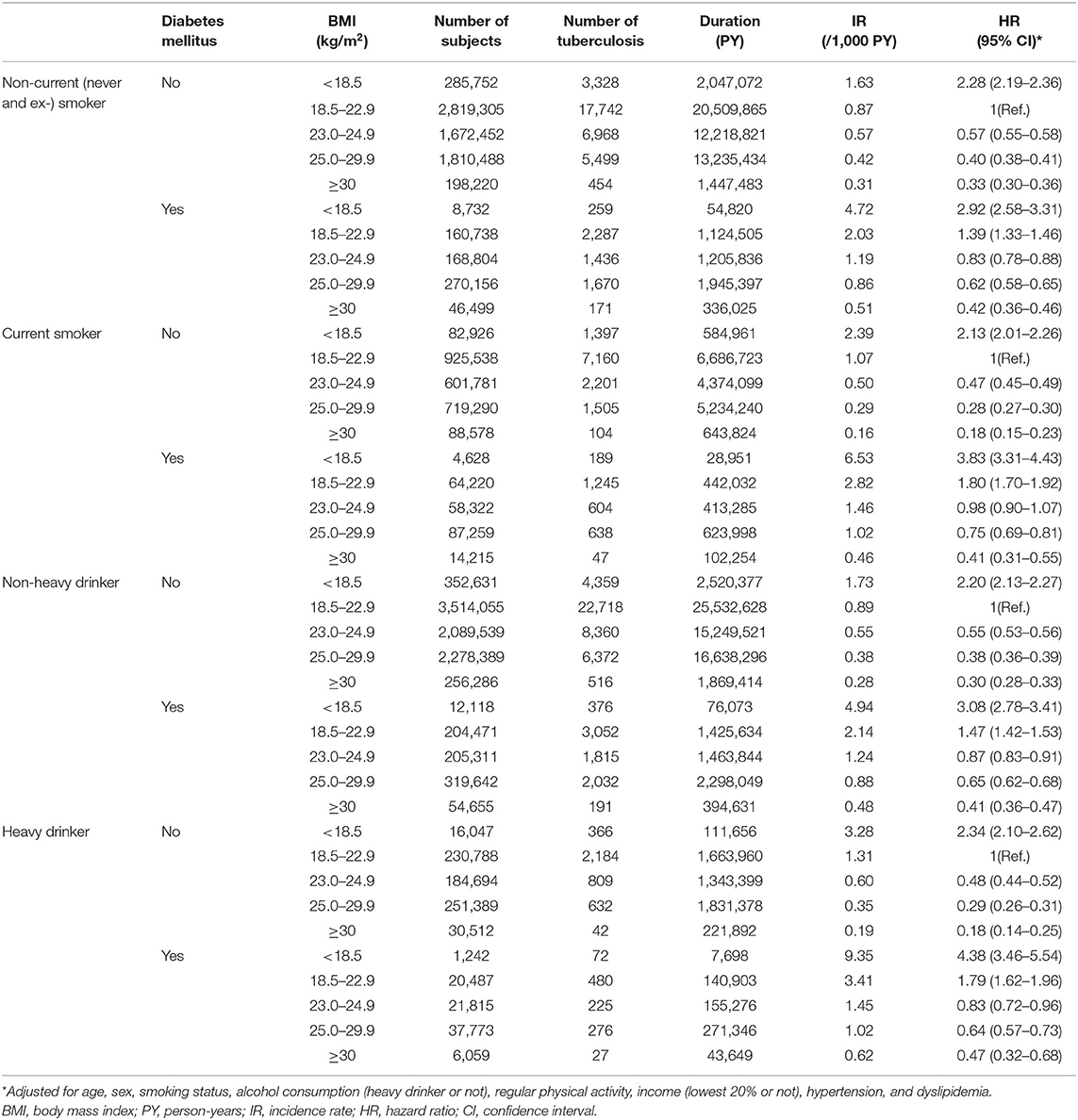
Table 4. Hazard ratios and 95% confidence intervals for incident tuberculosis according to diabetes mellitus state and body mass index plus stratified by smoking status and alcohol consumption.
Regarding the joint effects of underweight and DM on the risk of incident TB, a multiplicative effect was observed in participants aged < 65 years, current smokers, and heavy drinkers; and there were no significant interactions between BMI and DM in males, females, those aged ≥ 65 years, non-current (never and ex-) smokers, and non-heavy drinkers (Figure 3).
