Abstract
Recently, squamous cell carcinoma of the head and neck (SCCHN) chemoprevention research has made major advances with novel clinical trial designs suited for the purpose, use of biomarkers to identify high-risk patients, and the emergence of numerous molecularly targeted agents and natural dietary compounds. Among many natural compounds, green tea polyphenols (GTPs), particularly (−)-epigallocatechin-3-gallate (EGCG), possess remarkable potential as chemopreventive agents. EGCG modulates several key molecular signaling pathways at multiple levels and has synergistic or additive effects when combined with many other natural or synthetic compounds. This review will provide an update of the potential of GTPs, particularly EGCG, for chemoprevention of SCCHN.
Keywords: Squamous cell carcinoma of the head and neck, Chemoprevention, EGCG, Natural compounds, Molecular targets
Introduction
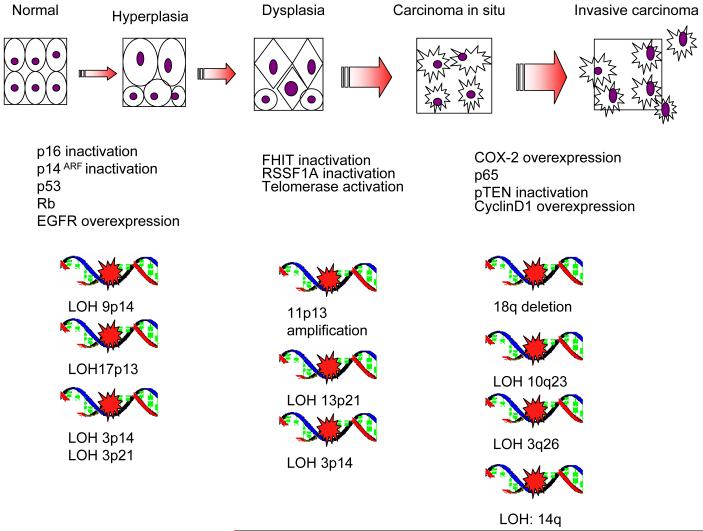
Cancer is the leading cause of death for people under the age of 85 (1). About 47,000 cases of squamous cell carcinoma of the head and neck (SCCHN) were estimated to occur in the year 2009, with an expected 11,000 deaths from the disease (1, 2). SCCHN causes significant morbidity and mortality with a five-year survival rate of less than 50% (2-5). A common cause of mortality in SCCHN survivors is second primary tumor, which occurs at an annual rate of 3-5% (6-8). SCCHN occurs as a consequence of accumulating genetic instabilities from exposure to various carcinogens, such as cigarettes, alcohol, marijuana and betel chewing (3, 9-11). Figure 1 illustrates the molecular progression of SCCHN.
Figure 1. Model of genetic instability and progression in head and neck cancer.
Genetic instability increases and accumulates as a lesion acquires its malignant potential. With increasing heterogeneity of genetic instability, inactivation of tumor suppressor genes and activation of oncogenes account for the phenotypic changes of SCCHN carcinogenesis through normal histology, hyperplasia, dysplasia, carcinoma in situ and invasive carcinoma.
Recently, SCCHN chemoprevention research has made major advances, including novel clinical trial designs suited for chemoprevention (12), use of biomarkers to identify high-risk patients (12), use of molecularly targeted agents and natural compounds in chemoprevention trials, and the development of an oral-specific carcinogenesis animal model (13). An ideal chemopreventive agent should be nontoxic, potent, inexpensive and easily available. Research over the last several decades has identified numerous natural compounds, many of which are present in the diet and have the potential to suppress the development of multiple cancers (14). Among many natural compounds, green tea polyphenols (GTPs) including (−)-epigallocatechin-3-gallate (EGCG) exhibit high promise for chemoprevention in epidemiological, preclinical and early clinical studies. EGCG also shows strong synergistic or additive antitumor activities with many natural or synthetic compounds. This review will provide an update of the potential of GTPs, particularly EGCG for chemoprevention of SCCHN.
Introduction to Green Tea Extract (GTE) and EGCG
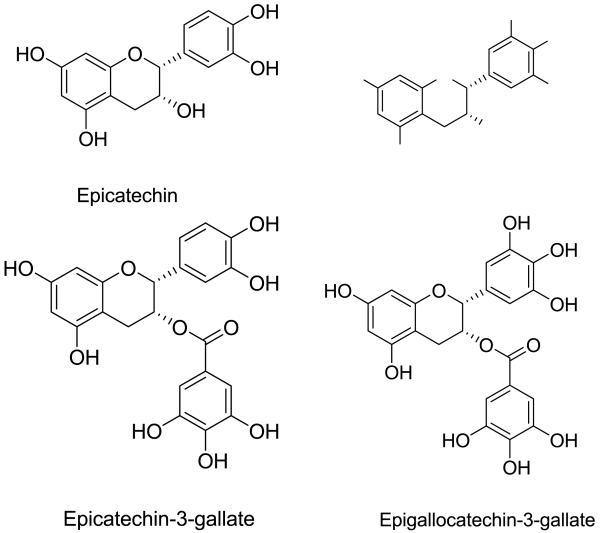
Green tea is produced from the non-fermented leaves of the plant Camellia sinensis. Figure 2 showed the four major polyphenols present in green tea extract (GTE). EGCG is the most potent and abundant antioxidant present in green tea and has been extensively investigated for its chemopreventive and therapeutic potential (15). Several formulations of GTE have been used in clinical trials, in which the EGCG content varies from 13 to 70% (Table 1).
Figure 2. Chemical structures of green tea polyphenols.
Table 1. Clinical Trials with Green Tea Extracts.
