Abstract
Background
Serum procalcitonin (PCT) evaluation has been proposed for early diagnosis and accurate staging and to guide decisions regarding patients with sepsis, severe sepsis and septic shock, with possible reduction in mortality.
Objectives
To assess the effectiveness and safety of serum PCT evaluation for reducing mortality and duration of antimicrobial therapy in adults with sepsis, severe sepsis or septic shock.
Search methods
We searched the Central Register of Controlled Trials (CENTRAL; 2015, Issue 7); MEDLINE (1950 to July 2015); Embase (Ovid SP, 1980 to July 2015); Latin American Caribbean Health Sciences Literature (LILACS via BIREME, 1982 to July 2015); and the Cumulative Index to Nursing and Allied Health Literature (CINAHL; EBSCO host, 1982 to July 2015), and trial registers (ISRCTN registry, ClinicalTrials.gov and CenterWatch, to July 2015). We reran the search in October 2016. We added three studies of interest to a list of ‘Studies awaiting classification' and will incorporate these into formal review findings during the review update.
Selection criteria
We included only randomized controlled trials (RCTs) testing PCT‐guided decisions in at least one of the comparison arms for adults (≥ 18 years old) with sepsis, severe sepsis or septic shock, according to international definitions and irrespective of the setting.
Data collection and analysis
Two review authors extracted study data and assessed the methodological quality of included studies. We conducted meta‐analysis with random‐effects models for the following primary outcomes: mortality and time spent receiving antimicrobial therapy in hospital and in the intensive care unit (ICU), as well as time spent on mechanical ventilation and change in antimicrobial regimen from a broad to a narrower spectrum.
Main results
We included 10 trials with 1215 participants. Low‐quality evidence showed no significant differences in mortality at longest follow‐up (risk ratio (RR) 0.81, 95% confidence interval (CI) 0.65 to 1.01; I2 = 10%; 10 trials; N = 1156), at 28 days (RR 0.89, 95% CI 0.61 to 1.31; I2 = 0%; four trials; N = 316), at ICU discharge (RR 1.03, 95% CI 0.50 to 2.11; I2 = 49%; three trials; N = 506) and at hospital discharge (RR 0.98, 95% CI 0.75 to 1.27; I2 = 0%; seven trials; N = 805; moderate‐quality evidence). However, mean time receiving antimicrobial therapy in the intervention groups was ‐1.28 days (95% CI to ‐1.95 to ‐0.61; I2 = 86%; four trials; N = 313; very low‐quality evidence). No primary study has analysed the change in antimicrobial regimen from a broad to a narrower spectrum.
Authors' conclusions
Up‐to‐date evidence of very low to moderate quality, with insufficient sample power per outcome, does not clearly support the use of procalcitonin‐guided antimicrobial therapy to minimize mortality, mechanical ventilation, clinical severity, reinfection or duration of antimicrobial therapy of patients with septic conditions.
Plain language summary
Procalcitonin evaluation for reducing mortality in adults with sepsis
Review question
Is procalcitonin evaluation effective in reducing mortality and time receiving antimicrobial therapy in adults with sepsis?
Background
Sepsis is defined as confirmed or suspected infection associated with a systemic inflammatory response syndrome (SIRS). This condition can evolve to an acute organ dysfunction, known as 'severe sepsis'; or to persistent hypotension, even after adequate fluid replacement, known as 'septic shock'. Procalcitonin (PCT) is a biological indicator in the blood that has been found to increase during blood infection. We wanted to assess whether evaluation of PCT can reduce mortality and time receiving antimicrobial therapy in adults with blood infection. To this end, we compared PCT versus nothing, versus standard care (only usual clinical judgement) and versus other blood chemical indicators. Nowadays, other chemical indicators include C‐reactive protein (CRP), interleukins and neopterin.
Study characteristics
The evidence is current to July 2015. However, we reran the search in October 2016 and will incorporate the three studies of interest when we update the review. For this version, we included 10 studies in this review. These studies were carried out in Australia, Brazil, China, Czech Republic, France, Germany, Indonesia and Switzerland. Researchers evaluated participants from academic and non‐academic surgical, general and trauma intensive care units (ICUs) and emergency departments. All studies analysed adults with confirmed or presumed blood infection. Comparisons were most commonly based on ‘standard care’, but one trial used CRP‐guided antibiotic therapy. In six trials, study authors had worked as consultants for, and/or received payments from, companies involved in the procalcitonin analysis.
Key results
Results showed no significant differences in mortality at longest follow‐up (124/573; 21.6% versus 152/583; 26.1%), at 28 days (37/160; 23.1% versus 39/156; 25%), at ICU discharge (28/247; 11.3% versus 25/259; 9.6%) or at hospital discharge (82/398; 20.6% versus 81/407; 19.9%), respectively, for PCT and non‐PCT groups. Also, researchers found no differences in mechanical ventilation, clinical severity, reinfection or duration of antimicrobial therapy. No study provided information about participants for whom the antimicrobial regimen was changed from a broad to a narrower spectrum.
Quality of the evidence
We considered the body of available evidence as having very low to moderate quality owing to absence of methods to prevent errors during studies or absence of information about such methods, as well as possibly insufficient numbers of studies and patients per outcome. Additionally, the authors of most studies worked as consultants and/or received payments from companies involved in the procalcitonin analysis.
Summary of findings
Summary of findings for the main comparison. PCT versus non‐PCT (standard care or CRP: primary outcomes) for reducing mortality in adult patients with sepsis, severe sepsis and septic shock.
| Patient or population: adult patients with sepsis, severe sepsis and septic shock Settings: emergency departments, as well as general medical and surgical, academic and non‐academic ICUs from Australia, Brazil, China, Czech Republic, France, Germany, Indonesia and Switzerland Intervention: PCT versus non‐PCT (standard care or CRP: primary outcomes) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with non‐PCT (standard care or CRP: primary outcomes) | Risk with PCT | |||||
| Mortality at longest follow‐up | Study population | RR 0.81 (0.65 to 1.01) | 1156 (10 RCTs) | ⊕⊕⊝⊝ Lowa | 70% of studies (7/10) were considered to have high risk of bias in at least 2 criteria, 50% (5/10) low risk of bias in at least 3 criteria and 50% (5/10) unclear risk of bias in at least 1 criterion, including randomization. We observed no asymmetry in the funnel plot. |
|
| 261 per 1000 | 211 per 1000 (169 to 263) | |||||
| Mortality at 28 days | Study population | RR 0.89 (0.61 to 1.31) | 316 (4 RCTs) | ⊕⊕⊝⊝ Lowb | 25% of studies (1/4) were considered to have unclear risk of bias for random sequence generation, 25% (1/4) unclear risk of bias for allocation concealment and 100% (4/4) unclear or high risk of bias for blinding of participants and outcome assessors. Confidence interval was considered relatively high (from 0.61 to 1.31). |
|
| 250 per 1000 | 223 per 1000 (153 to 328) | |||||
| Mortality at ICU discharge | Study population | RR 1.03 (0.50 to 2.11) | 506 (3 RCTs) | ⊕⊕⊝⊝ Lowb | All studies (3/3) were considered to have low risk of bias for random sequence generation, 33% (1/3) high risk of bias for allocation concealment and 100% (3/3) unclear or high risk of bias for blinding of participants and outcome assessors. I2 = 49% (heterogeneity test). Relatively large confidence interval was 0.50 to 2.11. |
|
| 97 per 1000 | 99 per 1000 (48 to 204) | |||||
| Mortality at hospital discharge | Study population | RR 0.98 (0.75 to 1.27) | 805 (7 RCTs) | ⊕⊕⊕⊝ Moderatec | 28% of studies (2/7) were considered to have unclear risk of bias for random sequence generation, 42.8% (3/7) unclear or high risk of bias for allocation concealment and 100% (7/7) unclear or high risk of bias for blinding of participants and outcome assessors. | |
| 199 per 1000 | 195 per 1000 (149 to 253) | |||||
| Time receiving antimicrobial therapy (days) ‐ mean (SD) | The mean time receiving antimicrobial therapy (days) ‐ mean (SD) was 8.09 (1.36) days | The mean time receiving antimicrobial therapy (days) ‐ mean (SD) in the intervention group was 1.28 days lower (1.95 lower to 0.61 lower) | ‐ | 313 (4 RCTs) | ⊕⊝⊝⊝ Very lowd | 75% of studies (3/4) were considered to have unclear risk of bias for both random sequence generation and allocation concealment; 100% (4/4) unclear or high risk of bias for blinding of both participants and outcome assessors and 25% (1/4) unclear risk of bias for incomplete outcome data. I2 > 50% (heterogeneity test indicating important heterogeneity between studies). Relatively large 95% confidence interval was 0.61 to 1.95 days. Combined study results show relevant reductions in time receiving antimicrobial therapy of 1.28 days, which varied from 0.61 days to 1.95 days; individual studies showed mean differences from 0.9 to 2 days. |
| Participants with antimicrobial regimen changed from a broad to a narrower spectrum | Not available from primary studies | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: We are very confident that the true effect lies close to the estimate of effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of effect but may be substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
a GRADE was downrated by two levels for risk of bias.
b GRADE was downrated by two levels: by one level for risk of bias; and by one level for imprecision.
c GRADE was downrated by one level for risk of bias.
d GRADE was downrated by four levels: by one level for risk of bias; by one level four imprecision; and by two levels for inconsistency.
Background
Description of the condition
Sepsis is defined as confirmed or suspected infection associated with a systemic inflammatory response syndrome (SIRS) (Dellinger 2013). This condition can evolve to an acute organ dysfunction or tissue hypoperfusion, known as severe sepsis, or to persistent hypotension or vasopressor requirement, even after adequate fluid resuscitation, known as septic shock (Bone 2009; Dellinger 2013).
According to a comprehensive review, the occurrence of septic conditions has been considered high, with incidence rates ranging from 11 to 300/100,000 inhabitants per year, depending on the severity of the systemic infection and the geographic region of patients (Jawad 2012), with 15 to 19 million cases worldwide reported per year (Adhikari 2010). Mortality rates can reach approximately 30% for sepsis, 50% for severe sepsis and 80% for septic shock (Jawad 2012; Salvo 1995; Silva 2004). However, studies evaluating the incidence, prevalence and mortality of sepsis can be biased strongly by the absence of rigour or even the lack of adequate knowledge among healthcare professionals about identification of SIRS, sepsis, severe sepsis and septic shock (Assunção 2010; Klein 2012). Moreover, such conditions are associated with high costs (Lagu 2012; Vaughan‐Sarrazin 2011) and bad prognoses, including low quality of life and high mortality, even after hospital discharge (Azevedo 2012; Cuthbertson 2013; Karlsson 2009).
Therapeutic approaches for sepsis include early and appropriate antimicrobial agents, fluid resuscitation and strategies for achieving adequate blood (arterial and venous) pressure, myocardial function, glucose levels and control of infectious foci (Dellinger 2013; Kuehn 2013; Machado 2013; Rivers 2012). However, the success of such early therapeutic approaches depends on rapid results of clinical and laboratory assessments, which usually include body temperature, heart rate, glycaemia, respiratory rate, mental status, white blood cells, partial pressure of oxygen in arterial blood, creatinine and lactate (Dellinger 2013; Levy 2003). Therefore, clinicians have used additional biomarkers in an attempt to diagnose the condition and drive the best therapeutic strategies at the most appropriate moment for patients with sepsis. The most thoroughly investigated biomarkers for specific infectious diseases are the interleukins, C‐reactive protein (CRP), procalcitonin (PCT) and neopterin (Tasdelen 2010; Tsalik 2012; Uusitalo‐Seppälä 2011). Some of these have been planned to be rigorously evaluated in other Cochrane systematic reviews (Shaikh 2011; Suresh 2013).
Description of the intervention
During the course of an inflammatory event, including systemic infection, several physiological and biochemical changes occur (Hosein 2011; Lichtenstern 2012; Salluh 2011). One of these changes is an increase in production of PCT, especially, but not exclusively, in cases of bacterial infection (Chalupa 2011; Gendrel 1999; McCann 2012; Redl 2000; te Witt 2012). However, some non‐infectious conditions, such as trauma, surgery, hyperthermia and neoplasm, can be associated with elevated procalcitonin levels (Becker 2008). The peptide PCT is a precursor of the calcitonin hormone, which is responsible for control of blood concentrations of calcium. Under physiological conditions, PCT is produced by the thyroid gland, but in inflammatory conditions, such as sepsis, virtually any type of cell can synthesize PCT (Morgenthaler 2003). Expression and liberation of PCT probably are stimulated by different cytokines and microbial by‐products (Zannoni 2012).