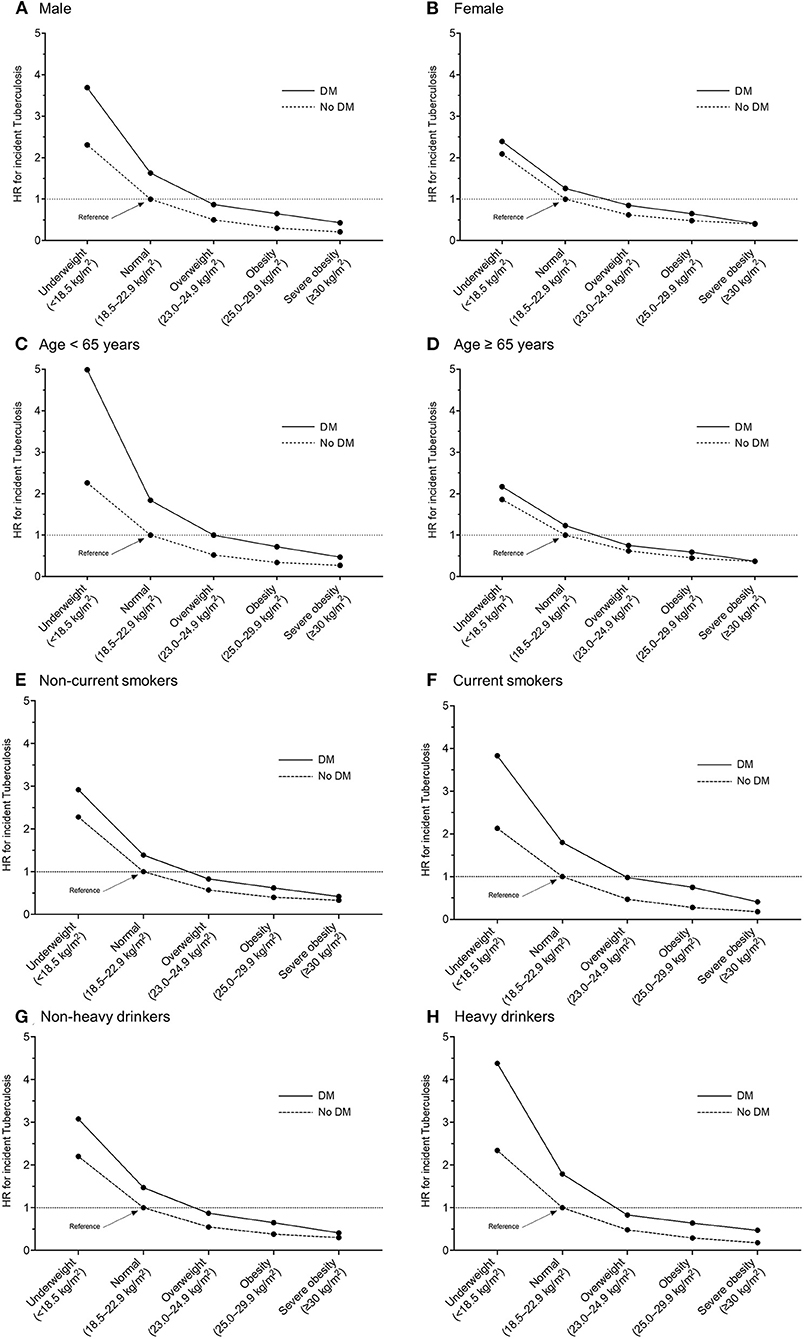
Figure 3. Multivariable hazard ratios of incident tuberculosis in participants with or without diabetes mellitus according to body mass index category, with a reference of normal weight participants without diabetes mellitus. Stratified by sex and age: (A) male, (B) female, (C) age < 65 years, (D) age ≥ 65 years, (E) non-current smokers, (F) current smokers, (G) non-heavy drinkers, and (H) heavy drinkers. HR, hazard ratio; DM, diabetes mellitus.