| Organ Site | Type of Clinical Trial |
Subjects | GTE Formulation | Treatments | Results | Comments |
|---|---|---|---|---|---|---|
| Oral Leukoplakia Li 1999 (80) |
Randomized, double-blind, placebo- controlled. |
59 patients with oral mucosa leukoplakia |
Mixed tea capsule prepared by the Institute of Tea Science and Research, Chinese Academy of Agricultural Science. Each capsule contained 40% green tea polyphenols EGCG content not reported. |
3 grams of mixed tea a day (760 mg mixed tea capsule, QD) plus topical treatment with mixed tea in glycerin at 10%, TID. |
37.9 % response rate in treatment arm vs 10% in control arm |
n/a |
| High Risk OPLs Tsao 2009 (35) |
Phase II trial | 41 patients with high risk OPLs |
THEA-FLAN 30 ARG supplied by Ito En Ltd. Each capsule contains 350mg of GTE, 13.2% EGCG |
Placebo TID, vs GTE capsule 500mg/m2 TID vs 750mg/m2 TID vs 1000mg/m2 TID (132 mg/m2 TID, EGCG) |
50% response rate in treatment arm vs 18.2% in placebo arm |
Higher 12- week histologic response rate in never drinkers (p=0.01) Neither clinical or histologic response to GTE intervention was associated with oral cancer development |
| Refractory Solid Tumors Pisters 2001 (81) |
Phase I trial | 49 patients with solid tumors refractory to standard treatment |
GTE capsule Supplied by Ito En Ltd. Each capsule contained 13.2% EGCG |
GTE capsule, 0.5 to 5.05g/m2 a day |
No major clinical response. Maximally tolerated dose was 4.2 g/ m2 of GTE a day for up to 6 months. Dose limiting toxicities were caffeine- related. |
Optimal dose suggested was 1 g/m2 TID. |
| Advanced Lung Cancer Laurie 2005 (92) |
Phase I trial | 17 patients with advanced lung cancer |
GTE capsule Supplied by Ito En Ltd. Each capsule contained 13.2% EGCG |
0.5 g/m2 GTE with dose escalation- scheme upto 8 g/m2 GTE. |
No major clinical response MTD was 3 g/m2 of oral GTE, once daily. |
n/a |
| High risk Cervical Lesions Ahn 2003 (85) |
Pilot study | 51 patients with cervical lesions |
Content of Poly E ointment or capsule was not reported. |
Poly E ointment twice a week vs. Poly E 200mg oral daily + Poly E ointment twice a week vs., Poly E 200mg orally daily, vs., EGCG 200mg orally daily vs Untreated |
Overall 69% (35/51) in treatment arms vs 10% (4/39) patients in untreated control (p<0.05) |
n/a |
| Colorectal Adenomas Shimizu 2008 (84) |
Randomized trial |
136 patients status 1 year post polypectomy |
GTE tablet provided by the Green Tea Union of Saitama. One 500mg tablet contains 52.5mg EGCG. |
GTE tablet 1500mg (157.5 mg EGCG) a day for 12 months |
Incidence of metachronous adenoma was 31% in the control group and 15% in the GTE group. (p<0.05) |
n/a |
| Prostate Cancer McLarty 2009 (36) |
Single arm phase II trial |
26 men with positive prostate biopsy awaiting prostectomy |
Polyphenon E provided by Mitsui- Norin Co Ltd / Polyphenon E International,Inc. Each Polyphenon E 325mg capsule contains 200mg EGCG |
Polyphenon E 1300mg (800mg EGCG) a day during the interval between the prostate biopsy and radical prostectomy. Median period was 34.5 days |
Serum levels of HGF, VEGF, IGF-BP3, IGF- I and PSA were decreased No liver toxicities were observed. |
n/a |
| High-Grade Prostate Intraepitheli al Neoplasia Bettuzzi 2006 (82) |
Double-blind placebo- controlled trial |
60 patients with HG-PIN |
Green tea catechin capsules. Each capsule contains 51.8% EGCG. |
Green tea catechins capsule 600mgs a day (311 mg, EGCG) vs. placebo for 1 year |
Incidence of prostate cancer was 30.0% in the control group vs 3.3% in the GTE group (p<0.01) |
GTE treatment did not significantly affect PSA values |
One of the first pieces of evidence of EGCG’s chemopreventive effect was reported in 1987, when the inhibitory effects of EGCG on teleocidin-induced tumor promotion in mouse skin were demonstrated (16). Its antitumor effects were also demonstrated by regression of experimentally-induced skin papilloma in mice by orally administered green tea, intraperitoneally (i.p.)-administered GTP fraction or i.p. EGCG (17). Topical application of EGCG was shown to induce apoptosis in UVB-induced skin tumors in mice (18). Numerous preclinical studies have followed since showing the mechanisms of antitumor effects of GTP and EGCG in various cancer models.
Mechanisms of Action / Preclinical Studies
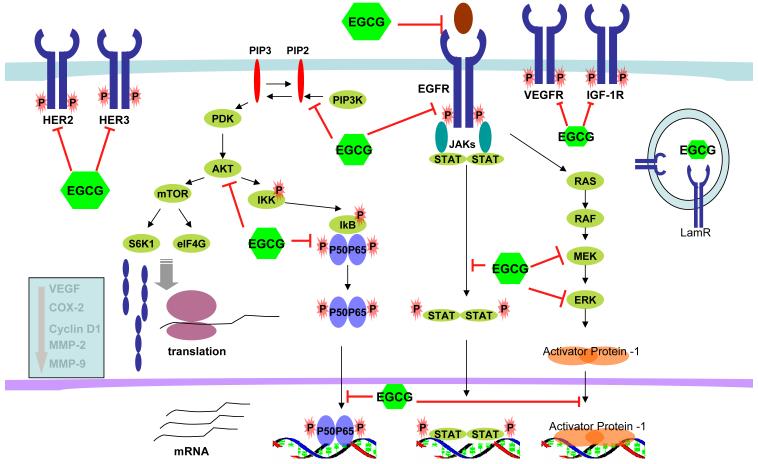
EGCG exerts its chemopreventive actions by modulating multiple signaling pathways at various cellular levels, the ultimate outcomes of which are apoptosis, cell cycle arrest, growth inhibition, anti-angiogenesis and inhibition of metastasis. Figure 3 illustrates the molecular targets modulated by EGCG. At the cell membrane, EGCG inhibits activation of receptor tyrosine kinases, such as epidermal growth factor receptor (EGFR), human epidermal growth factor receptor (HER)-2 and HER-3, insulin-like growth factor-1 receptor (IGF-1R) and vascular endothelial growth factor receptor (VEGFR), and their downstream effectors such as pAkt and pERK (19-29). Of these, EGFR appears to be the most active target of EGCG in both in vitro and in vivo SCCHN models (19, 25, 28). A correlation between pEGFR inhibition, Akt phosphorylation and tumor growth inhibition was observed in SCCHN xenografted tumor tissues (19). EGCG in combination with curcumin inhibited VEGFR-1 activation in a breast cancer xenograft model (30). EGCG also inhibited VEGFR-2 activation in a hepatocellular carcinoma xenograft model (31). Inhibition of VEGF by green tea preparations was also observed in animal models of breast (32, 33) and prostate cancers (34). Downregulation of angiogenic stromal VEGF was observed in a clinical trial with GTE (35). GTP treatment also decreased serum VEGF levels in prostate cancer patients (36). Laminin receptor was identified as a potential receptor for EGCG to modulate several important intracellular signaling pathways (37-39).
Figure 3. Molecular targets of EGCG.
Several signaling pathways are affected by EGCG at multiple levels. EGCG inhibits the ligand binding of EGFR and inhibits phosphorylation/activation of the receptor tyrosine kinases. It also inhibits several intracellular signaling pathways, including PI3K/Akt/mTOR, JAK/STAT, RAF/MEK/ERK/AP-1, and Akt/NF-κB. At the nuclear level, EGCG also inhibits the DNA binding of effector transcription factors, such as NF-κB, AP-1 and STAT. As a consequence, expression of molecules that are involved in cell proliferation, angiogenesis, invasion and inflammation are reduced.
The effects of EGCG on cytoplasmic signaling molecules in cell culture and animal models include inhibition of Akt, extracellular signal-related kinase (ERK)1/2 and MAP kinase or ERK kinase (MEK) phosphorylation (40-42), in addition to modulating phosphoinositide 3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) (41, 43), and signal transducer and activator of transcription (STAT)-3 (25).