According to results from up to 30 studies included in two systematic reviews, serum PCT evaluation has revealed values of sensitivity ranging from 55% to 97% (with pooled sensitivity of 77%) and values of specificity from 55% to 93% (with pooled specificity of 79%), as compared with definitions provided by the American College of Chest Physicians (ACCP)/Society of Critical Care Medicine (SCCM) Consensus Conference, the German Sepsis Society or microbiological culture (Tang 2007; Wacker 2013). However, irrespective of accuracy properties, serum PCT evaluation is an important health technology that should be evaluated in the area of 'stratified medicine research' (Hingorani 2013). Serum procalcitonin evaluation can possibly permit early detection of sepsis and determination of the appropriate antimicrobial regimen, including, but not restricted to, antimicrobial timing and spectrum. An important outcome already observed with the serum procalcitonin evaluation can include lower hospital costs, but studies have reported no consistent differences in mortality nor in length of stay in the intensive care unit (ICU) (Maravić‐Stojković 2011; Schuetz 2012; Tang 2009).
How the intervention might work
Serum PCT evaluation has been proposed for early diagnosis and accurate staging of sepsis, which can contribute to early decisions, optimal care (Kenzaka 2012; Matthaiou 2012) and, consequently, better outcomes for patients with sepsis, severe sepsis and septic shock (Kumar 2010). Thus, the core ‘action mechanism’ of serum PCT evaluation consists of altered decisions in the care of patients with sepsis, severe sepsis and septic shock, based on test results, with possible reduction in the risk of bad outcomes. The core of this logical sequence of events is that serum PCT evaluation, not drugs or usual care, is the technology being tested (Hingorani 2013).
Why it is important to do this review
According to Rodger 2012, evaluation of the accuracy of a diagnostic test is not sufficient to prove its effectiveness, safety or efficiency. Corroborating this concept, Hingorani 2013 emphasized that the existence of a factor that predicts differential treatment response does not guarantee that it will be effective when used as a test in clinical practice to inform therapeutic decisions. Therefore, it is of extreme importance that all available evidence on the effectiveness, safety and efficiency of the serum PCT evaluation as a health technology for patients with sepsis, severe sepsis and septic shock is scrutinized. We plan to perform a Cochrane systematic review of randomized controlled trials (RCTs) on this clinical question that can be updated to summarize the main findings for clinical practice.
Objectives
To assess the effectiveness and safety of serum PCT evaluation for reducing mortality and duration of antimicrobial therapy in adults with sepsis, severe sepsis or septic shock.
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs and quasi‐randomized trials (allocation not considered strictly random), irrespective of language and publication year. We excluded cross‐over trials because of the nature of both the intervention and the clinical condition of interest for this review.
Types of participants
We included adults (≥ 18 years old) with sepsis, severe sepsis or septic shock, according to international definitions, irrespective of health specialty (e.g. ward, outpatient clinic, ICU, emergency department). We accepted the following definitions.
Sepsis: confirmed or suspected infection associated with a systemic inflammatory response syndrome (SIRS).
Severe sepsis: sepsis associated with acute organ dysfunction.
Septic shock: sepsis associated with tissue hypoperfusion and persistent hypotension, or vasopressor requirement, even after adequate fluid resuscitation (Bone 2009; Dellinger 2013).
Types of interventions
We considered studies that performed serum PCT evaluation in at least one comparison group. We expected three possible comparison groups based on diagnostic and staging methods: (i) standard methods used routinely to diagnose and stage sepsis; (ii) serum PCT evaluation or PCT‐guided therapy algorithm; and (iii) other biomarkers (e.g. CRP, interleukins, pentraxin). On the basis of these groups, we expected the following possible comparisons.
i + ii versus i.
i + ii versus i + iii.
i + ii + iii versus i.
i + ii + iii versus i + iii.
i + ii versus i + ii (different PCT‐guided therapy algorithms).
Types of outcome measures
Primary outcomes
Mortality at up to 28 days, in the ICU, in hospital (from sepsis or all causes) and at longest follow‐up.
Time receiving antimicrobial therapy (in days) or quantity (volume) of antimicrobial agents received.
Change in antimicrobial regimen from a broad to a narrower spectrum.
Secondary outcomes
Hospital length of stay (days).
ICU length of stay (days).
Clinical severity of participant's condition, assessed by validated instruments such as the Acute Physiology and Chronic Health Evaluation II (APACHE II) and the Sequential Organ Failure Assessment (SOFA).
New infection, as defined by a new SIRS event by reason of a new micro‐organism detected after resolution of the initial infection, involving the same infectious focus or a different infectious focus; or reinfection, as defined by a new SIRS event by reason of the same micro‐organism detected after resolution of the initial infection, involving the same infectious focus or a different infectious focus.
Duration of mechanical ventilation (days).
Search methods for identification of studies
Electronic searches
Two review authors searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2015, Issue 7; Appendix 1); MEDLINE (via PubMed, 1950 to July 2015; Appendix 2); Embase (Ovid SP, 1980 to July 2015; Appendix 3); the Cumulative Index to Nursing and Allied Health Literature (CINAHL; EBSCO host, 1982 to July 2015); and the Latin American Caribbean Health Sciences Literature (LILACS via BIREME, 1982 to July 2015; Appendix 5). Additionally, we reran the search in October 2016. We added three new studies of interest to a list of Studies awaiting classification and will incorporate these into formal review findings during the review update.
We used a systematic and sensitive search strategy with search terms for sepsis, severe sepsis, septic shock, procalcitonin evaluation and randomized controlled trials (Appendix 2). We applied no restrictions based on language or date of publication.
Searching other resources
We handsearched the reference lists of reviews, randomized and non‐randomized studies and editorials to look for additional studies. We contacted the lead authors of studies and experts in this field to ask about missed, unreported or ongoing studies. We searched for ongoing clinical trials and unpublished studies on the following Internet sites (July 2015).
Data collection and analysis
Selection of studies
After excluding duplicates, two review authors (BNGA and RBA) independently assessed all titles and abstracts of studies retrieved by the search strategy to determine their relevance for possible inclusion. We resolved disagreements by discussion with a third review author (RS).
Data extraction and management
Two review authors (BNGA and RBA) independently extracted data from each study using a previously prepared data extraction form that includes specific characteristics of each study (Appendix 6). We described as the ‘primary reference’ the first publication of each study with more than one publication, and as ‘secondary references’ all other publications, but we extracted data from all references onto the same extraction sheet.
Assessment of risk of bias in included studies
Two review authors (RBA and BNGA) assessed risk of bias on the basis of criteria described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We assessed the quality of RCTs according to the following domain‐based evaluation.
Was the allocation sequence adequately generated?
Was allocation adequately concealed?
Was knowledge of the allocated intervention adequately prevented during the study?
Were incomplete outcome data adequately addressed?
Are reports of the study free of the suggestion of selective outcome reporting?
Was the study apparently free of other problems that could put it at high risk of bias?
We classified each domain as ‘low risk of bias’ when the authors of primary studies reported methods to prevent bias; as ‘unclear risk of bias’ when risk of bias was uncertain; and as ‘high risk of bias’ when the authors of primary studies clearly had not prevented risk of bias.
We reported these assessments for each individual study in the ‘Risk of bias’ table.
We contacted study author(s) to ask for clarification if we had any uncertainty regarding study data.
Measures of treatment effect
For dichotomous data (e.g. mortality rates), we calculated risk ratios (RRs). In case the effect estimates were statistically significant, we calculated the number needed to treat for an additional beneficial outcome (NNTB) (Christensen 2006). We calculated mean differences (MDs) for continuous data (e.g. hospital length of stay, ICU length of stay). However, some data were presented in isolation, as they were reported in the primary studies. Thus, we reported some continuous data as medians and respective ranges or interquartile ranges (e.g. time receiving antimicrobial therapy, hospital and ICU length of stay, duration of mechanical ventilation in days). Similarly, we presented some dichotomous data as hazard ratios (HRs) with their respective 95% confidence intervals (CIs) (e.g. antibiotic therapy discontinuation). We reported effect estimates from continuous and dichotomous data as P values and 95% CIs for both individual and pooled data (see Data synthesis).
We planned to contact study author(s) to ask for clarification if we had any uncertainty regarding estimated effects (including but not restricted to data reported only in graphs).
Unit of analysis issues
The individual participant was the unit of analysis inclusively in analyses of cluster‐randomized controlled trials. When this was the case, we used direct effect estimates obtained from individual studies (and respective confidence intervals) and combined them in a meta‐analysis using the generic inverse variance method.
Dealing with missing data
If it was possible to assess the real number of randomly assigned participants (by reading the publication or by contacting study authors), we intended to perform intention‐to‐treat (ITT) analyses for dichotomous data. We assumed the worst outcome for all participants who withdrew from/dropped out of the study (see Sensitivity analysis).
Assessment of heterogeneity
We evaluated the consistency of estimated effects from individual studies by calculating I2 (Higgins 2011). The I2 statistic describes approximately the proportion of variation in point estimates that is due to heterogeneity rather than to sampling error. We evaluated the degree of heterogeneity according to the following thresholds.
0% to 40%: may not be important.
30% to 60%: may represent moderate heterogeneity.
50% to 90%: may represent substantial heterogeneity.
75% to 100%: represents considerable heterogeneity.
Assessment of reporting biases
We planned to assess reporting bias by visually inspecting the funnel plot to detect the presence of asymmetry, if we included in the review more than 10 studies per outcome.
Data synthesis
Meta‐analytical data synthesis
When more than one study reported continuous and dichotomous data, we pooled results by using the fixed‐effect meta‐analysis model when we noted no substantial statistical heterogeneity, and the random‐effects meta‐analysis model when statistical heterogeneity between included studies was substantial (see Assessment of heterogeneity). We pooled continuous data by using the weighted average of differences between comparison groups, wherein outcomes published for more than one study were assessed on the same scales. If data were reported on different scales that could not be adjusted to a uniform scale, we planned to analyse them by using the standardized mean difference (SMD). We performed a post hoc trial sequential analysis (TSA) to quantify the reliability of cumulative data in meta‐analyses (Brok 2009; Wetterslev 2008).
Synthesis of dichotomous and continuous data without sufficient information to insert into a forest plot
When estimated effects were reported without sufficient information for insertion into a forest plot, such as numbers of participants, numbers of events, means, standard deviations and standard errors, as well as effect estimates for non‐parametric data (e.g. range, median, percentiles), we reported the data separately in tables in the Data collection and analysis section (not in forest plots).
Synthesis of the quality of the body of evidence
We used the principles of the GRADE approach (Guyatt 2008) to assess the quality of the body of evidence for our primary outcomes of mortality at 28 days, mortality at ICU discharge, mortality at hospital discharge and time receiving antimicrobial therapy. We imported effect estimates from RevMan 5.3 to GRADE profiler (GRADEpro 2014) to create Table 1. This table provides outcome‐specific information concerning the overall quality of evidence from studies included in the comparison, the magnitude of effect of interventions examined and the sum of available data on outcomes that we considered. The GRADE approach appraises the quality of a body of evidence according to the extent to which one can be confident that an estimate of effect or association reflects the item assessed. The quality of a body of evidence is based on different items, which reflect within‐study risk of bias (methodological quality), directness of evidence, heterogeneity of data, precision of effect estimates and risk of publication bias. Thus, we considered each of these items as having 'no limitation', 'serious limitation' or 'very serious limitation' (by downgrading them respectively for one or two levels), resulting in one of the following four overall qualities of evidence for each outcome: 'high', 'moderate', 'low' or 'very low' quality.
Subgroup analysis and investigation of heterogeneity
We planned to compare the possible subgroups below.
Diagnostic criteria for sepsis, severe sepsis and septic shock.
Participants with sepsis, severe sepsis or septic shock.
Infection foci, including respiratory, surgical, bloodstream, catheter, urinary and others.
Different cut‐off points for PCT to guide the antimicrobial regimen for any absolute reduction in PCT level, any relative reduction in PCT level or any threshold of PCT level.
Participants attended by different health specialties (e.g. emergency, ICU, ward).