Discussion
This population-based longitudinal study evaluated the effects of DM and BMI on the risk of incident TB. Compared to the normal weight without DM, the normal weight with DM, the underweight without DM, and the underweight with DM showed a 1.5-fold, a 2.2-fold, and a 3.2-fold increased risk of TB, respectively. Although there was no significant interaction between BMI and DM on the risk of TB in the overall population, the synergistic effect of underweight and DM was evident in subgroups of aged <65 years, current smokers, and heavy drinkers.
DM is a well-known risk factor of TB development; a previous meta-analysis showed a three-fold increased risk of TB incidence (8). There have been several studies suggesting biological plausibility to explain the underlying mechanism. Studies in animal models revealed that diabetic mice had higher mycobacterial loads in the early course of Mycobacterium tuberculosis infection than euglycemic mice did (19, 20). Additionally, diabetic mice revealed decreased levels of interferon-γ and interleukin-12 during M. tuberculosis infection than euglycemic mice, which denotes diminished T helper 1 adaptive immunity (19). Furthermore, DM patients' neutrophils showed reduced chemotaxis and oxidative killing potential, indicating reduced innate immunity (21). In agreement with previous reports, our study confirms the increased risk of TB incidence in patients with DM. Additionally, DM control status did not have a significant impact on the association between TB risk in participants with DM in this study; therefore, DM itself is more important for the risk of TB compared with DM control status.
In addition to DM, underweight has also been known to increase TB incidence. A previous meta-analysis demonstrated a log-linear inverse relationship between TB incidence and BMI (4); our study also showed that underweight subjects had an increased risk of TB incidence and that the chance was inversely correlated with BMI. The possible mechanistic link between underweight and TB incidence can be explained by the fact that low nutritional status has harmful effects on a cell-mediated immune system. Experimental animal studies revealed that protein deficiency, in particular, could have devastating consequences regarding T-cell trafficking and antigen-induced proliferation, inability to form mature granulomas, and diminished production of protective cytokines (4, 22). In contrast, in line with previous reports (4, 23, 24), this study also showed that overweight and obese individuals had a significantly lower risk of TB than normal-weight individuals. Although the biological mechanism of the phenomenon is not established, leptin, an adipose tissue-driven energy-regulating hormone, might affect the T-helper 1/T-helper 2 balance of the host immune system, subsequently altering the risk of TB infection (5, 25, 26). Specifically, a considerable proportion of Asian patients with DM was underweight or normal weight, unlike Western populations, where most DM cases are attributable to obesity or overweight (10, 27). Hence, considering BMI is essential when assessing TB risk in Asian patients with DM; however, as a previous study suggested, there is a complex interplay between body weight and DM on the risk of TB (5).
In this context, we should assess the joint effects of DM and BMI on the TB risk. A recent study from Singapore also investigated a joint effect of DM and underweight on TB development compared to obesity. However, the study had two major limitations: (1) subjects with obesity, not those with normal weight, were the reference group for the analysis, and (2) the overall study population was small, which limited statistical power and further stratified analyses (10). While overcoming the limitations of the previous study, we reassessed the joint effect in a nationwide, population-based cohort. This study showed that there was no significant interaction between BMI and DM on the risk of incident TB in the overall population.
Although a stratified analysis revealed no significant joint effects of underweight and DM on TB risk in either sex group, underweight male participants showed a higher TB risk than underweight females when compared with normal weight participants in each group. In line with our findings, previous research suggested that the male predominance of TB infections was mostly attributed to socio-cultural factors leading to a higher risk of exposure to M. tuberculosis in males (28–30). Furthermore, this study may reinforce the support for the male predominance of TB, as a multivariable analysis was adjusted for well-known risk factors for the male predominance of TB, including alcohol abuse, smoking, and socioeconomic deprivation (31).
Interestingly, in the stratified analysis, the joint effects of underweight and DM on TB risk were synergistic only in participants younger than 65 years old. This finding may be explained by the heterogeneity of the participants without DM between the age groups. Because baseline glucose tolerance is lower in elderly participants without DM, elderly controls might have an elevated risk of incident TB compared to younger controls (32), thus reducing the evident effect of DM (8). Additionally, types of DM may affect the results. Because this study did not distinguish between type 1 and type 2 DM, the younger age group might include a higher proportion of subjects with type 1 DM, a more severe form of DM with a juvenile onset, compared to the older age group (33). Although there are some caveats when interpreting the synergistic effects of underweight and DM on TB risk, this study is meaningful in terms of providing information on whom to screen for TB. Taken together, clinicians should assess TB risk more carefully in young and thin patients with DM.