EGCG also modulates the function of certain transcription factors, namely nuclear factor-κB (NF-κB) and activator protein (AP)-1 in cell culture and animal studies (44-48). NF-κB facilitates the transcription of genes involved in inflammation, immunity, and carcinogenesis. In a normal human epidermal keratinocyte model, pretreatment with EGCG caused significant inhibition of UVB-induced NF-κB/p65 activation and its nuclear translocation (47). As a consequence of AP-1 inhibition, expression of its target molecules, such as cyclin D1 and cyclooxygenase (COX)-2 are reduced, inducing apoptosis and reducing inflammatory response (21, 25, 49). Furthermore, EGCG induces apoptosis and G0/G1 arrest in several cell lines via activation of p53 and its downstream targets p21, p57 and Bax (50-53). Evidence also suggests that EGCG induces the expression of p73, which is important for apoptosis and expression of a subset of p53-target genes (54, 55).
EGCG has shown a dose-dependent inhibition of invasion and migration of human oral cancer, which is thought to be related to decreased production of matrix metalloproteinase (MMP)-2/9 and urokinase plasminogen secretion (56). Topically administered GTP in UVB-induced tumors also inhibited the expression of MMP-2 and MMP-9 (57).
It seems that EGCG modulates multiple molecular targets, which largely depend on the experimental context. Moreover, the molecular targets affected by EGCG in cell cultures, animal models, and clinical samples are sometimes different. Furthermore, the doses of EGCG and administration routes used in these three situations are different. Although it is not currently clear which of these EGCG targets are most critical for its chemopreventive effects observed in clinical trials, this is an important issue that may become better elucidated once more clinical trial results are available.
Combination of EGCG with other compounds for SCCHN chemoprevention
EGCG and curcumin
Curcumin is another popular natural compound that has been studied extensively (58-62). EGCG showed synergistic effects with curcumin in SCCHN cells (63). The median effect analysis revealed that the combination of EGCG and curcumin exhibited synergistic growth inhibition of premalignant and malignant cells (63). Combination of topical curcumin and oral green tea also resulted in superior antitumor effects in 7,12-dimethylbenz[a]anthracene-induced carcinogenesis in Syrian hamsters (64). The combination significantly decreased the number and volume of visible oral tumors, purportedly mediated through suppression of cell proliferation, induction of apoptosis and inhibition of angiogenesis (64). This combination regimen has also demonstrated enhanced or synergistic effects in other cancer models such as a hormone receptor-positive breast cancer model (30). The combination induced greater tumor volume reduction and inhibition of VEGFR protein compared to the single agents in nude mice (30).
It also appears that the sequence of administration of these agents affects the synergistic effect. In an in vitro study in chronic lymphocytic leukemia-B cells, although each agent alone was active, simultaneous administration of the agents reduced apoptosis (65). However, treatment with EGCG followed by curcumin showed synergistic apoptotic effects (65).
EGCG and EGFR tyrosine kinase inhibitors
EGFR overexpression appears to play a role in the early part of SCCHN carcinogenesis, correlates with progression of dysplasia (66) and associates with poor clinical outcome in invasive SCCHN (67, 68). Our laboratory has demonstrated synergistic growth inhibition of SCCHN by EGCG and erlotinib, mediated through greater inhibition of pEGFR and pAkt (19). The combination of erlotinib and EGCG was associated with greater tumor growth inhibition compared to single agent treatments (19). The mechanism of synergy was thought to be mediated through more sustained inhibition of Akt phosphorylation as compared with single agent treatment (19). A subsequent study suggested a critical role of activation of p53 and inhibition of NF-κB signaling pathways (69). The same combination also demonstrated enhanced anti-proliferative effects in several erlotinb-sensitive and erlotinb-resistant non-small cell lung cancer (NSCLC) cell lines and in SCID mice bearing erlotinib-resistant NSCLC tumors (70).
Combination of EGCG with other compounds in other cancer models
The apoptosis-inducing effects of EGCG were drastically enhanced by sulindac and tamoxifen in lung cancer cell lines (71). Co-treatment with EGCG plus celecoxib induced synergistic apoptosis in lung cancer cell lines (72). Although neither EGCG nor celecoxib alone induced growth arrest or the expression of DNA damage-inducible-153 (GADD153) mRNA and protein, co-treatment with both compounds strongly induced the expression of both GADD153 mRNA and protein. Moreover, inhibition of ERK1/2 activation using chemical inhibitors inhibited the expression of GADD153 and apoptosis, suggesting that combination of EGCG and celecoxib induced synergistic apoptosis by upregulating GADD153 via ERK1/2 (72). Combined treatment with GTE and sulindac resulted in a significantly greater reduction in tumor number (from 72.3 +/− 28.3 to 32.0 +/− 18.7) than that achieved with either single agent alone (to 56.7 +/− 3.5 and 49.0 +/− 12.7 with GTE or sulindac alone, respectively) in multiple intestinal neoplasia mice that harbored a mutated Apc gene (73).
EGCG combined with the selective cyclooxygenase-2 inhibitor NS-398 resulted in synergistic cell growth inhibition, apoptosis induction, and inhibition of NF-κB in prostate cancer cell lines and enhanced tumor growth inhibition with reduced serum levels of prostate specific antigen (PSA) and insulin-like growth factor-1 in nude mice bearing androgen-sensitive prostate cancer cells (74).
Several studies have also demonstrated enhanced or synergistic anti-tumor effects in both in vitro and in vivo models of breast cancers when EGCG was combined with tamoxifen or soy phytochemical concentrate (75-77). Furthermore, in prostate cancer models, dihydrotesterone increased the sensitivity of prostate cancer cells to EGCG (78). Moreover, in a carcinogen-induced lung tumorigenesis mouse model, the combination of atorvastatin and EGCG at relatively low doses was able to induce synergistic tumor growth inhibition (79)
SCCHN Chemoprevention Clinical Trials with Green Tea Extracts
Despite mounting preclinical evidence to support the efficacy of GTPs, only a few clinical trials have been conducted (Table 1). The first clinical trial using green tea for oral premalignant lesions (OPL) was a double-blind, placebo-controlled randomized trial in patients with oral leukoplakia receiving either 760mg of mixed tea (40% GTPs) capsules q.i.d. plus 10% mixed tea ointment topically, or placebo plus topical glycerin (80). After 6 months of treatment, treatment groups achieved a response rate of 37.9%, compared to 10% in the control group (80). This finding correlated with histopathologic results showing a reduced number of EGFR-positive cells (80). Pisters et al. reported a phase I trial using a GTE in an attempt to find the maximum tolerated dose (81). The percentage of total catechins, EGCG, EGC, ECG, EC and caffeine contents of the GTE preparation were 26.9, 13.2, 8.3, 3.3, 2.2 and 6.8, respectively. Patients were given GTE either once or thrice a day for 4 weeks, up to a maximum of 6 months (81). The dose limiting toxicities were tremors, cough, constipation, and headache, which were thought to be related to caffeine components of the GTE (81). Although there was no clinical response observed, oral GTE at 1.0 g/m2 thrice a day for at least 6 months was recommended (81).