PCT‐guided antibiotic commencement versus PCT‐guided antibiotic stewardship. It is important to note that multiple subgroup analyses may generate misleading results, but the review authors judged it improbable that included studies would provide sufficient information to permit analyses of all six of these subgroups.
However, we performed no subgroup analyses in this version of the review because of (1) the absence of statistical heterogeneity in the meta‐analysis or (2) the absence of a sufficient number of studies with the same specific characteristics to be combined in the same subgroup.
Sensitivity analysis
We planned to examine the robustness of results by excluding and including trials on the basis of risk of bias of included studies, and by considering quasi‐randomized controlled trials. We also planned to compare random‐effects and fixed‐effect estimates only for the primary outcomes, as well as intention‐to‐treat analysis versus available data analysis (refer to Dealing with missing data). However, risks of bias were highly diverse among the included studies, no quasi‐RCT was localized and studies were clinically and methodologically heterogeneous, which justified the use of random‐effects meta‐analysis only. Thus, we performed sensitivity analysis only for ITT versus available data analyses for the present version of this systematic review. We assumed poor outcomes for missing data, as supported by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Results
Description of studies
Results of the search
The search across all databases yielded 1068 titles. When we excluded duplicate references, we found that we had 740 articles. Of these 740 articles, we excluded 699 because they did not focus on the use of procalcitonin for adults with sepsis, severe sepsis or septic shock, as stated in their titles or abstracts. Of the remaining 41 full‐text articles, we excluded 25 that were derived from 22 studies because of study design. Thus, 16 articles had the potential to be included in the review (Figure 1). Of these 16 articles, we obtained four (derived from three studies) through the search strategy that we reran across all databases in October 2016 (Bloos 2016; de Jong 2016; Najafi 2015). We contacted the main authors of four of the remaining 12 articles (Dharaniyadewi 2013; Hochreiter 2009; Liu 2013; Schroeder 2009) to request further information, as outlined in the Characteristics of included studies table.
1.
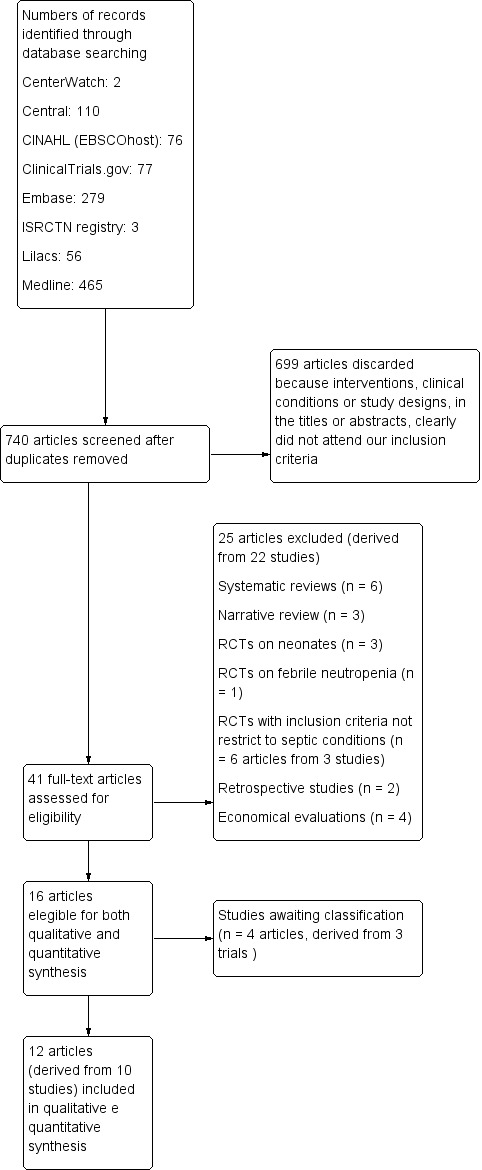
Study flow diagram.
Included studies
We included in this review 12 articles derived from 10 trials (1215 participants). These trials were carried out in France (one; Annane 2013), Brazil (two; Deliberato 2013; Oliveira 2013), Indonesia (one; Dharaniyadewi 2013), Germany (two; Hochreiter 2009; Schroeder 2009), China (one; Liu 2013), Switzerland (one; Nobre 2008), Czech Republic (one; Svoboda 2007) and Australia (one; Shehabi 2014). Five trials were multi‐centre RCTs (Annane 2013; Nobre 2008; Oliveira 2013; Shehabi 2014; Svoboda 2007). Three studies included participants from surgical ICUs (Deliberato 2013; Hochreiter 2009; Schroeder 2009), but Schroeder 2009 specifically considered participants who had undergone abdominal surgery. Nobre 2008 included participants from both general/medical and surgical ICUs. Svoboda 2007 included participants with trauma. Three trials were carried out in academic health services (Liu 2013; Nobre 2008; Oliveira 2013). One trial (Shehabi 2014) referred to its participants as derived from both academic and non‐academic ICUs.
Although they showed some variation in their manner of describing the inclusion criteria, all trials included in this systematic review mentioned adults with confirmed or presumed sepsis, severe sepsis and/or septic shock, according to criteria usually accepted by international consensus (ACCP/SCCM Consensus Conference Committee 1992; Bone 1992; Levy 2003).
The procalcitonin algorithms were relatively diverse. In general, the authors used PCT drops from 25% to 90% together with PCT thresholds raging from 0.1 to 2.0 ng/mL (Annane 2013; Deliberato 2013; Hochreiter 2009; Liu 2013; Nobre 2008; Oliveira 2013; Schroeder 2009; Shehabi 2014; Svoboda 2007). Four authors have also considered other clinical signs and symptoms (Hochreiter 2009; Schroeder 2009; Shehabi 2014; Svoboda 2007). Dharaniyadewi 2013 provided no PCT algorithm in the paper or in the protocol available from Clinical Trials.gov (NCT01862185).
Control arms were referred to most commonly as 'standard care', which was generally based on local epidemiology and susceptibility of micro‐organisms, infectious foci, routine clinical evaluation or other criteria based on different guidelines previously implemented in the health service (Annane 2013; Deliberato 2013; Dharaniyadewi 2013; Hochreiter 2009; Liu 2013; Nobre 2008; Svoboda 2007). Just one study used a CRP‐guided algorithm, in which antimicrobial therapy was stopped when CRP levels dropped by > 50% or when CRP < 25 mg/dL was reached but the participant's PCT concentrations were not known (Oliveira 2013).
For more detailed information about the studies included in this review, please refer to Characteristics of included studies.
Excluded studies
On the basis of study design and inclusion criteria, we excluded 25 articles that had been generated from 22 studies. Six were systematic reviews (Kopterides 2010; Mann 2011; Prkno 2013; Sandifer 2012; Schuetz 2011; Soni 2013); three were narrative reviews (Pantelidou 2015; Schuetz 2013; Ternhag 2010); three RCTs included neonates (Kolici 2013; Stocker 2010a; Stocker 2010b); one RCT had inclusion criteria strict for febrile neutropenia (Lima 2016); three were RCTs (which generated six articles) with inclusion criteria not specific for sepsis, severe sepsis and/or septic shock (Bouadma 2010; Jensen 2011; Layios 2012); two used a retrospective study design (Bodmann 2016; Kiehntopf 2011); and four performed economic evaluations (Bréchot 2015; Harrison 2015; Kip 2015; Westwood 2015).
For more detailed information about the excluded studies, please refer to the Characteristics of excluded studies table.
Studies awaiting classification
Although results of this systematic review are based on the search from July 2015, a new search carried out in October 2016 revealed three new studies of interest, which we will incorporate into formal review findings during the review update. Two multi‐centre (Bloos 2016; de Jong 2016) and one single‐centre (Najafi 2015) RCTs were carried out in 35 medical ICUs in Germany (Bloos 2016), 15 in Netherlands (de Jong 2016) and one in Tehran (Najafi 2015). These trials included a total of 2695 participants. Bloos 2016 was the only 2 × 2 factorial study that included use of high‐dose intravenous sodium selenite in patients with severe sepsis or septic shock, according to ACCP/SCCM criteria. The other two studies included participants with an antimicrobial regimen initiated for suspected or proven infection on admission or during ICU stay (de Jong 2016) and for SIRS (Najafi 2015).
The interventions offered by Bloos 2016 consisted of a PCT‐based algorithm (with or without sodium selenite) versus antimicrobial therapy, according to the discretion of the responsible physician (with no PCT usage), also with or without sodium selenite. de Jong 2016 and Najafi 2015 randomized participants to PCT‐guided antimicrobial therapy via different algorithms or to standard treatment (non‐PCT groups). These three studies measured mortality at different follow‐up times and in different settings (e.g. at 28 days, at one year, in ICU, in hospital). For additional details, see Characteristics of studies awaiting classification.
Ongoing studies
We found no studies awaiting classification.
Risk of bias in included studies
Please see Characteristics of included studies, Figure 2 and Figure 3.
2.
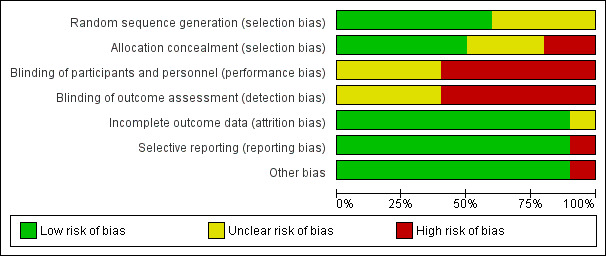
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
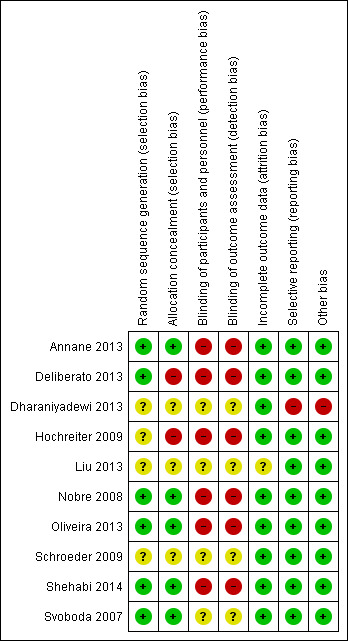
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
We considered six trials as having low risk of bias regarding random sequence generation because study authors used random methods. The most frequently reported method was based on electronic random processes generally referred to as “computer‐generated random numbers” (Annane 2013; Nobre 2008; Oliveira 2013; Shehabi 2014; Svoboda 2007), but Deliberato 2013 reported that two authors of the trial randomly drew folders from a black box to assign participants to a “PCT group” or a “standard group”. We considered the other four trials to have unclear risk of bias because they did not specify any method of randomization (Dharaniyadewi 2013; Hochreiter 2009; Liu 2013; Schroeder 2009).
We tried without success to contact the authors of four trials by email to obtain detailed information about the method used for random sequence generation (Dharaniyadewi 2013; Hochreiter 2009; Liu 2013; Schroeder 2009) (please refer to Characteristics of included studies for details).
Allocation concealment
Three trials provided no information regarding allocation concealment methods (Dharaniyadewi 2013; Liu 2013; Schroeder 2009), thus we considered them as having unclear risk of bias. Four trials used methods associated with low risk of bias for allocation concealment (Annane 2013; Nobre 2008; Shehabi 2014; Svoboda 2007). Annane 2013 and Shehabi 2014 reported the use of web‐based central randomization. Two trials used opaque, sealed and numbered envelopes (Nobre 2008; Svoboda 2007). We considered two trials as having high risk of bias (Deliberato 2013; Hochreiter 2009). One of them used a method that does not prevent exemption in the randomization process because study authors performed randomization by drawing folders from a box to assign participants to comparison groups (Deliberato 2013). Given that Hochreiter 2009 provided no description of the method used to randomize participant assignments, and that study authors clearly reported the study as open label, we have also assumed that this study has high risk of bias.
We tried without success to contact the authors of four trials by email to obtain detailed information about the method used for allocation concealment (Dharaniyadewi 2013; Hochreiter 2009; Liu 2013; Schroeder 2009).
Blinding
Performance bias
Personnel involved in the trials were clearly unblinded to assignments in six trials (Annane 2013; Deliberato 2013; Hochreiter 2009; Nobre 2008; Oliveira 2013; Shehabi 2014), thus we considered these trials as having high risk of performance bias. We classified four trials as having unclear risk of performance bias because study authors provided no information regarding blinding of personnel and participants (Dharaniyadewi 2013; Liu 2013; Schroeder 2009; Svoboda 2007).