Smoking and alcohol consumption are well-known risk factors for TB (34, 35). Not surprisingly, there was also a synergistic effect between DM and BMI on the development of TB among current smokers and heavy drinkers in this study. The underweight with DM who currently smoke and drink heavily had a higher risk of TB than non-current smokers and non-heavy drinkers, respectively. Potential underlying mechanisms of high TB risk among current smokers may include decreased activity of alveolar macrophages, impaired mucociliary clearance, decreased immune response of pulmonary lymphocytes, modified pulmonary dendritic cell activity, and reduction in the cytotoxic activity of natural killer cells (36). Similarly, there are two possible pathways to explain the association between alcohol consumption and the risk of TB: impaired immune system (compromised response to newly introduced pathogens in alveolar macrophages) and aggravated malnutrition (37). Accordingly, it seems logical that smoking status and alcohol consumptions potentiate the effect of underweight on TB development in patients with DM. From a public health perspective, these findings imply that smoking cessation and avoiding heavy alcohol consumption would help improve general health and reduce the incidence of TB among underweight patients with DM.
The major strength of this study was to assess the joint effects of BMI and DM on the risk of incident TB, using a nationwide population-based longitudinal cohort with exact measurements of exposures (DM and BMI). However, there were also limitations to this study. First, the diagnoses of DM and comorbidities were based on ICD-10 codes and medications. Thus, there might be potential errors in diagnosing diseases. Second, this study was performed in Korea, an intermediate-TB-burden country. Our findings might not be generalizable to patients in other countries. Third, since we assessed the BMI of each participant upon enrollment in this study, the impact of temporal changes in BMI during the follow-up period on TB risk could not be assessed in this study. Fourth, this study could not analyze the detailed clinical courses of subjects with DM potentially affecting BMI change and TB risk, which include glucose control trajectories, types of DM, and classifications of DM medications.
In conclusion, compared to the normal weight individuals without DM, the normal weight with DM, the underweight without DM, and the underweight with DM showed a 1.5-fold, a 2.2-fold, and a 3.2-fold increased risk of TB, respectively. Although no significant interaction was observed between BMI and DM on the risk of TB among the overall population, the synergistic effect of underweight and DM was evident in participants younger than 65 years, current smokers, and heavy drinkers, suggesting that a focused screening of incident TB in patients with DM may be beneficial according to this study's results.
Data Availability Statement
The datasets presented in this article are not readily available because restrictions apply to the availability of these data, which were used under license for this study. Requests to access the datasets should be directed to Dong Wook Shin, dwshin.md@gmail.com.
Ethics Statement
The studies involving human participants were reviewed and approved by Institutional Review Board of Hallym University Kangnam Sacred Heart Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
DS is the guarantor of this study. HC, JY, HL, and DS drafted the manuscript. All authors listed have provided substantial contributions to the conception, design, data acquisition, analysis, or interpretation of this work, and participated in revising the manuscript after a critical review.
Funding
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, Information, and Communications Technologies (Grant Nos. 2020R1F1A1070468 and 2021M3E5D1A01015176 to HL and 2019R1G1A1008692 to HC) and the Korean Ministry of Education (Grant No. 2021R1I1A3052416 to HC). The funders had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