Recently, Tsao et al. reported a randomized phase II trial using the same GTE preparation as Pisters et al. (81) for patients with high risk OPLs (35). Patients were randomized into one of the four arms – 500, 750 or 1,000 mg/m2 GTE, or placebo, t.i.d. for 12 weeks, and clinical response was measured by the Response Evaluation Criteria in Solid Tumors (35). At 12 weeks, there was 50% OPL clinical response rate in the treatment arms, vs. 18.2% in the control arm. A higher response rate was observed in the two higher dose GTE arms, of 58.8% vs 36.4% in the lower dose arm, suggesting dose-response effects of GTE (35). GTE was very well tolerated with only three grade 3 toxicities, including insomnia, diarrhea and oral/neck pain, and no grade 4 toxicity. Analysis of patients’ demographics among clinical responders showed a higher response rate in never-drinkers (p = 0.001). Other demographic characteristics did not affect the clinical response. At a median follow-up of 27.5 months, there was no difference in oral cancer-free survival between the GTE and placebo arms. Baseline biomarker characteristics, such as higher stromal VEGF expression, were associated with clinical but not histologic response. Interestingly, stromal VEGF and cyclin D1 expression were downregulated in clinically responsive GTE patients and upregulated in nonresponsive patients (35). These biomarker characteristics would be important predictive factors in profiling the patient population that would benefit most from GTE treatment.
Patients with high grade-prostate intraepithelial neoplasia (HG-PIN) received either 200mg of green tea catechin (GTC) containing approximately 103.6mg of EGCG, orally, t.i.d., or placebo. The primary endpoint was prevalence of prostate cancer. After 1 year follow-up, 3.3% patients were diagnosed with prostate cancer in the treatment group compared with 30% in the placebo group (82). Multivariate analysis including age, PSA, prostate volume, HG-PIN, and monofocal or plurifocal HG-PIN lesions showed no significant differences between the two arms. In a 2-year follow-up, only one prostate cancer was diagnosed among 13 GTC-treated patients and 2 among 9 placebo-treated patients (83). A daily dose of 311 mg of EGCG in GTC formulation was able to induce a clinical response in these patients. Although the results of this clinical study for prostate cancer prevention are dramatic and highly promising, the data need to be validated in larger randomized trials.
Oral administration of GTE, three 500 mg tablets, each containing 52.5 mg EGCG, 12.3 mg EC, 34.6 mg EGC, 11.1 mg ECG, and 15.7 mg caffeine, daily for 12 months, in addition to a tea drinking lifestyle, demonstrated its efficacy in preventing incidence of metachronous adenoma in patients 1 year post polypectomy (84). Twelve-month follow-up colonoscopy showed 31% incidence of colonic adenoma in the control arm vs. 15% in the GTE arm (84). It was not reported whether the GTE treatment had any impact on the incidence of colon cancer. Furthermore, patients with HPV-infected cervical premalignant lesions were treated with various formulations of GTE: polyphenon E 200mg orally t.i.d., EGCG 200mg orally t.i.d., polyphenon E 200mg orally t.i.d. plus topical polyphenon E twice a week, or polyphenon E topical treatment only twice a week for 8 to 12 weeks (85). Compared with 4 out of 39 untreated patients, 35 out of 51 treated patients achieved a clinical response in reduction in HPV DNA titer or improvement in cytology or tissue biopsy after treatment (85).
Although these clinical trials used different GTE formulations with different percentages of EGCG content, none have encountered serious adverse effects. It appears that a GTE formulation containing EGCG levels as low as 158mg a day for 12 months was able to induce a clinical response (84). GTE is also well tolerated at doses as high as 4200mg/m2, containing 554.4mg/m2 of EGCG, a day for at least 6 months (81).
Future Directions and Conclusions
Since the first clinical chemoprevention study in 1986 (86), the field of SCCHN chemoprevention has made remarkable advances and entered into mainstream cancer research. Although none of the agents has yet been translated into clinical practice, there are several agents under clinical investigation that hold strong promise. Amongst the most promising compounds are GTPs. The recent phase II clinical trial by Tsao et al, showing a dose-response relationship of GTE against OPLs that correlates with biomarker response, has generated a tangible momentum in SCCHN chemoprevention (35). Several approaches should be pursued in order to maximize yields from this promising agent in future investigations. First, GTE should be used in combination to generate synergy and to reduce toxicity since this agent has shown synergistic/additive antitumor effects in many cancer models (19, 63, 65, 72, 74, 76, 79).
Second, investigations to enhance the bioavailability and potency of GTE should be encouraged and validated more vigorously in preclinical studies. One approach is the use of a pro-drug formulation of EGCG, many of which have shown increased activity and bioavailability in vitro and in vivo (87-90). Another promising approach is to formulate nanoparticles for effective delivery. Siddiqui et al, have demonstrated about 10-fold higher potency of nanoparticle-encapsulated EGCG (91). Both of these approaches are novel and hold high promise for chemoprevention, thus warranting further validation in animal studies and clinical settings. However, efforts must be made not to compromise the safety or cost of this agent.