Detection bias
Four trials (Dharaniyadewi 2013; Liu 2013; Schroeder 2009; Svoboda 2007) provided no information with regard to blinding of outcome assessors, and we considered them as having unclear risk of detection bias. We considered six trials as having high risk of detection bias because study authors reported that outcome assessors were not blinded (Annane 2013; Deliberato 2013; Hochreiter 2009; Nobre 2008; Oliveira 2013 ;Shehabi 2014).
Incomplete outcome data
We considered all included trials as having low risk of attrition bias because they described a clear flow of participants from randomization to outcome assessment, along with low withdrawal rates, with the exception of one trial with unclear risk of attrition bias (Liu 2013), which provided neither information about withdrawals nor a description of a clear flow of participants within the trial.
Selective reporting
We considered all trials as having low risk of reporting bias because investigators evaluated clinically relevant outcomes. Additionally, four trials made their protocols available along with previously planned outcomes in an electronic repository of research protocols (clinicaltrials.gov) (Annane 2013; Nobre 2008; Oliveira 2013; Shehabi 2014).
Other potential sources of bias
Nine trials were associated with no suspected additional source of bias, and we considered them as having low risk of bias (Annane 2013; Deliberato 2013; Hochreiter 2009; Liu 2013; Nobre 2008; Oliveira 2013; Schroeder 2009; Shehabi 2014; Svoboda 2007), along with one study for which the authors did not provide a detailed PCT algorithm for dealing with antimicrobial therapies (Dharaniyadewi 2013).
Effects of interventions
See: Table 1
Primary outcomes
1. Mortality
1.1 Mortality at longest follow‐up
Ten trials when combined into a meta‐analysis (Annane 2013; Deliberato 2013; Dharaniyadewi 2013; Hochreiter 2009; Liu 2013; Nobre 2008; Oliveira 2013; Schroeder 2009; Shehabi 2014; Svoboda 2007) showed no significant differences in mortality at longest follow‐up between PCT (124/573; 21.6%) and non‐PCT (152/583; 26.1%) groups with RR of 0.81 (95% CI 0.65 to 1.01; I2 = 10%; Analysis 1.1). One trial compared mortality rates between the procalcitonin group (21/49; 42.8%) and the CRP‐monitoring group (21/45; 46.6%) (Oliveira 2013) and reported no differences between comparison groups (RR 0.92, 95% CI 0.59 to 1.44). We downgraded the evidence from high to low quality because risk of bias from primary studies was downgraded by two levels.
1.1. Analysis.
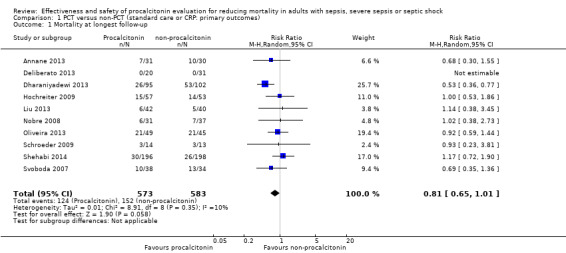
Comparison 1 PCT versus non‐PCT (standard care or CRP: primary outcomes), Outcome 1 Mortality at longest follow‐up.
A post hoc TSA for mortality at longest follow‐up with inclusion of trials with no events (zero event adjustment of 0.001 constant), type 1 error of 5% and power of 80% resulted in a TSA‐adjusted RR of 0.80 (95% CI 0.65 to 0.98; I2 = 0%; diversity (D2) = 0%). On the basis of mortality incidence of 26.07% in the control arm and risk reduction of 17.1%, the required information size is 2853. With 1156 participants included at this time, only 40.52% of the required information size has been reached. Additionally, TSA is designed for trials with low risk of bias, and given that all included trials had high risk of bias, the true required information size may very well be higher than reported here, but a large trial with low risk of bias in favour of the intervention may equally reduce the required information size. Additionally, the TSA figure shows that the conventional boundary was not crossed and no significant benefit favoured the intervention (Figure 4).
4.
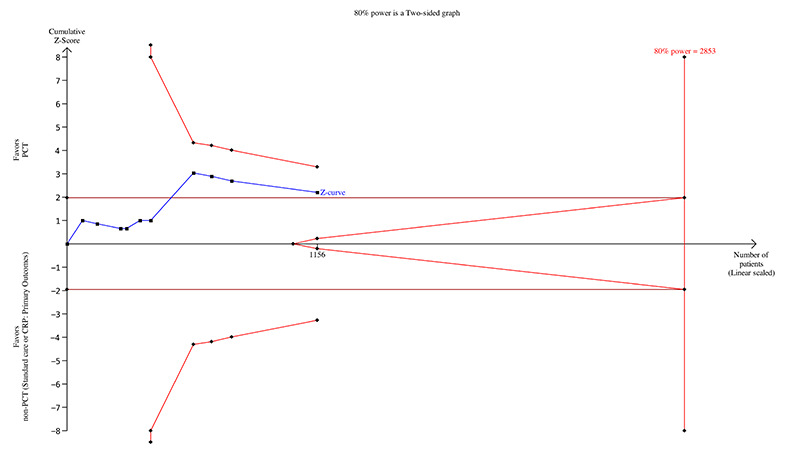
Trial sequential analysis for mortality at longest follow‐up (available data analysis).
1.2 Mortality at 28 days
Four trials combined into a meta‐analysis (Liu 2013; Nobre 2008; Oliveira 2013; Svoboda 2007) showed no significant differences in mortality at 28 days between PCT (37/160; 23.1%) and non‐PCT (39/156; 25.0%) comparison groups (RR 0.89, 95% CI 0.61 to 1.31; four trials; N = 316; I2 = 0%) (Analysis 1.2). One trial compared mortality rates between the procalcitonin group (16/49; 32.6%) and the CRP‐monitoring group (15/45; 33.3%) (Oliveira 2013) and reported no difference between comparison groups (RR 0.98, 95% CI 0.55 to 1.74). We downgraded the evidence from high to low quality because risk of bias from primary studies was downgraded by one level, and imprecision was downgraded by one level.
1.2. Analysis.
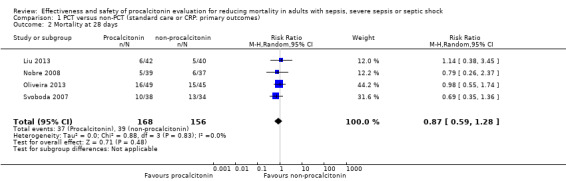
Comparison 1 PCT versus non‐PCT (standard care or CRP: primary outcomes), Outcome 2 Mortality at 28 days.
1.3 Mortality at ICU discharge
After we combined effect estimates from three trials (Annane 2013; Deliberato 2013; Shehabi 2014) in a meta‐analysis, we found no significant differences in mortality at ICU discharge (RR 1.03, 95% CI 0.50 to 2.11; I2 = 49%) (Analysis 1.3). We downgraded the evidence from high to low quality because risk of bias and imprecision from primary studies were downgraded by one level.
1.3. Analysis.
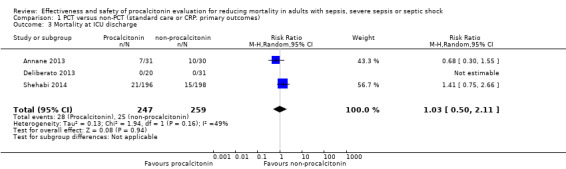
Comparison 1 PCT versus non‐PCT (standard care or CRP: primary outcomes), Outcome 3 Mortality at ICU discharge.
1.4 Mortality at hospital discharge
Irrespective of whether comparison arms provided procalcitonin versus non‐procalcitonin (standard care) or procalcitonin versus CRP‐guided antimicrobial therapy (Oliveira 2013), a meta‐analysis combining seven trials (Annane 2013; Deliberato 2013; Hochreiter 2009; Nobre 2008; Oliveira 2013; Schroeder 2009; Shehabi 2014) showed absence of differences between them (RR 0.98, 95% CI 0.75 to 1.27; I2 = 0%; Analysis 1.4). We downgraded the evidence from high to moderate quality because risk of bias and imprecision from primary studies were downgraded by one level.
1.4. Analysis.
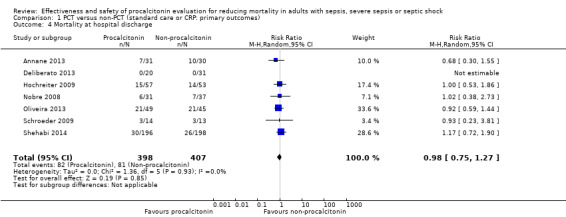
Comparison 1 PCT versus non‐PCT (standard care or CRP: primary outcomes), Outcome 4 Mortality at hospital discharge.
2. Time receiving antimicrobial therapy (in days) or quantity (volume) of antimicrobial agents received
A meta‐analysis that combined four trials evaluating the duration of antimicrobial therapy in days (Hochreiter 2009; Oliveira 2013; Liu 2013; Schroeder 2009) resulted in a reduction of ‐1.28 mean days (95% CI ‐1.95 to ‐0.61; I2 = 86%) in the procalcitonin group as compared with the non‐procalcitonin group (Analysis 1.5).
1.5. Analysis.
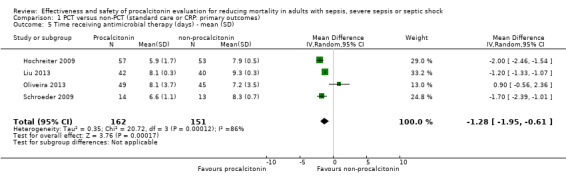
Comparison 1 PCT versus non‐PCT (standard care or CRP: primary outcomes), Outcome 5 Time receiving antimicrobial therapy (days) ‐ mean (SD).
Even after Oliveira 2013 was removed from the analysis because investigators compared procalcitonin versus CRP, heterogeneity remained and the effect estimate did not change significantly (‐1.60 mean days, 95% CI ‐2.18 to ‐1.01; I2 = 84%). When we combined only Hochreiter 2009 and Schroeder 2009 in a meta‐analysis, the inconsistency test (I2 statistic) dropped to 0%, but the effect estimate remained very close to that observed before Liu 2013 and Oliveira 2013 were excluded, as shown by a mean reduction of ‐1.91 days (95% CI ‐2.29 to ‐1.52; I2 = 0%). Still with regard to duration of antibiotic treatment, Oliveira 2013 observed 13 median days (interquartile range (IQR) 7 to 18) in the procalcitonin group and eight median days (IQR 6 to 18) in the CRP‐guided antimicrobial therapy group, but without statistical significance in the comparison between groups (P = 0.183), as reported by study authors (Analysis 1.6).
1.6. Analysis.
Comparison 1 PCT versus non‐PCT (standard care or CRP: primary outcomes), Outcome 6 Time receiving antimicrobial therapy (days) ‐ median (IQR).
| Time receiving antimicrobial therapy (days) ‐ median (IQR) | |||||
|---|---|---|---|---|---|
| Study | Procalcitonin, median (range / interquartile range [IQR]) | non‐Procalcitonin, median (range / interquartile range [IQR]) | Difference between medians | P‐value | Favoured group |
| Annane 2013 | 5 (IQR: 2–5) | 5 (IQR: 3–5) | 0 days | 0.52 | Procalcionin |
| Deliberato 2013 | 9 (Range: 5–24) | 13 (Range: 3–45) | 4 days | 0.008 | Procalcionin |
| Oliveira 2013 | 13 (IQR: 7–18) | 8 (IQR: 6–18) | 5 days | 0.183 | non‐Procalcionin |
We downgraded the evidence from high to very low quality because risk of bias was downgraded by one level, imprecision by one level and inconsistency by two levels.
3. Participants with change in antimicrobial regimen from a broad to a narrower spectrum
No study made available sufficient and comparable information on participants who had their antimicrobial regimen changed from a broad to a narrower spectrum.
Secondary outcomes
Hospital length of stay (days)
With the exception of Oliveira 2013, other trials (Annane 2013; Deliberato 2013; Nobre 2008; Shehabi 2014) showed results favouring the procalcitonin groups, with differences in median values ranging from two days to seven days, but found no statistical significance (Analysis 2.1). However, another study (Liu 2013) showed results favouring the procalcitonin group (27.0 mean days; standard deviation (SD) = 4.9) as compared with the non‐procalcitonin group (32.0 mean days; SD = 5.4), with a statistically significant difference between comparison groups (‐5.00 days, 95% CI ‐7.24 to ‐2.76; P < 0.0001; Analysis 2.1).