This study was performed using the National Health Insurance System database, and the results do not necessarily represent the opinion of the National Health Insurance Corporation.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2021.739766/full#supplementary-material
References
2. Choi H. Nosocomial exposure to tuberculosis: a snapshot of South Korea. Korean J Intern Med. (2021) 36:1061–2. doi: 10.3904/kjim.2021.367
3. Falagas ME, Kompoti M. Obesity and infection. Lancet Infect Dis. (2006) 6:438–46. doi: 10.1016/S1473-3099(06)70523-0
4. Lönnroth K, Williams BG, Cegielski P, Dye C. A consistent log-linear relationship between tuberculosis incidence and body mass index. Int J Epidemiol. (2010) 39:149–55. doi: 10.1093/ije/dyp308
5. Lin HH, Wu CY, Wang CH, Fu H, Lönnroth K, Chang YC, et al. Association of obesity, diabetes, and risk of tuberculosis: two population-based cohorts. Clin Infect Dis. (2018) 66:699–705. doi: 10.1093/cid/cix852
6. Maggio CA, Pi-Sunyer FX. Obesity and type 2 diabetes. Endocrinol Metab Clin North Am. (2003) 32:805–22, viii. doi: 10.1016/S0889-8529(03)00071-9
7. Chan JM, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. Obesity, fat distribution, and weight gain as risk factors for clinical diabetes in men. Diabetes Care. (1994) 17:961–9. doi: 10.2337/diacare.17.9.961
8. Jeon CY, Murray MB. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS Med. (2008) 5:e152. doi: 10.1371/journal.pmed.0050152
9. Ronacher K, van Crevel R, Critchley JA, Bremer AA, Schlesinger LS, Kapur A, et al. Defining a research agenda to address the converging epidemics of tuberculosis and diabetes: part 2: underlying biologic mechanisms. Chest. (2017) 152:174–80. doi: 10.1016/j.chest.2017.02.032
10. Soh AZ, Chee CBE, Wang YT, Yuan JM, Koh WP. Diabetes and body mass index in relation to risk of active tuberculosis: a prospective population-based cohort. Int J Tuberc Lung Dis. (2019) 23:1277–82. doi: 10.5588/ijtld.19.0094
11. Pan WH, Yeh WT. How to define obesity? Evidence-based multiple action points for public awareness, screening, and treatment: an extension of Asian-Pacific recommendations. Asia Pac J Clin Nutr. (2008) 17:370–4.
12. Shin DW, Cho B, Guallar E. Korean national health insurance database. JAMA Intern Med. (2016) 176:138. doi: 10.1001/jamainternmed.2015.7110
13. NHIS. National Health Examination Statistical Yearbook 2014. Seoul: National Health Insurence Service, 2014 (2014).
14. Jeong SM, Han K, Kim D, Rhee SY, Jang W, Shin DW. Body mass index, diabetes, and the risk of Parkinson's disease. Mov Disord. (2020) 35:236–44. doi: 10.1002/mds.27922
15. American Diabetes A. Standards of medical care in diabetes–2009. Diabetes Care. (2009) 32 (Suppl. 1):S13–61. doi: 10.2337/dc09-S013
16. Go U, Park M, Kim UN, Lee S, Han S, Lee J, et al. Tuberculosis prevention and care in Korea: evolution of policy and practice. J Clin Tuberc Other Mycobact Dis. (2018) 11:28–36. doi: 10.1016/j.jctube.2018.04.006
17. Choi H, Yang B, Nam H, Kyoung DS, Sim YS, Park HY, et al. Population-based prevalence of bronchiectasis and associated comorbidities in South Korea. Eur Respir J. (2019) 54:1900194. doi: 10.1183/13993003.00194-2019
18. Marshall SW. Power for tests of interaction: effect of raising the type I error rate. Epidemiol Perspect Innov. (2007) 4:4. doi: 10.1186/1742-5573-4-4
19. Martens GW, Arikan MC, Lee J, Ren F, Greiner D, Kornfeld H. Tuberculosis susceptibility of diabetic mice. Am J Respir Cell Mol Biol. (2007) 37:518–24. doi: 10.1165/rcmb.2006-0478OC
20. Yamashiro S, Kawakami K, Uezu K, Kinjo T, Miyagi K, Nakamura K, et al. Lower expression of Th1-related cytokines and inducible nitric oxide synthase in mice with streptozotocin-induced diabetes mellitus infected with Mycobacterium tuberculosis. Clin Exp Immunol. (2005) 139:57–64. doi: 10.1111/j.1365-2249.2005.02677.x
21. Delamaire M, Maugendre D, Moreno M, Le Goff MC, Allannic H, Genetet B. Impaired leucocyte functions in diabetic patients. Diabet Med. (1997) 14:29–34. doi: 10.1002/(SICI)1096-9136(199701)14:1<29::AID-DIA300>3.0.CO;2-V
22. Cegielski JP, McMurray DN. The relationship between malnutrition and tuberculosis: evidence from studies in humans and experimental animals. Int J Tuberc Lung Dis. (2004) 8:286–98.