Supplementary Material
Acknowledgements
This work was supported by grants from the NIH (P50 CA128613, U01 CA101244, and R01 CA112643). DMS is Distinguished Cancer Scholar of the Georgia Cancer Coalition (GCC). ARA is a recipient of SPORE Career Development Award. We also wish to give our thanks and appreciation to Dr. Anthea Hammond for her critical and editorial review of this article.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 3.Vokes EE, Weichselbaum RR, Lippman SM, Hong WK. Head and neck cancer. N Engl J Med. 1993;328:184–94. doi: 10.1056/NEJM199301213280306. [DOI] [PubMed] [Google Scholar]
- 4.Forastiere AA, Leong T, Rowinsky E, et al. Phase III comparison of high-dose paclitaxel + cisplatin + granulocyte colony-stimulating factor versus low-dose paclitaxel + cisplatin in advanced head and neck cancer: Eastern Cooperative Oncology Group Study E1393. J Clin Oncol. 2001;19:1088–95. doi: 10.1200/JCO.2001.19.4.1088. [DOI] [PubMed] [Google Scholar]
- 5.Haddad RI, Shin DM. Recent advances in head and neck cancer. N Engl J Med. 2008;359:1143–54. doi: 10.1056/NEJMra0707975. [DOI] [PubMed] [Google Scholar]
- 6.Kelloff GJ, Lippman SM, Dannenberg AJ, et al. Progress in chemoprevention drug development: the promise of molecular biomarkers for prevention of intraepithelial neoplasia and cancer--a plan to move forward. Clin Cancer Res. 2006;12:3661–97. doi: 10.1158/1078-0432.CCR-06-1104. [DOI] [PubMed] [Google Scholar]
- 7.Sturgis EM, Miller RH. Second primary malignancies in the head and neck cancer patient. Ann Otol Rhinol Laryngol. 1995;104:946–54. doi: 10.1177/000348949510401206. [DOI] [PubMed] [Google Scholar]
- 8.Braakhuis BJ, Tabor MP, Leemans CR, van der Waal I, Snow GB, Brakenhoff RH. Second primary tumors and field cancerization in oral and oropharyngeal cancer: molecular techniques provide new insights and definitions. Head Neck. 2002;24:198–206. doi: 10.1002/hed.10042. [DOI] [PubMed] [Google Scholar]
- 9.Mao L, Oh Y. Does marijuana or crack cocaine cause cancer? J Natl Cancer Inst. 1998;90:1182–4. doi: 10.1093/jnci/90.16.1182. [DOI] [PubMed] [Google Scholar]
- 10.Mao L, Hong WK. How does human papillomavirus contribute to head and neck cancer development? J Natl Cancer Inst. 2004;96:978–80. doi: 10.1093/jnci/djh209. [DOI] [PubMed] [Google Scholar]
- 11.Mao L, Hong WK, Papadimitrakopoulou VA. Focus on head and neck cancer. Cancer Cell. 2004;5:311–6. doi: 10.1016/s1535-6108(04)00090-x. [DOI] [PubMed] [Google Scholar]
- 12.Lippman SM, Heymach JV. The convergent development of molecular-targeted drugs for cancer treatment and prevention. Clin Cancer Res. 2007;13:4035–41. doi: 10.1158/1078-0432.CCR-07-0063. [DOI] [PubMed] [Google Scholar]
- 13.Czerninski R, Amornphimoltham P, Patel V, Molinolo AA, Gutkind JS. Targeting mammalian target of rapamycin by rapamycin prevents tumor progression in an oral-specific chemical carcinogenesis model. Cancer Prev Res (Phila Pa) 2009;2:27–36. doi: 10.1158/1940-6207.CAPR-08-0147. [DOI] [PubMed] [Google Scholar]
- 14.Amin AR, Kucuk O, Khuri FR, Shin DM. Perspectives for cancer prevention with natural compounds. J Clin Oncol. 2009;27:2712–25. doi: 10.1200/JCO.2008.20.6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang CS, Chung JY, Yang G, Chhabra SK, Lee MJ. Tea and tea polyphenols in cancer prevention. J Nutr. 2000;130:472S–8S. doi: 10.1093/jn/130.2.472S. [DOI] [PubMed] [Google Scholar]
- 16.Yoshizawa S, Horiuchi T, Fujiki H, Yoshida T, Okuda T, Sugimura T. Antitumor promoting activity of (−)-epigallocatechin gallate, the main constituent of Tannin in green tea. Phytother Res. 1987;1:44–7. [Google Scholar]
- 17.Wang ZY, Huang MT, Ho CT, et al. Inhibitory effect of green tea on the growth of established skin papillomas in mice. Cancer Res. 1992;52:6657–65. [PubMed] [Google Scholar]
- 18.Lu YP, Lou YR, Xie JG, et al. Topical applications of caffeine or (−)-epigallocatechin gallate (EGCG) inhibit carcinogenesis and selectively increase apoptosis in UVB-induced skin tumors in mice. Proc Natl Acad Sci U S A. 2002;99:12455–60. doi: 10.1073/pnas.182429899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang X, Zhang H, Tighiouart M, et al. Synergistic inhibition of head and neck tumor growth by green tea (−)-epigallocatechin-3-gallate and EGFR tyrosine kinase inhibitor. Int J Cancer. 2008;123:1005–14. doi: 10.1002/ijc.23585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shimizu M, Shirakami Y, Sakai H, et al. EGCG inhibits activation of the insulin-like growth factor (IGF)/IGF-1 receptor axis in human hepatocellular carcinoma cells. Cancer Lett. 2007 doi: 10.1016/j.canlet.2007.11.026. [DOI] [PubMed] [Google Scholar]
- 21.Shimizu M, Deguchi A, Lim JT, Moriwaki H, Kopelovich L, Weinstein IB. (−)-Epigallocatechin gallate and polyphenon E inhibit growth and activation of the epidermal growth factor receptor and human epidermal growth factor receptor-2 signaling pathways in human colon cancer cells. Clin Cancer Res. 2005;11:2735–46. doi: 10.1158/1078-0432.CCR-04-2014. [DOI] [PubMed] [Google Scholar]
- 22.Pierce AL, Shimizu M, Beckman BR, Baker DM, Dickhoff WW. Time course of the GH/IGF axis response to fasting and increased ration in chinook salmon (Oncorhynchus tshawytscha) Gen Comp Endocrinol. 2005;140:192–202. doi: 10.1016/j.ygcen.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 23.Lee YK, Shanafelt TD, Bone ND, Strege AK, Jelinek DF, Kay NE. VEGF receptors on chronic lymphocytic leukemia (CLL) B cells interact with STAT 1 and 3: implication for apoptosis resistance. Leukemia. 2005;19:513–23. doi: 10.1038/sj.leu.2403667. [DOI] [PubMed] [Google Scholar]
- 24.Neuhaus T, Pabst S, Stier S, et al. Inhibition of the vascular-endothelial growth factor-induced intracellular signaling and mitogenesis of human endothelial cells by epigallocatechin-3 gallate. Eur J Pharmacol. 2004;483:223–7. doi: 10.1016/j.ejphar.2003.10.029. [DOI] [PubMed] [Google Scholar]
- 25.Masuda M, Suzui M, Lim JT, Weinstein IB. Epigallocatechin-3-gallate inhibits activation of HER-2/neu and downstream signaling pathways in human head and neck and breast carcinoma cells. Clin Cancer Res. 2003;9:3486–91. [PubMed] [Google Scholar]