2.1. Analysis.
Comparison 2 PCT versus non‐PCT (standard care or CRP: secondary outcomes), Outcome 1 Hospital length of stay (days) ‐ median (IQR) or mean (SD).
| Hospital length of stay (days) ‐ median (IQR) or mean (SD) | ||||||
|---|---|---|---|---|---|---|
| Study |
Procalcitonin median (Range / interquartile range IQR) or mean (SD) |
non‐Procalcitonin median (Range / interquartile range IQR) or mean (SD) |
Mean difference | Diference between medians | P‐value | Favoured group |
| Annane 2013 | 27 (IQR: 9–49) | 33.0 (IQR: 11–69) | not informed | 6 days | 0.22 | procalcitonin |
| Deliberato 2013 | 10.5 (Range: 5–547) | 14.0 (Range: 2–82) | not informed | 3.5 days | 0.34 | procalcitonin |
| Liu 2013 | 27 (4.9 SD) | 32 (5.4 SD) | 5 days | not applied | <0.0001 (Z‐test) | procalcitonin |
| Nobre 2008 | 14.0 (Range: 5–64) | 21.0 (Range: 5–89) | not informed | 7 days | 0.16 | procalcitonin |
| Oliveira 2013 | 36 (IQR: 20–59) | 25 (IQR: 13–52) | not informed | 11 days | 0.175 | non‐procalcitonin |
| Shehabi 2014 | 15 (IQR: 9‐29) | 17 (IQR: 10‐32) | not informed | 2 days | 0.19 | procalcitonin |
ICU length of stay (days)
Four trials evaluated mean days in the ICU (Hochreiter 2009; Liu 2013; Schroeder 2009; Svoboda 2007), resulting in a pooled effect that favoured the procalcitonin group (‐2.05 days, 95% CI ‐3.14 to ‐0.97; I2 = 0%; Analysis 2.2).
2.2. Analysis.
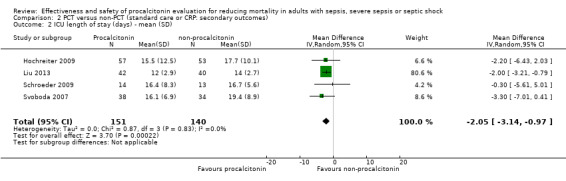
Comparison 2 PCT versus non‐PCT (standard care or CRP: secondary outcomes), Outcome 2 ICU length of stay (days) ‐ mean (SD).
Five other studies evaluated time in the ICU as median values with respective IQRs and P values (Annane 2013; Deliberato 2013; Nobre 2008; Oliveira 2013; Shehabi 2014). Four trials reported directions of effect favouring the procalcitonin groups, with differences between median values ranging from a half‐day to two days, but only Nobre 2008 found a statistically significant difference (0.03) between the procalcitonin group (median of 3 days; IQR 1 to 18) and the non‐procalcitonin group (median of 5 days; IQR 1 to 30). Oliveira 2013 found the opposite direction of effect favouring the non‐procalcitonin group (median of 12 days; IQR 7 to 18) as compared with the procalcitonin group (median of 14 days; IQR 9 to 24), with no statistically significant differences (P = 0.164; Analysis 2.3).
2.3. Analysis.
Comparison 2 PCT versus non‐PCT (standard care or CRP: secondary outcomes), Outcome 3 ICU length of stay (days) ‐ median (IQR).
| ICU length of stay (days) ‐ median (IQR) | |||||
|---|---|---|---|---|---|
| Study | Procalcitonin, median (range / interquartile range [IQR]) | non‐Procalcitonin, median (range / interquartile range [IQR]) | Diference between medians | P‐value | Favoured group |
| Annane 2013 | 22 (IQR: 8–42) | 23 (IQR: 10–60) | 1 day | 0.58 | Procalcitonin |
| Deliberato 2013 | 3.5 (Range: 1–57) | 4 (Range: 1–28) | 0.5 days | 0.80 | Procalcitonin |
| Nobre 2008 | 3 (Range: 1–18) | 5 (Range: 1–30) | 2 days | 0.03 | Procalcitonin |
| Oliveira 2013 | 14 (IQR: 9–24) | 12 (IQR: 7–18) | 2 days | 0.164 | non‐Procalcitonin |
| Shehabi 2014 | 6 (IQR: 3‐9.5) | 6 (IQR: 4‐10) | 0 days | 0.87 | Procalcitonin |
Clinical severity of participant's condition
Svoboda 2007 reported SOFA scores at day 28 as means and respective standard deviations, resulting in a borderline statistically non‐significant difference in means of ‐1.40 (95% CI ‐2.82 to 0.02; P = 0.5) in favour of the procalcitonin group (Analysis 2.4).
2.4. Analysis.
Comparison 2 PCT versus non‐PCT (standard care or CRP: secondary outcomes), Outcome 4 SOFA score during ICU stay.
| SOFA score during ICU stay | |||||
|---|---|---|---|---|---|
| Study |
Procalcitonin, Mean (SD) (N = 38) |
non‐Procalcitonin, Mean (SD) (N = 34) |
Mean difference (95% CI) | P‐value | Favoured group |
| Svoboda 2007 | 7.9 (2.8) | 9.3 (3.3) | ‐1.40 [‐2.82, 0.02] | 0.05 | Procalcitonin |
Annane 2013 also evaluated SOFA scores at days three and five but found no statistically significant differences (Analysis 2.5; Analysis 2.6). Schroeder 2009 analysed the SOFAmax, defined as the highest sequential failure assessment score, during the period of observation but found a statistically non‐significant difference between comparison groups, as shown in Analysis 2.7; and Liu 2013 found other non‐significant difference while evaluating APACHE II (Analysis 2.8).
2.5. Analysis.
Comparison 2 PCT versus non‐PCT (standard care or CRP: secondary outcomes), Outcome 5 SOFA score at day 3.
| SOFA score at day 3 | |||
|---|---|---|---|
| Study | Procalcitonin, median (interquartile range [IQR]) | non‐Procalcitonin, median (interquartile range [IQR]) | P‐value |
| Annane 2013 | 8 (5–10) | 8 (7–11) | 0.85 |
2.6. Analysis.
Comparison 2 PCT versus non‐PCT (standard care or CRP: secondary outcomes), Outcome 6 SOFA score at day 5.
| SOFA score at day 5 | |||
|---|---|---|---|
| Study | Procalcitonin, median (interquartile range [IQR]) | non‐Procalcitonin, median (interquartile range [IQR]) | P‐value |
| Annane 2013 | 8 (5–9) | 8 (7–11) | 0.61 |
2.7. Analysis.
Comparison 2 PCT versus non‐PCT (standard care or CRP: secondary outcomes), Outcome 7 SOFAmax score.
| SOFAmax score | |||||
|---|---|---|---|---|---|
| Study |
Procalcitonin, Mean (95% CI) (N = 14) |
non‐Procalcitonin, Mean (95% CI) (N = 13) |
Mean difference (95% cI) | P‐value | Favoured group |
| Schroeder 2009 | 7.3 (3.5) | 8.4 (4.2) | ‐1.00 (‐3.93, 1.93) | 0.50 | Procalcitonin |
2.8. Analysis.
Comparison 2 PCT versus non‐PCT (standard care or CRP: secondary outcomes), Outcome 8 APACHE II score.
| APACHE II score | |||||
|---|---|---|---|---|---|
| Study |
Procalcsditonin, Mean (SD) (N = 42) |
non‐Procalcitonin, Mean (SD) (N = 40) |
Mean difference (95% cI) | P‐value | Favoured group |
| Liu 2013 | 5.7 (0.9) | 6.2 (1.3) | 0.50 (3.54, 4.54) | 0.81 | non‐procalcitonin |
New infection or reinfection
After the results of Deliberato 2013, Nobre 2008 and Oliveira 2013 were combined, the meta‐analysis revealed a higher but non‐significant risk of reinfection in the procalcitonin group (5/100; 5.0%) as compared with the non‐procalcitonin group (3/113; 2.65%) with a risk ratio of 1.84 (95% CI 0.43 to 7.89; I2 = 0%; Analysis 2.9).
2.9. Analysis.
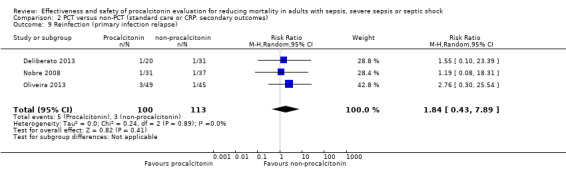
Comparison 2 PCT versus non‐PCT (standard care or CRP: secondary outcomes), Outcome 9 Reinfection (primary infection relapse).
Duration of mechanical ventilation (days)
Three trials (Annane 2013; Shehabi 2014; Svoboda 2007) found no statistically significant differences between comparison groups, although effects favoured procalcitonin in Annane 2013 and Svoboda 2007 (Analysis 2.10).
2.10. Analysis.
Comparison 2 PCT versus non‐PCT (standard care or CRP: secondary outcomes), Outcome 10 Duration of mechanical ventilation (days).
| Duration of mechanical ventilation (days) | |||||
|---|---|---|---|---|---|
| Study |
Procalcitonin, median (interquartile range [IQR]) or mean (SD) |
non‐Procalcitonin, median (interquartile range [IQR]) or mean (SD) |
Difference between medians or Difference between means (95% CI) |
P‐value | Favoured group |
| Annane 2013 | 11 (IQR: 5–25) | 14 (IQR: 8–25) | 3 days | 0.56 | procalcitonin |
| Shehabi 2014 | 4 (IQR: 2‐9) | 4 (IQR: 2‐11) | 0 days | 0.99 | procalcitonin |
| Svoboda 2007 | 10.3 (7.8 SD) | 13.9 (9.4 SD) | 3.6 days (0.42, 7.62) | 0.08 | procalcitonin |
Other outcomes of potential interest
After we had extracted all estimates of effects from the primary studies, we had an excess of 56 dependent variables on which to base our comparisons between procalcitonin and non‐procalcitonin groups (standard care or CRP), as shown in Analysis 3.1 to Analysis 3.38. However, we observed significant effects in favour of procalcitonin‐guided antimicrobial therapy in only eight variables related to mortality at 14 days (Analysis 3.2), in empirical antibiotic initiation (Analysis 3.12; Analysis 3.13) and in an additional five ways of measuring time of antimicrobial usage (Analysis 3.23; Analysis 3.28; Analysis 3.29; Analysis 3.30; Analysis 3.32).
3.1. Analysis.
Comparison 3 PCT versus non‐PCT ‐ other outcomes of potential interest, Outcome 1 Mortality at 5 days.
| Mortality at 5 days | |||||
|---|---|---|---|---|---|
| Study | Procalcitonin (n/N) | non‐Procalcitonin (n/N) | OR (95% CI) | P‐value | Favoured group |
| Annane 2013 | 3/31 | 3/31 | 1.00 (0.22, 4.58) | 1.0 | no group |
3.38. Analysis.
Comparison 3 PCT versus non‐PCT ‐ other outcomes of potential interest, Outcome 38 Clinical cure*.