23. Leung CC, Lam TH, Chan WM, Yew WW, Ho KS, Leung G, et al. Lower risk of tuberculosis in obesity. Arch Intern Med. (2007) 167:1297–304. doi: 10.1001/archinte.167.12.1297
24. Pealing L, Wing K, Mathur R, Prieto-Merino D, Smeeth L, Moore DA. Risk of tuberculosis in patients with diabetes: population based cohort study using the UK clinical practice research datalink. BMC Med. (2015) 13:135. doi: 10.1186/s12916-015-0381-9
25. Díaz BB, Rodríguez IM, González DA, Pérez MCR, de León AC. An overview of leptin and the Th1/Th2 balance. Open J Immunol. (2014) 2014. doi: 10.4236/oji.2014.42006
26. Matarese G, Moschos S, Mantzoros CS. Leptin in immunology. J Immunol. (2005) 174:3137–42. doi: 10.4049/jimmunol.174.6.3137
27. Chan JC, Malik V, Jia W, Kadowaki T, Yajnik CS, Yoon KH, et al. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA. (2009) 301:2129–40. doi: 10.1001/jama.2009.726
28. Diwan VK, Thorson A. Sex, gender, and tuberculosis. Lancet. (1999) 353:1000–1. doi: 10.1016/S0140-6736(99)01318-5
29. Holmes CB, Hausler H, Nunn P. A review of sex differences in the epidemiology of tuberculosis. Int J Tuberc Lung Dis. (1998) 2:96–104.
30. Hudelson P. Gender differentials in tuberculosis: the role of socio-economic and cultural factors. Tuber Lung Dis. (1996) 77:391–400. doi: 10.1016/S0962-8479(96)90110-0
31. Marçôa R, Ribeiro AI, Zão I, Duarte R. Tuberculosis and gender - factors influencing the risk of tuberculosis among men and women by age group. Pulmonology. (2018) 24:199–202. doi: 10.1016/j.pulmoe.2018.03.004
32. Harris MI, Flegal KM, Cowie CC, Eberhardt MS, Goldstein DE, Little RR, et al. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults. The third national health and nutrition examination survey, 1988–1994. Diabetes Care. (1998) 21:518–24. doi: 10.2337/diacare.21.4.518
33. Zaccardi F, Webb DR, Yates T, Davies MJ. Pathophysiology of type 1 and type 2 diabetes mellitus: a 90-year perspective. Postgrad Med J. (2016) 92:63–9. doi: 10.1136/postgradmedj-2015-133281
34. Kolappan C, Gopi PG. Tobacco smoking and pulmonary tuberculosis. Thorax. (2002) 57:964–6. doi: 10.1136/thorax.57.11.964
35. Rehm J, Samokhvalov AV, Neuman MG, Room R, Parry C, Lonnroth K, et al. The association between alcohol use, alcohol use disorders and tuberculosis (TB). A systematic review. BMC Public Health. (2009) 9:450. doi: 10.1186/1471-2458-9-450
36. Khan AH, Sulaiman SAS, Hassali MA, Khan KU, Ming LC, Mateen O, et al. Effect of smoking on treatment outcome among tuberculosis patients in Malaysia; a multicenter study. BMC Public Health. (2020) 20:854. doi: 10.1186/s12889-020-08856-6
Keywords: diabetes mellitus, tuberculosis, body mass index, epidemiology, nutritional status
Citation: Choi H, Yoo JE, Han K, Choi W, Rhee SY, Lee H and Shin DW (2021) Body Mass Index, Diabetes, and Risk of Tuberculosis: A Retrospective Cohort Study. Front. Nutr. 8:739766. doi: 10.3389/fnut.2021.739766
Received: 11 July 2021; Accepted: 29 October 2021;
Published: 01 December 2021.
Edited by:
Weimin Ye, Karolinska Institutet (KI), SwedenReviewed by:
Alex Kojo Anderson, University of Georgia, United StatesDae Jung Kim, Ajou University, South Korea
Copyright © 2021 Choi, Yoo, Han, Choi, Rhee, Lee and Shin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hyun Lee, namuhanayeyo@hanynang.ac.kr; Dong Wook Shin, dwshin.md@gmail.com
†These authors have contributed equally to this work
‡These authors have contributed equally to this work and share senior authorship
 Hayoung Choi
Hayoung Choi Jung Eun Yoo2†
Jung Eun Yoo2† Sang Youl Rhee
Sang Youl Rhee Hyun Lee
Hyun Lee Dong Wook Shin
Dong Wook Shin