- 26.Pianetti S, Guo S, Kavanagh KT, Sonenshein GE. Green tea polyphenol epigallocatechin-3 gallate inhibits Her-2/neu signaling, proliferation, and transformed phenotype of breast cancer cells. Cancer Res. 2002;62:652–5. [PubMed] [Google Scholar]
- 27.Liang YC, Lin-shiau SY, Chen CF, Lin JK. Suppression of extracellular signals and cell proliferation through EGF receptor binding by (−)-epigallocatechin gallate in human A431 epidermoid carcinoma cells. J Cell Biochem. 1997;67:55–65. doi: 10.1002/(sici)1097-4644(19971001)67:1<55::aid-jcb6>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 28.Masuda M, Suzui M, Lim JT, Deguchi A, Soh JW, Weinstein IB. Epigallocatechin-3-gallate decreases VEGF production in head and neck and breast carcinoma cells by inhibiting EGFR-related pathways of signal transduction. J Exp Ther Oncol. 2002;2:350–9. doi: 10.1046/j.1359-4117.2002.01062.x. [DOI] [PubMed] [Google Scholar]
- 29.Adachi S, Nagao T, Ingolfsson HI, et al. The inhibitory effect of (−)-epigallocatechin gallate on activation of the epidermal growth factor receptor is associated with altered lipid order in HT29 colon cancer cells. Cancer Res. 2007;67:6493–501. doi: 10.1158/0008-5472.CAN-07-0411. [DOI] [PubMed] [Google Scholar]
- 30.Somers-Edgar TJ, Scandlyn MJ, Stuart EC, Le Nedelec MJ, Valentine SP, Rosengren RJ. The combination of epigallocatechin gallate and curcumin suppresses ER alpha-breast cancer cell growth in vitro and in vivo. Int J Cancer. 2008;122:1966–71. doi: 10.1002/ijc.23328. [DOI] [PubMed] [Google Scholar]
- 31.Shirakami Y, Shimizu M, Adachi S, et al. (−)-Epigallocatechin gallate suppresses the growth of human hepatocellular carcinoma cells by inhibiting activation of the vascular endothelial growth factor-vascular endothelial growth factor receptor axis. Cancer Sci. 2009;100:1957–62. doi: 10.1111/j.1349-7006.2009.01241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leong H, Mathur PS, Greene GL. Inhibition of mammary tumorigenesis in the C3(1)/SV40 mouse model by green tea. Breast Cancer Res Treat. 2008;107:359–69. doi: 10.1007/s10549-007-9568-x. [DOI] [PubMed] [Google Scholar]
- 33.Leong H, Mathur PS, Greene GL. Green tea catechins inhibit angiogenesis through suppression of STAT3 activation. Breast Cancer Res Treat. 2009;117:505–15. doi: 10.1007/s10549-008-0196-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siddiqui IA, Zaman N, Aziz MH, et al. Inhibition of CWR22Rnu1 tumor growth and PSA secretion in athymic nude mice by green and black teas. Carcinogenesis. 2006;27:833–9. doi: 10.1093/carcin/bgi323. [DOI] [PubMed] [Google Scholar]
- 35.Tsao AS, Liu D, Martin J, et al. Phase II randomized, placebo-controlled trial of green tea extract in patients with high-risk oral premalignant lesions. Cancer Prev Res (Phila Pa) 2009;2:931–41. doi: 10.1158/1940-6207.CAPR-09-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McLarty J, Bigelow RL, Smith M, Elmajian D, Ankem M, Cardelli JA. Tea polyphenols decrease serum levels of prostate-specific antigen, hepatocyte growth factor, and vascular endothelial growth factor in prostate cancer patients and inhibit production of hepatocyte growth factor and vascular endothelial growth factor in vitro. Cancer Prev Res (Phila Pa) 2009;2:673–82. doi: 10.1158/1940-6207.CAPR-08-0167. [DOI] [PubMed] [Google Scholar]
- 37.Tachibana H, Koga K, Fujimura Y, Yamada K. A receptor for green tea polyphenol EGCG. Nat Struct Mol Biol. 2004;11:380–1. doi: 10.1038/nsmb743. [DOI] [PubMed] [Google Scholar]
- 38.Fujimura Y, Umeda D, Kiyohara Y, Sunada Y, Yamada K, Tachibana H. The involvement of the 67 kDa laminin receptor-mediated modulation of cytoskeleton in the degranulation inhibition induced by epigallocatechin-3-O-gallate. Biochem Biophys Res Commun. 2006;348:524–31. doi: 10.1016/j.bbrc.2006.07.086. [DOI] [PubMed] [Google Scholar]
- 39.Umeda D, Yano S, Yamada K, Tachibana H. Green tea polyphenol epigallocatechin-3-gallate signaling pathway through 67-kDa laminin receptor. J Biol Chem. 2008;283:3050–8. doi: 10.1074/jbc.M707892200. [DOI] [PubMed] [Google Scholar]
- 40.Chung JY, Park JO, Phyu H, Dong Z, Yang CS. Mechanisms of inhibition of the Ras-MAP kinase signaling pathway in 30.7b Ras 12 cells by tea polyphenols (−)-epigallocatechin-3-gallate and theaflavin-3,3′-digallate. FASEB J. 2001;15:2022–4. doi: 10.1096/fj.01-0031fje. [DOI] [PubMed] [Google Scholar]
- 41.Pan MH, Lin CC, Lin JK, Chen WJ. Tea polyphenol (−)-epigallocatechin 3-gallate suppresses heregulin-beta1-induced fatty acid synthase expression in human breast cancer cells by inhibiting phosphatidylinositol 3-kinase/Akt and mitogen-activated protein kinase cascade signaling. J Agric Food Chem. 2007;55:5030–7. doi: 10.1021/jf070316r. [DOI] [PubMed] [Google Scholar]
- 42.Sah JF, Balasubramanian S, Eckert RL, Rorke EA. Epigallocatechin-3-gallate inhibits epidermal growth factor receptor signaling pathway. Evidence for direct inhibition of ERK1/2 and AKT kinases. J Biol Chem. 2004;279:12755–62. doi: 10.1074/jbc.M312333200. [DOI] [PubMed] [Google Scholar]
- 43.Syed DN, Afaq F, Kweon MH, et al. Green tea polyphenol EGCG suppresses cigarette smoke condensate-induced NF-kappaB activation in normal human bronchial epithelial cells. Oncogene. 2007;26:673–82. doi: 10.1038/sj.onc.1209829. [DOI] [PubMed] [Google Scholar]
- 44.Lee JS, Oh TY, Kim YK, et al. Protective effects of green tea polyphenol extracts against ethanol-induced gastric mucosal damages in rats: stress-responsive transcription factors and MAP kinases as potential targets. Mutat Res. 2005;579:214–24. doi: 10.1016/j.mrfmmm.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 45.Nomura M, Ma W, Chen N, Bode AM, Dong Z. Inhibition of 12-O-tetradecanoylphorbol-13-acetate-induced NF-kappaB activation by tea polyphenols, (−)-epigallocatechin gallate and theaflavins. Carcinogenesis. 2000;21:1885–90. doi: 10.1093/carcin/21.10.1885. [DOI] [PubMed] [Google Scholar]
- 46.Gupta S, Hastak K, Afaq F, Ahmad N, Mukhtar H. Essential role of caspases in epigallocatechin-3-gallate-mediated inhibition of nuclear factor kappa B and induction of apoptosis. Oncogene. 2004;23:2507–22. doi: 10.1038/sj.onc.1207353. [DOI] [PubMed] [Google Scholar]