3.2. Analysis.
Comparison 3 PCT versus non‐PCT ‐ other outcomes of potential interest, Outcome 2 Mortality at 14 days.
| Mortality at 14 days | |||||
|---|---|---|---|---|---|
| Study | Procalcitonin (n/N) | non‐Procalcitonin (n/N) | RR (95% CI) | P‐value | Favoured group |
| Dharaniyadewi 2013 | 26/95 | 53/102 | 0.53 (0.36, 0.77) | 0.00086 | Procalcitonin |
3.12. Analysis.
Comparison 3 PCT versus non‐PCT ‐ other outcomes of potential interest, Outcome 12 Empirical antibiotic initiation ≤ 6 hours.
| Empirical antibiotic initiation ≤ 6 hours | |||||
|---|---|---|---|---|---|
| Study | Procalcitonin (n/N) | non‐Procalcitonin (n/N) | OR (95% CI) | P‐value | Favoured group |
| Dharaniyadewi 2013 | 83/95 | 36/102 | 2.48 (1.88, 3.25) | <0.00001 | Procalcitonin |
3.13. Analysis.
Comparison 3 PCT versus non‐PCT ‐ other outcomes of potential interest, Outcome 13 Empirical antibiotic initiation > 6 hours.
| Empirical antibiotic initiation > 6 hours | |||||
|---|---|---|---|---|---|
| Study | Procalcitonin (n/N) | non‐Procalcitonin (n/N) | OR (95% CI) | P‐value | Favoured group |
| Dharaniyadewi 2013 | 12/95 | 66/102 | 0.20 (0.11, 0.34) | <0.00001 | Procalcitonin |
3.23. Analysis.
Comparison 3 PCT versus non‐PCT ‐ other outcomes of potential interest, Outcome 23 Antibiotic therapy‐free days (mean, SD) ‐ PCT vs standard care.
| Antibiotic therapy‐free days (mean, SD) ‐ PCT vs standard care | |||||
|---|---|---|---|---|---|
| Study |
Procalcitonin Mean (SD), N = 31 |
non‐Procalcitonin Mean (SD), N = 37 |
Meand difference (95% CI) | P‐value | Favoured group |
| Nobre 2008 | 17.4 (7.6) | 13.6 (7.6) | 3.80 (0.17, 7.43) | 0.04 | Procalcitonin |
3.28. Analysis.
Comparison 3 PCT versus non‐PCT ‐ other outcomes of potential interest, Outcome 28 All antibiotics total daily defined dose Irrespective of the antimicrobial.
| All antibiotics total daily defined dose Irrespective of the antimicrobial | ||||
|---|---|---|---|---|
| Study | Procalcitonin, median (interquartile range [IQR]) | non‐Procalcitonin, median (interquartile range [IQR]) | P‐value | Favoured group |
| Shehabi 2014 | 1200 (500‐3000) | 1500 (750‐4000) | 0.001 | Procalcitonin |
3.29. Analysis.
Comparison 3 PCT versus non‐PCT ‐ other outcomes of potential interest, Outcome 29 Antibiotics "stopped earlier" (hazard ratio).
| Antibiotics "stopped earlier" (hazard ratio) | |||
|---|---|---|---|
| Study | Procalcitonin versus non‐procalcitonin, hazard ratio (95% CI) | P‐value | Favoured group |
| Nobre 2008 | 1.9 (1.2–3.1) | 0.009 | Procalcitonin |
3.30. Analysis.
Comparison 3 PCT versus non‐PCT ‐ other outcomes of potential interest, Outcome 30 Antibiotics "stopped earlier" (hazard ratio) adjusted for disease severity.
| Antibiotics "stopped earlier" (hazard ratio) adjusted for disease severity | |||
|---|---|---|---|
| Study | Procalcitonin versus non‐procalcitonin, hazard ratio (95% CI) | P‐value | Favoured group |
| Nobre 2008 | 1.9 (1.2–3.2) | 0.009 | Procalcitonin |
3.32. Analysis.
Comparison 3 PCT versus non‐PCT ‐ other outcomes of potential interest, Outcome 32 Duration of first episode of antibiotic treatment (days).
| Duration of first episode of antibiotic treatment (days) | |||||
|---|---|---|---|---|---|
| Study | Procalcitonin, median (range) | Non‐procalcitonin, median (range) | mean difference (95% CI) | P‐value | Favoured group |
| Nobre 2008 | 6 (4–16) | 10 (3–33) | 3.2 (1.1 to 5.4) | 0.003 | non‐Procalcitonin |
| Oliveira 2013 | 7 (6.0–8.5) | 6 (5.0–7.0) | not informed | 0.06 | procalcitonin |
As a probable consequence of more rational antimicrobial usage, two study authors reported relevant reductions in costs associated with procalcitonin‐guided antimicrobial therapy. Deliberato 2013, for example, reported a reduction in total costs of antibiotics from USD 42,397.00 in the standard care group to USD10,608.00 in the procalcitonin‐guided antimicrobial therapy group, corresponding to an approximate reduction in cost with antimicrobials of 75% (Analysis 3.39), as well as a mean cost with antibiotics plus PCT kits per participant of USD 977.40 against USD1367.64 in the non‐procalcitonin group, corresponding to an approximate cost reduction of 28% (Analysis 3.40). Schroeder 2009 reported an important reduction of 17% in costs of antibiotic treatment associated with procalcitonin‐guided antimicrobial therapy as compared with non‐procalcitonin‐guided treatment (P < 0.01) (Analysis 3.41).
3.39. Analysis.
Comparison 3 PCT versus non‐PCT ‐ other outcomes of potential interest, Outcome 39 Total costs with antibiotics per comparison group (USD).
| Total costs with antibiotics per comparison group (USD) | |||||
|---|---|---|---|---|---|
| Study | Procalcitonin | non‐procalcitonin | Between‐group absolute difference | P‐value | Favoured group |
| Deliberato 2013 | US$ 10 608.00 | US$ 42 397.00 | US$ 31 789.00 | not available | procalcitonin |
3.40. Analysis.
Comparison 3 PCT versus non‐PCT ‐ other outcomes of potential interest, Outcome 40 Mean cost with antibiotics + PCT kit per participant (USD).
| Mean cost with antibiotics + PCT kit per participant (USD) | |||||
|---|---|---|---|---|---|
| Study | Procalcitonin | non‐procalcitonin | Between‐group absolute difference | P‐value | Favoured group |
| Deliberato 2013 | 977.4 | 1367.64 | 390.24 | not available | procalcitonin |
3.41. Analysis.
Comparison 3 PCT versus non‐PCT ‐ other outcomes of potential interest, Outcome 41 Cost reduction for antibiotic treatment.
| Cost reduction for antibiotic treatment | |||
|---|---|---|---|
| Study | Percentage of reduction | P‐Vaue | Favoured group |
| Schroeder 2009 | 17.8% | <0.01 | Procalcitonin |
Assessment of reporting biases
We explored publication bias for mortality at longest follow‐up by using the funnel plot. Visual inspection of the funnel plot (Figure 5) revealed no apparent influence (tendency) of small studies leading to more or less beneficial intervention effect estimates (Higgins 2011). Thus, we consider publication bias improbable at the present version of this systematic review.
5.
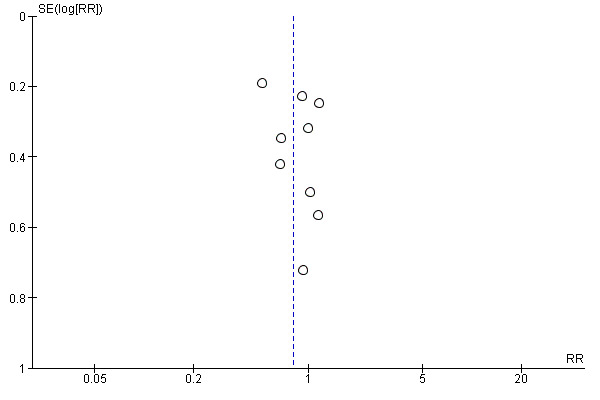
Funnel plot of comparison: 1 PCT versus non‐PCT (standard care or CRP: primary outcomes), outcome: 1.1 Mortality at longest follow‐up.
Sensitivity analysis
Sensitivity analysis 1. Imputing missing data with mortality versus available data analysis in "Mortality at longest follow‐up" outcome
We performed a sensitivity analysis to observe the effects of imputing missing data with poor outcomes in the analysis of our primary outcome of "Mortality at longest follow‐up" (Higgins 2011). Our ITT analysis showed no significant differences in mortality at longest follow‐up between PCT (164/613; 26.7%) and non‐PCT (171/602; 28.4%) comparison groups (RR 0.98, 95% CI 0.73 to 1.32; I2 = 56%; Analysis 4.1), as in the available data analysis (RR of 0.81, 95% CI 0.65 to 1.01; I2 = 10%; Analysis 1.1).
4.1. Analysis.
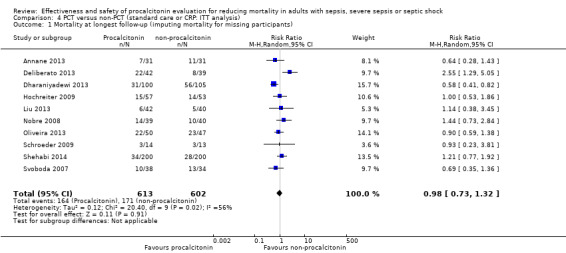
Comparison 4 PCT versus non‐PCT (standard care or CRP: ITT analysis), Outcome 1 Mortality at longest follow‐up (imputing mortality for missing participants).
We performed additional post hoc sensitivity analyses to test the effects of including three studies with inclusion criteria not specific for sepsis, severe sepsis or septic shock, as previously planned in our protocol (Bouadma 2010; Jensen 2011; Layios 2012) and as shown in Sensitivity analyses 2, 3 and 4 below.
Sensitivity analysis 2. Mortality at longest follow‐up in studies with inclusion criteria not specific for sepsis, severe sepsis or septic shock
Inclusion of Bouadma 2010, Jensen 2011 and Layios 2012 in a meta‐analysis of 13 trials (Annane 2013; Bouadma 2010; Deliberato 2013; Dharaniyadewi 2013; Hochreiter 2009; Jensen 2011; Layios 2012; Liu 2013; Nobre 2008; Oliveira 2013; Schroeder 2009; Shehabi 2014; Svoboda 2007) revealed no significant differences in mortality at longest follow‐up between PCT (435/1742; 24.9%) and non‐PCT (460/1744; 26.3%) comparison groups (RR 0.92, 95% CI 0.81 to 1.05; I2 = 11%; Figure 6) as compared with included studies (RR 0.81, 95% CI 0.65 to 1.01; I2 = 10%; Analysis 1.1).
6.
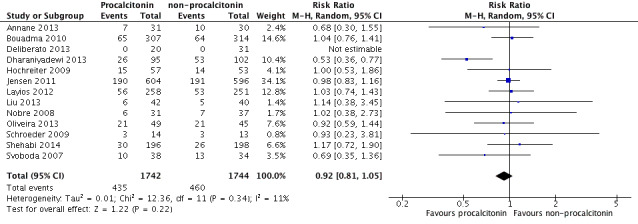
Sensitivity analysis including Bouadma 2010, Jensen 2011 and Layios 2012: 1 PCT versus non‐PCT (standard care or CRP: primary outcomes), outcome: 1.1 Mortality at longest follow‐up (Analysis 1.1).
Sensitivity analysis 3. Mortality at 28 days in studies with inclusion criteria not specific for sepsis, severe sepsis or septic shock
Inclusion of Jensen 2011 and Bouadma 2010 in a meta‐analysis of six studies (Bouadma 2010; Jensen 2011; Liu 2013; Nobre 2008; Oliveira 2013; Svoboda 2007) revealed no significant differences in mortality at 28 days between PCT (292/1071; 27.2%) and non‐PCT (294/1066; 27.6%) comparison groups (RR 0.98, 95% CI 0.86 to 1.12; I2 = 0%; Figure 7) as compared with included studies (RR 0.89, 95% CI 0.61 to 1.31; four trials; N = 316; I2 = 0%; Analysis 1.2).
7.
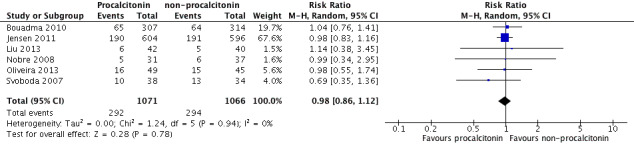
Sensitivity analysis including Bouadma 2010 and Jensen 2011: 1 PCT versus non‐PCT (standard care or CRP: primary outcomes), outcome: 1.2 Mortality at 28 days (Analysis 1.2).
Sensitivity analysis 4. Mortality at ICU discharge in studies with inclusion criteria not specific for sepsis, severe sepsis or septic shock
Inclusion of Layios 2012 in a meta‐analysis of four studies (Annane 2013; Deliberato 2013; Layios 2012; Shehabi 2014) revealed no significant differences in mortality at ICU discharge (RR 1.04, 95% CI 0.79 to 1.38; I2 = 0%; Figure 8) compared with included studies (RR 1.03, 95% CI 0.50 to 2.11; I2 = 49%; Analysis 1.3).
8.