- 47.Afaq F, Adhami VM, Ahmad N, Mukhtar H. Inhibition of ultraviolet B-mediated activation of nuclear factor kappaB in normal human epidermal keratinocytes by green tea Constituent (−)-epigallocatechin-3-gallate. Oncogene. 2003;22:1035–44. doi: 10.1038/sj.onc.1206206. [DOI] [PubMed] [Google Scholar]
- 48.Dong Z, Ma W, Huang C, Yang CS. Inhibition of tumor promoter-induced activator protein 1 activation and cell transformation by tea polyphenols, (−)-epigallocatechin gallate, and theaflavins. Cancer Res. 1997;57:4414–9. [PubMed] [Google Scholar]
- 49.Masuda M, Suzui M, Weinstein IB. Effects of Epigallocatechin-3-gallate on Growth, Epidermal Growth Factor Receptor Signaling Pathways, Gene Expression, and Chemosensitivity in Human Head and Neck Squamous Cell Carcinoma Cell Lines. Clin Cancer Res. 2001;7:4220–9. [PubMed] [Google Scholar]
- 50.Hsu S, Lewis JB, Borke JL, et al. Chemopreventive effects of green tea polyphenols correlate with reversible induction of p57 expression. Anticancer Res. 2001;21:3743–8. [PubMed] [Google Scholar]
- 51.Hastak K, Gupta S, Ahmad N, Agarwal MK, Agarwal ML, Mukhtar H. Role of p53 and NF-kappaB in epigallocatechin-3-gallate-induced apoptosis of LNCaP cells. Oncogene. 2003;22:4851–9. doi: 10.1038/sj.onc.1206708. [DOI] [PubMed] [Google Scholar]
- 52.Hastak K, Agarwal MK, Mukhtar H, Agarwal ML. Ablation of either p21 or Bax prevents p53-dependent apoptosis induced by green tea polyphenol epigallocatechin-3-gallate. FASEB J. 2005;19:789–91. doi: 10.1096/fj.04-2226fje. [DOI] [PubMed] [Google Scholar]
- 53.Yamamoto T, Digumarthi H, Aranbayeva Z, et al. EGCG-targeted p57/KIP2 reduces tumorigenicity of oral carcinoma cells: role of c-Jun N-terminal kinase. Toxicol Appl Pharmacol. 2007;224:318–25. doi: 10.1016/j.taap.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 54.Amin AR, Thakur VS, Paul RK, et al. SHP-2 tyrosine phosphatase inhibits p73-dependent apoptosis and expression of a subset of p53 target genes induced by EGCG. Proc Natl Acad Sci U S A. 2007;104:5419–24. doi: 10.1073/pnas.0700642104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shammas MA, Neri P, Koley H, et al. Specific killing of multiple myeloma cells by (−)-epigallocatechin-3-gallate extracted from green tea: biologic activity and therapeutic implications. Blood. 2006;108:2804–10. doi: 10.1182/blood-2006-05-022814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ho YC, Yang SF, Peng CY, Chou MY, Chang YC. Epigallocatechin-3-gallate inhibits the invasion of human oral cancer cells and decreases the productions of matrix metalloproteinases and urokinase-plasminogen activator. J Oral Pathol Med. 2007;36:588–93. doi: 10.1111/j.1600-0714.2007.00588.x. [DOI] [PubMed] [Google Scholar]
- 57.Mantena SK, Roy AM, Katiyar SK. Epigallocatechin-3-gallate inhibits photocarcinogenesis through inhibition of angiogenic factors and activation of CD8+ T cells in tumors. Photochem Photobiol. 2005;81:1174–9. doi: 10.1562/2005-04-11-RA-487. [DOI] [PubMed] [Google Scholar]
- 58.Lin JK. Suppression of protein kinase C and nuclear oncogene expression as possible action mechanisms of cancer chemoprevention by Curcumin. Arch Pharm Res. 2004;27:683–92. doi: 10.1007/BF02980135. [DOI] [PubMed] [Google Scholar]
- 59.Plummer SM, Holloway KA, Manson MM, et al. Inhibition of cyclo-oxygenase 2 expression in colon cells by the chemopreventive agent curcumin involves inhibition of NF-kappaB activation via the NIK/IKK signalling complex. Oncogene. 1999;18:6013–20. doi: 10.1038/sj.onc.1202980. [DOI] [PubMed] [Google Scholar]
- 60.Gopalakrishna R, Jaken S. Protein kinase C signaling and oxidative stress. Free Radic Biol Med. 2000;28:1349–61. doi: 10.1016/s0891-5849(00)00221-5. [DOI] [PubMed] [Google Scholar]
- 61.Dorai T, Gehani N, Katz A. Therapeutic potential of curcumin in human prostate cancer. II. Curcumin inhibits tyrosine kinase activity of epidermal growth factor receptor and depletes the protein. Mol Urol. 2000;4:1–6. [PubMed] [Google Scholar]
- 62.Lin JK, Chen YC, Huang YT, Lin-Shiau SY. Suppression of protein kinase C and nuclear oncogene expression as possible molecular mechanisms of cancer chemoprevention by apigenin and curcumin. J Cell Biochem Suppl. 1997;28-29:39–48. [PubMed] [Google Scholar]
- 63.Khafif A, Schantz SP, Chou TC, Edelstein D, Sacks PG. Quantitation of chemopreventive synergism between (−)-epigallocatechin-3-gallate and curcumin in normal, premalignant and malignant human oral epithelial cells. Carcinogenesis. 1998;19:419–24. doi: 10.1093/carcin/19.3.419. [DOI] [PubMed] [Google Scholar]
- 64.Li N, Chen X, Liao J, et al. Inhibition of 7,12-dimethylbenz[a]anthracene (DMBA)-induced oral carcinogenesis in hamsters by tea and curcumin. Carcinogenesis. 2002;23:1307–13. doi: 10.1093/carcin/23.8.1307. [DOI] [PubMed] [Google Scholar]
- 65.Ghosh AK, Kay NE, Secreto CR, Shanafelt TD. Curcumin inhibits prosurvival pathways in chronic lymphocytic leukemia B cells and may overcome their stromal protection in combination with EGCG. Clin Cancer Res. 2009;15:1250–8. doi: 10.1158/1078-0432.CCR-08-1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rubin Grandis J, Tweardy DJ, Melhem MF. Asynchronous modulation of transforming growth factor alpha and epidermal growth factor receptor protein expression in progression of premalignant lesions to head and neck squamous cell carcinoma. Clin Cancer Res. 1998;4:13–20. [PubMed] [Google Scholar]
- 67.Rubin Grandis J, Melhem MF, Gooding WE, et al. Levels of TGF-alpha and EGFR protein in head and neck squamous cell carcinoma and patient survival. J Natl Cancer Inst. 1998;90:824–32. doi: 10.1093/jnci/90.11.824. [DOI] [PubMed] [Google Scholar]
- 68.Ang KK, Berkey BA, Tu X, et al. Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Cancer Res. 2002;62:7350–6. [PubMed] [Google Scholar]
- 69.Amin AR, Khuri FR, Chen ZG, Shin DM. Synergistic Growth Inhibition of Squamous Cell Carcinoma of the Head and Neck by Erlotinib and Epigallocatechin-3-Gallate: The Role of p53-Dependent Inhibition of Nuclear Factor-{kappa}B. Cancer Prev Res (Phila Pa) 2009 doi: 10.1158/1940-6207.CAPR-09-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Milligan SA, Burke P, Coleman DT, et al. The green tea polyphenol EGCG potentiates the antiproliferative activity of c-Met and epidermal growth factor receptor inhibitors in non-small cell lung cancer cells. Clin Cancer Res. 2009;15:4885–94. doi: 10.1158/1078-0432.CCR-09-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Suganuma M, Okabe S, Kai Y, Sueoka N, Sueoka E, Fujiki H. Synergistic effects of (−−)-epigallocatechin gallate with (−−)-epicatechin, sulindac, or tamoxifen on cancer-preventive activity in the human lung cancer cell line PC-9. Cancer Res. 1999;59:44–7. [PubMed] [Google Scholar]
- 72.Suganuma M, Kurusu M, Suzuki K, Tasaki E, Fujiki H. Green tea polyphenol stimulates cancer preventive effects of celecoxib in human lung cancer cells by upregulation of GADD153 gene. Int J Cancer. 2006;119:33–40. doi: 10.1002/ijc.21809. [DOI] [PubMed] [Google Scholar]
- 73.Suganuma M, Ohkura Y, Okabe S, Fujiki H. Combination cancer chemoprevention with green tea extract and sulindac shown in intestinal tumor formation in Min mice. J Cancer Res Clin Oncol. 2001;127:69–72. doi: 10.1007/s004320000189. [DOI] [PubMed] [Google Scholar]
- 74.Adhami VM, Malik A, Zaman N, et al. Combined inhibitory effects of green tea polyphenols and selective cyclooxygenase-2 inhibitors on the growth of human prostate cancer cells both in vitro and in vivo. Clin Cancer Res. 2007;13:1611–9. doi: 10.1158/1078-0432.CCR-06-2269. [DOI] [PubMed] [Google Scholar]
- 75.Chisholm K, Bray BJ, Rosengren RJ. Tamoxifen and epigallocatechin gallate are synergistically cytotoxic to MDA-MB-231 human breast cancer cells. Anticancer Drugs. 2004;15:889–97. doi: 10.1097/00001813-200410000-00010. [DOI] [PubMed] [Google Scholar]
- 76.Zhou JR, Yu L, Mai Z, Blackburn GL. Combined inhibition of estrogen-dependent human breast carcinoma by soy and tea bioactive components in mice. Int J Cancer. 2004;108:8–14. doi: 10.1002/ijc.11549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Scandlyn MJ, Stuart EC, Somers-Edgar TJ, Menzies AR, Rosengren RJ. A new role for tamoxifen in oestrogen receptor-negative breast cancer when it is combined with epigallocatechin gallate. Br J Cancer. 2008;99:1056–63. doi: 10.1038/sj.bjc.6604634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Thomas F, Patel S, Holly JM, Persad R, Bahl A, Perks CM. Dihydrotestosterone sensitises LNCaP cells to death induced by epigallocatechin-3-Gallate (EGCG) or an IGF-I receptor inhibitor. Prostate. 2009;69:219–24. doi: 10.1002/pros.20873. [DOI] [PubMed] [Google Scholar]
- 79.Lu G, Xiao H, You H, et al. Synergistic inhibition of lung tumorigenesis by a combination of green tea polyphenols and atorvastatin. Clin Cancer Res. 2008;14:4981–8. doi: 10.1158/1078-0432.CCR-07-1860. [DOI] [PubMed] [Google Scholar]
- 80.Li N, Sun Z, Han C, Chen J. The chemopreventive effects of tea on human oral precancerous mucosa lesions. Proc Soc Exp Biol Med. 1999;220:218–24. doi: 10.1046/j.1525-1373.1999.d01-37.x. [DOI] [PubMed] [Google Scholar]
- 81.Pisters KM, Newman RA, Coldman B, et al. Phase I trial of oral green tea extract in adult patients with solid tumors. J Clin Oncol. 2001;19:1830–8. doi: 10.1200/JCO.2001.19.6.1830. [DOI] [PubMed] [Google Scholar]
- 82.Bettuzzi S, Brausi M, Rizzi F, Castagnetti G, Peracchia G, Corti A. Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: a preliminary report from a one-year proof-of-principle study. Cancer Res. 2006;66:1234–40. doi: 10.1158/0008-5472.CAN-05-1145. [DOI] [PubMed] [Google Scholar]
- 83.Brausi M, Rizzi F, Bettuzzi S. Chemoprevention of human prostate cancer by green tea catechins: two years later. A follow-up update. Eur Urol. 2008;54:472–3. doi: 10.1016/j.eururo.2008.03.100. [DOI] [PubMed] [Google Scholar]
- 84.Shimizu M, Fukutomi Y, Ninomiya M, et al. Green tea extracts for the prevention of metachronous colorectal adenomas: a pilot study. Cancer Epidemiol Biomarkers Prev. 2008;17:3020–5. doi: 10.1158/1055-9965.EPI-08-0528. [DOI] [PubMed] [Google Scholar]
- 85.Ahn WS, Yoo J, Huh SW, et al. Protective effects of green tea extracts (polyphenon E and EGCG) on human cervical lesions. Eur J Cancer Prev. 2003;12:383–90. doi: 10.1097/00008469-200310000-00007. [DOI] [PubMed] [Google Scholar]
- 86.Hong WK, Endicott J, Itri LM, et al. 13-cis-retinoic acid in the treatment of oral leukoplakia. N Engl J Med. 1986;315:1501–5. doi: 10.1056/NEJM198612113152401. [DOI] [PubMed] [Google Scholar]
- 87.Yang H, Sun DK, Chen D, et al. Antitumor activity of novel fluoro-substituted (−)-epigallocatechin-3-gallate analogs. Cancer Lett. 2009 doi: 10.1016/j.canlet.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yu Z, Qin XL, Gu YY, et al. Prodrugs of Fluoro-Substituted Benzoates of EGC as Tumor Cellular Proteasome Inhibitors and Apoptosis Inducers. Int J Mol Sci. 2008;9:951–61. doi: 10.3390/ijms9060951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Waleh NS, Chao WR, Bensari A, Zaveri NT. Novel D-ring analog of epigallocatechin-3-gallate inhibits tumor growth and VEGF expression in breast carcinoma cells. Anticancer Res. 2005;25:397–402. [PubMed] [Google Scholar]
- 90.Lam WH, Kazi A, Kuhn DJ, et al. A potential prodrug for a green tea polyphenol proteasome inhibitor: evaluation of the peracetate ester of (−)-epigallocatechin gallate [(−)-EGCG] Bioorg Med Chem. 2004;12:5587–93. doi: 10.1016/j.bmc.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 91.Siddiqui IA, Adhami VM, Bharali DJ, et al. Introducing nanochemoprevention as a novel approach for cancer control: proof of principle with green tea polyphenol epigallocatechin-3-gallate. Cancer Res. 2009;69:1712–6. doi: 10.1158/0008-5472.CAN-08-3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Laurie SA, Miller VA, Grant SC, Kris MG, Ng KK. Phase I study of green tea extract in patients with advanced lung cancer. Cancer Chemother Pharmacol. 2005;55:33–8. doi: 10.1007/s00280-004-0859-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.