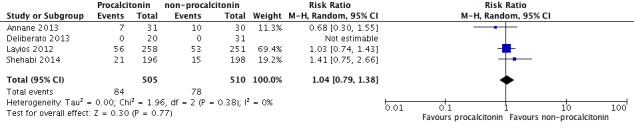
Sensitivity analysis including Layios 2012: 1 PCT versus non‐PCT (standard care or CRP: primary outcomes), outcome: 1.3 Mortality at ICU discharge (Analysis 1.3).
Discussion
Summary of main results
Through our comprehensive search strategy, we retrieved 486 articles. Twelve articles met our inclusion criteria but were generated from 10 studies. This relatively small number of studies provided 59 dependent variables. Seventeen were related to primary and secondary outcomes previously planned for this systematic review. We did not omit the remaining 42 outcomes from this review because we considered them to be of potential interest to readers, including researchers and decision makers.
Primary outcomes
For mortality at 28 days, at intensive care unit (ICU) discharge, at hospital discharge and at longest follow‐up, included studies were consistent in terms of showing absence of differences between procalcitonin‐guided and non‐procalcitonin‐guided antimicrobial therapy (standard care or C‐reactive protein (CRP)‐guided antimicrobial therapy) (Annane 2013; Deliberato 2013; Hochreiter 2009; Liu 2013; Nobre 2008; Oliveira 2013; Schroeder 2009; Shehabi 2014; Svoboda 2007), with the exception of Dharaniyadewi 2013.
Three out of four trials (Hochreiter 2009; Liu 2013; Schroeder 2009) showed that duration of antimicrobial therapy was reduced by more than one day, but one study (Oliveira 2013) showed non‐significant statistical differences that favoured CRP‐guided antimicrobial therapy. Additionally, Deliberato 2013 noted a statistically significant reduction of four days in duration of antimicrobial therapy associated with the procalcitonin group, measured as median values; however, Annane 2013 did not observe such a significant reduction in the time it took to receive antimicrobial therapy.
Secondary outcomes
Liu 2013 showed a statistically significant reduction of five days of stay in the hospital in the procalcitonin group. Annane 2013, Deliberato 2013, Nobre 2008, Oliveira 2013 and Shehabi 2014 reported length of hospital stay as median values. Four of these studies showed statistically non‐significant reductions in favour of procalcitonin groups, which varied from two to six days of stay in the hospital (Annane 2013; Deliberato 2013; Nobre 2008; Shehabi 2014).
Four studies reported more optimistic results for ICU length of stay, with an approximate mean difference of two days in favour of the procalcitonin groups (Hochreiter 2009; Liu 2013; Schroeder 2009; Svoboda 2007), but Oliveira 2013 observed non‐significant results. Annane 2013, Deliberato 2013, Nobre 2008, Oliveira 2013 and Shehabi 2014 reported results as median values, but only Nobre 2008 noted a significant difference of two days in favour of the procalcitonin group.
Although participants in some included studies showed a propensity for a shorter stay in both hospital and ICU, all studies that evaluated the clinical severity of the participant's condition (Annane 2013; Liu 2013; Schroeder 2009; Svoboda 2007), reinfection (Deliberato 2013; Nobre 2008; Oliveira 2013) and duration of mechanical ventilation (Annane 2013; Shehabi 2014; Svoboda 2007) showed no relevant effect associated with procalcitonin‐guided antimicrobial therapy. However, the higher proportion of reinfection among procalcitonin groups is supposed to be caused by reduced antibiotic exposure.
Other outcomes of potential interest for the user
Investigators reported significant effects in favour of procalcitonin‐guided antimicrobial therapy for other outcomes not planned for this systematic review, specifically, mortality at day 14 (Dharaniyadewi 2013) and empirical antibiotic initiation (Dharaniyadewi 2013) and five additional ways of measuring the time receiving antimicrobial treatment (Nobre 2008; Oliveira 2013; Shehabi 2014). More promising findings were the cost reductions associated with procalcitonin‐guided antimicrobial therapy, which varied from 17% to 75%, depending on the method of cost evaluation applied (Deliberato 2013; Schroeder 2009). However, all of these results are limited and should be read with caution.
Overall completeness and applicability of evidence
Evidence presented in this systematic review shows absence of a clear effect of procalcitonin‐guided antimicrobial therapy in minimizing mortality, reinfection, clinical severity or mechanical ventilation of patients with sepsis, severe sepsis or septic shock. However, procalcitonin evaluation has relevant potential for reducing the duration of antimicrobial therapy, as well as patient stay in both hospital and ICU.
The reader should consider the possibility of insufficient sample power for all outcomes because of the low number of included studies, which totalled at most 1156 participants for one outcome in primary studies: mortality at longest follow‐up.Otherwise, a post hoc sensitivity analysis performed to test the effects of including three studies with inclusion criteria not specific for sepsis, severe sepsis or septic shock (Bouadma 2010; Jensen 2011; Layios 2012) had no significant effect on measures of mortality.
It is important to consider that, although we found low mortality rates of around 20% in both comparison groups, all studies had included high percentages of participants with severe and/or septic shock, with the exception of Hochreiter 2009 and Liu 2013, which did not specify these proportions.
Although it was not previously planned as a primary or secondary outcome, our analysis revealed promising effects of procalcitonin‐guided antimicrobial therapy in reducing costs as a probable consequence of reducing time on the antimicrobial regimen, but such findings should be confirmed/refuted by future experimental or observational studies in which investigators perform economic analysis.
Another important issue involves the diagnostic accuracy of procalcitonin for septic conditions and their prognosis. Despite its limitations, no better biomarker for sepsis and its prognosis is known (García de Guadiana‐Romualdo 2015; Garnacho‐Montero 2014; Hoeboer 2015; Leli 2014; Liu 2015; Nargis 2014).
Quality of the evidence
According to Table 1, we considered the evidence to be of low quality for mortality at longest follow‐up, mortality at 28 days and mortality at ICU discharge, and of moderate quality for mortality at hospital discharge, with no significant effect of procalcitonin‐guided antimicrobial therapy, even when this approach was compared with standard care. Although we included 10 studies in this systematic review, only the outcome of "mortality at longest follow‐up" was reported by 10 studies, for which trial sequential analysis showed an actual sample size corresponding to approximately 40% of the required information size (1156 of 2853 participants). Moreover, although four studies reported relevant reduction in the time of antimicrobial therapy, these studies were associated with serious risk of bias, resulting in evidence of very low quality for this specific outcome.
Visual inspection of the funnel plot revealed no asymmetry, suggesting absence of publication bias. The possibility that investigators have not made their studies available for reasons of absence of effect is improbable because most of the studies included in this systematic review showed absence of differences for several other outcomes, including primary and secondary outcomes. Even so, we should not dismiss the possibility that investigators may not make available studies showing negative effects of procalcitonin‐guided antimicrobial therapy because studies with positive results are more likely to be published (Kicinski 2013).
One of the most important points to be stressed to the reader and the scientific community is that both the precision of our effect estimates and the quality of the evidence were affected by the large number of dependent variables that have been evaluated in studies published to this point. In this systematic review, we could find 59 outcomes in 10 studies. Thus, higher‐quality evidence will certainly be achieved if researchers concentrate their efforts on analysis of common and clinically relevant outcomes. Upon thinking of the large divergence of outcomes assessed in these studies, we provided estimates of effects for all outcomes reported in the primary studies included in this review.
Potential biases in the review process
Besides using a highly sensitive strategy in our search for studies, we applied no language restrictions, resulting in the inclusion of a publication written in Chinese (Liu 2013). After we reran the search (October 2016), we retrieved three additional studies of interest and included them in the list of Studies awaiting classification. We will incorporate these studies into our formal review findings during the review update; these findings will contribute 94.4% (2695 participants) of the required information size of 2853 participants, according to the trial sequential analysis. Although it is improbable that these studies will change estimated effects on mortality, they may change other relevant outcomes, especially hospital and ICU length of stay, as well as time on antimicrobial therapy and mechanical ventilation.
We could not minimize during the review process a source of bias that was precisely related to the evaluation of risk of bias for some studies because we had no success in obtaining additional information from the authors of four primary studies (Dharaniyadewi 2013; Hochreiter 2009; Liu 2013; Schroeder 2009). One of these studies is available only as an extended abstract (Dharaniyadewi 2013).
We included mortality at longest follow‐up as one additional primary outcome because absence of evidence on mortality often results from insufficient power, as well as from clinical and methodological heterogeneity between studies. However, we believe such an inclusion does not introduce potential bias into the review process because no substitution of outcomes occurred, and evidence of absence of effect could be reinforced by this new outcome.
Agreements and disagreements with other studies or reviews
In a narrative review with some elements of a systematic review (systematic search across relevant databases), Mann 2011 focused analysis on the accuracy of procalcitonin for diagnosing sepsis in critically ill burn patients. After analysing 14 observational studies and five systematic reviews, these review authors supported the discriminatory capacity of procalcitonin as an important tool to be combined with the clinical diagnosis of sepsis. Another narrative review by Schuetz 2013 not only supports procalcitonin evaluation as a valuable diagnostic tool for respiratory infection and sepsis but also suggests desired repercussions of procalcitonin evaluation in reducing the time it takes to receive antimicrobial therapy, without affecting mortality. Besides narrative reviews, the medical literature already includes several systematic reviews conducted to investigate the potential causal relationship between procalcitonin monitoring and relevant outcomes such as mortality, but these review authors included studies that used different inclusion criteria (Kopterides 2010; Prkno 2013; Schuetz 2011). These three systematic reviews yielded a total of 14 included randomized controlled trials (RCTs).
Two systematic reviews analysed studies consisting of critically ill adult and neonatal participants (Kopterides 2010), as well as adult participants from primary care, emergency department and ICU (Schuetz 2011), but review authors were not strictly interested in patients with sepsis, severe sepsis and/or septic shock at study entry, according to our inclusion criteria. Another systematic review by Prkno 2013 included RCTs and observational studies that evaluated adult participants with severe sepsis. Among the RCTs analysed by Prkno 2013, we noted the inclusion of Jensen 2011, which we did not consider in our systematic review. We clearly justified the exclusion of Jensen 2011 from our systematic review on the basis of broad inclusion criteria for patients admitted to the ICU, also without a strict interest in sepsis, severe sepsis or septic shock, which was similar to the approach of Kopterides 2010 and Schuetz 2011. Another difference from other systematic reviews was our inclusion of three additional studies that met our inclusion criteria (Liu 2013; Oliveira 2013; Shehabi 2014). Even under such methodological divergences, Prkno 2013, Kopterides 2010 and Schuetz 2011 provided conclusions consistent with those of our systematic review because they noted the same general evidence of absence of effect for mortality and shorter time receiving antibiotic treatment that we had observed in procalcitonin‐guided antimicrobial therapy as compared with non‐procalcitonin‐guided treatment. However, as opposed to Kopterides 2010 and Prkno 2013, our systematic review shows a probable reduction in the time patients stay in the hospital and in the ICU, which remains to be adequately proven.
Besides the above‐mentioned general agreement among systematic reviews, other updated and important observational and health economic studies have reported shorter hospital stay (Kip 2015) and less time receiving antibiotic therapy (Hohn 2013; Hohn 2015; Kip 2015; Maseda 2015), with a probable consequence of relevant cost reductions, as reported specifically by Kip 2015.
Finally, it is important to consider that, since 2012, one of the main existing guidelines for dealing with severe sepsis and septic shock (Dellinger 2013) has included recommending procalcitonin to support clinicians while they decide whether or not to discontinue empirical antimicrobial therapy for patients with septic conditions. However, Dellinger 2013 assumes low quality of available evidence for this recommendation.
Authors' conclusions
Implications for practice.
Evidence of low to moderate quality does not support the use of procalcitonin‐guided antimicrobial therapy to minimize mortality, reinfection, clinical severity, mechanical ventilation, or duration of antimicrobial therapy of patients with sepsis, severe sepsis or septic shock. However, the reader should consider the possibility of insufficient sample power for all outcomes.
Implications for research.
The findings of this systematic review suggest promising effects of procalcitonin‐guided antimicrobial therapy in reducing the stay of patients in both the hospital and the ICU, which deserve better confirmation from RCTs. Next trials should include an additional 1697 participants to confirm possible superiority of procalcitonin for mortality at longest follow‐up as compared with control. The possible reduction in costs (as a probable consequence of reduced time on the antimicrobial regimen) should also be confirmed/refuted by future studies in which investigators perform cost analyses.
What's new
| Date | Event | Description |
|---|---|---|
| 3 January 2019 | Amended | Editorial team changed to Cochrane Emergency and Critical Care |
Notes
Brenda NG Andriolo was previously known as Brenda NG Silva.
Acknowledgements
We would like to thank the Cochrane Anaesthesia, Critical and Emergency Care Group (ACE) for its support, and Arash Afshari (Content Editor), Nathan Pace (Statistical Editor) and Arturo J Martí‐Carvajal, Djillali Annane and James Washam (Peer Reviewers) for help and editorial advice provided during preparation of the protocol for this systematic review (Silva 2014); as well as Arash Afshari (Content Editor), Jing Xie (Statistical Editor), James Walsham and David T Huang (Peer Reviewers) and Brian Stafford (Consumer Referee) for help and editorial advice provided during preparation of this systematic review, and Gustavo Porfírio (Brazilian Cochrane Centre) for assistance in the search for studies.
Appendices
Appendix 1. CENTRAL, the Cochrane Library search strategy
#1 procalcitonin or (calcitonin near (precursor* or polyprotein* or polypeptide)) or ((CGRP* or CALC*) near protein) or (alpha protein near human) #2 MeSH descriptor: [Sepsis] explode all trees #3 MeSH descriptor: [Septicemia] explode all trees #4 MeSH descriptor: [Shock, Septic] explode all trees #5 (sepsis or septic* or blood?stream infection* or (shock adj3 (endotoxic or toxic))) #6 #2 or #3 or #4 or #5 #7 #1 and #6
Appendix 2. MEDLINE (PubMed) search strategy
#1 ("procalcitonin" [Supplementary Concept]) OR (calcitonin precursor polyprotein) OR (CGRP1 protein, mouse) OR (CALCA protein, human) OR (calcitonin/calcitonin‐related polypeptide, alpha protein, human) OR (CGRP1 protein, human) OR (CALC1 protein, human)
#2 ((Sepsis) OR (Septicemia) OR (Blood stream infection) OR (Septic shock) OR (Endotoxic Shock) OR (Toxic Shock) OR (Severe sepsis))
#3 ((randomized controlled trial [pt]) OR (controlled clinical trial [pt]) OR (randomized [tiab]) OR (placebo [tiab]) OR (drug therapy [sh]) OR (randomly [tiab]) OR (trial [tiab]) OR (groups [tiab])) AND (humans [mh])
#4 #1 and #2 and #3
Appendix 3. Embase (Ovid SP) search strategy
1. procalcitonin/ or (calcitonin adj3 (precursor* or polyprotein* or polypeptide)).ti,ab. or ((CGRP* or CALC*) adj3 protein).ti,ab. or (alpha protein adj3 human).ti,ab. 2. sepsis/ or septicemia/ or bloodstream infection/ or septic shock/ or (sepsis or septic* or blood stream infection* or (shock adj3 (endotoxic or toxic))).ti,ab. 3. (randomized‐controlled‐trial/ or randomization/ or controlled‐study/ or multicenter‐study/ or phase‐3‐clinical‐trial/ or phase‐4‐clinical‐trial/ or double‐blind‐procedure/ or single‐blind‐procedure/ or (random* or cross?over* or multicenter* or factorial* or placebo* or volunteer*).mp. or ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask*)).ti,ab. or (latin adj square).mp.) not (animals not (humans and animals)).sh. 4. 1 and 2 and 3
Appendix 4. CINAHL (EBSCOhost) search strategy
S1 procalcitonin or (calcitonin N5 (precursor* or polyprotein* or polypeptide)) or ((CGRP* or CALC*) N5 protein) or (alpha protein N3 human) S2 ( (MH "Sepsis+") OR (MH "Shock, Septic+") ) OR AB ( (sepsis or septic* or blood stream infection* or (shock N3 (endotoxic or toxic))) ) S3 random* or ((clinical or controlled) N3 trial*) or placebo* or multicenter* or prospective or ((blind* or mask*) N5 (single or double or triple or treble)) S4 S1 and S2 and S3
Appendix 5. LILACS (BIREME) search strategy
(calcitonin$ and (precursor$ or polyprotein$ or polypeptide)) or procalcitonin
Appendix 6. Data extraction form
Extraction sheet
Effectiveness and safety of procalcitonin evaluation for reducing mortality in adult patients with sepsis, severe sepsis and septic shock
Study ID:
Date of study (year):
Review ID:
Reviewer:
Author (last name):
Locale of study:
I ‐ ACTION
II ‐ PARTICIPANTS
Participants
a. N:
b. Age:
c. Diagnosis (sepsis, severe sepsis, septic shock)
d. Baseline disease:
f. Gender:
g. Setting:
III ‐ INTERVENTIONS
Intervention group (procalcitonin evaluation)
Control group (other biomarkers or no biomarker)
IV ‐ OUTCOMES
(final or change from baseline values)
Primary outcomes
1. Mortality (at different time points)
Intervention group (procalcitonin evaluation)
n:
N:
Mean:
Standard deviation:
Other statistics (e.g. median, odds ratio)
Control group (other biomarkers or no biomarker)
n:
N:
Mean:
Standard deviation :
Other statistics (e.g. median, odds ratio)
Secondary outcomes
2. Hospital length of stay
Intervention group (procalcitonin evaluation)
n:
N:
Mean:
Standard deviation:
Other statistics (e.g. median, odds ratio)
Control group (other biomarkers or no biomarker)
n:
N:
Mean:
Standard deviation:
Other statistics (e.g. median, odds ratio)
3. Intensive care unit (ICU) length of stay
Intervention group (procalcitonin evaluation)
n:
N:
Mean:
Standard deviation:
Other statistics (e.g. median, odds ratio)
Control group (other biomarkers or no biomarker)
n:
N:
Mean:
Standard deviation:
Other statistics (e.g. median, odds ratio)
4. Clinical severity
Intervention group (procalcitonin evaluation)
n:
N:
Mean:
Standard deviation:
Other statistics (e.g. median, odds ratio)
Control group (other biomarkers or no biomarker)
n:
N:
Mean:
Standard deviation:
Other statistics (e.g. median, odds ratio)
5. New infection/Reinfection
Intervention group (procalcitonin evaluation)
n:
N:
Mean:
Standard deviation:
Other statistics (e.g. median, odds ratio)
Control group (other biomarkers or no biomarker)
n:
N:
Mean:
Standard deviation:
Other statistics (e.g. median, odds ratio)
6. Use of antimicrobial agents
Intervention group (procalcitonin evaluation)
n:
N:
Mean:
Standard deviation:
Other statistics (e.g. median, odds ratio)
Control group (other biomarkers or no biomarker)
n:
N:
Mean:
Standard deviation:
Other statistics (e.g. median, odds ratio)
V ‐ METHODOLOGICAL QUALITY OF STUDY
Please mark the appropriate item and describe the reason.
1. Was the allocation sequence adequately generated? (please provide an explanation)
·'Low risk of bias' when the authors of primary studies report methods to prevent bias.
'Unclear risk of bias' when the risk of bias is uncertain.
'High risk of bias' when the authors of primary studies clearly have not prevented the risk of bias.
2. Was allocation adequately concealed? (please provide an explanation)
'Low risk of bias' when the authors of primary studies report methods to prevent bias.
'Unclear risk of bias' when the risk of bias is uncertain.
'High risk of bias' when the authors of primary studies clearly have not prevented the risk of bias.
3. Was knowledge of the allocated intervention adequately prevented during the study? (please provide an explanation)
'Low risk of bias' when the authors of primary studies report methods to prevent bias.
'Unclear risk of bias' when the risk of bias is uncertain.
'High risk of bias' when the authors of primary studies clearly have not prevented the risk of bias.
4. Were incomplete outcome data adequately addressed? (please provide an explanation)
'Low risk of bias' when the authors of primary studies report methods to prevent bias.
'Unclear risk of bias' when the risk of bias is uncertain.
'High risk of bias' when the authors of primary studies clearly have not prevented the risk of bias.
5. Are reports of the study free of the suggestion of selective outcome reporting? (please provide an explanation)
'Low risk of bias' when the authors of primary studies report methods to prevent bias.
'Unclear risk of bias' when the risk of bias is uncertain.
'High risk of bias' when the authors of primary studies clearly have not prevented the risk of bias.
6. Was the study apparently free of other problems that could put it at high risk of bias? (please provide an explanation)
'Low risk of bias' when the authors of primary studies report methods to prevent bias.
'Unclear risk of bias' when the risk of bias is uncertain.
'High risk of bias' when the authors of primary studies clearly have not prevented the risk of bias.
VI ‐ Observation (including non‐published data)
Data and analyses
Comparison 1. PCT versus non‐PCT (standard care or CRP: primary outcomes).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality at longest follow‐up | 10 | 1156 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.65, 1.01] |
| 2 Mortality at 28 days | 4 | 324 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.59, 1.28] |
| 3 Mortality at ICU discharge | 3 | 506 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.50, 2.11] |
| 4 Mortality at hospital discharge | 7 | 805 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.75, 1.27] |
| 5 Time receiving antimicrobial therapy (days) ‐ mean (SD) | 4 | 313 | Mean Difference (IV, Random, 95% CI) | ‐1.28 [‐1.95, ‐0.61] |
| 6 Time receiving antimicrobial therapy (days) ‐ median (IQR) | Other data | No numeric data |
Comparison 2. PCT versus non‐PCT (standard care or CRP: secondary outcomes).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Hospital length of stay (days) ‐ median (IQR) or mean (SD) | Other data | No numeric data | ||
| 2 ICU length of stay (days) ‐ mean (SD) | 4 | 291 | Mean Difference (IV, Random, 95% CI) | ‐2.05 [‐3.14, ‐0.97] |
| 3 ICU length of stay (days) ‐ median (IQR) | Other data | No numeric data | ||
| 4 SOFA score during ICU stay | Other data | No numeric data | ||
| 5 SOFA score at day 3 | Other data | No numeric data | ||
| 6 SOFA score at day 5 | Other data | No numeric data | ||
| 7 SOFAmax score | Other data | No numeric data | ||
| 8 APACHE II score | Other data | No numeric data | ||
| 9 Reinfection (primary infection relapse) | 3 | 213 | Risk Ratio (M‐H, Random, 95% CI) | 1.84 [0.43, 7.89] |
| 10 Duration of mechanical ventilation (days) | Other data | No numeric data |
Comparison 3. PCT versus non‐PCT ‐ other outcomes of potential interest.
3.3. Analysis.
Comparison 3 PCT versus non‐PCT ‐ other outcomes of potential interest, Outcome 3 Mortality at 90 days.
| Mortality at 90 days | |||||
|---|---|---|---|---|---|
| Study | Procalcitonin (n/N) | non‐Procalcitonin (n/N) | RR (95% CI) | P‐value | Favoured group |
| Shehabi 2014 | 36/196 | 31/198 | 1.14 (0.73, 1.77) | 0.56 | non‐Procalcitonin |
3.4. Analysis.
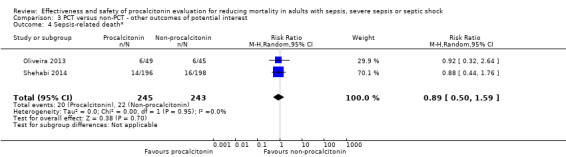
Comparison 3 PCT versus non‐PCT ‐ other outcomes of potential interest, Outcome 4 Sepsis‐related death*.
3.5. Analysis.
Comparison 3 PCT versus non‐PCT ‐ other outcomes of potential interest, Outcome 5 Septic shock‐related death.
| Septic shock‐related death | |||||
|---|---|---|---|---|---|
| Study | Procalcitonin (n/N) | non‐Procalcitonin (n/N) | OR (95% CI) | P‐value | Favoured group |
| Shehabi 2014 | 14/196 | 16/198 | 0.88 (0.44, 1.76) | 0.73 | non‐Procalcitonin |
3.6. Analysis.
Comparison 3 PCT versus non‐PCT ‐ other outcomes of potential interest, Outcome 6 Patients on antibiotics at day 5 (last information carried over for non‐survivors).
| Patients on antibiotics at day 5 (last information carried over for non‐survivors) | |||||
|---|---|---|---|---|---|
| Study | Procalcitonin (n/N) | non‐Procalcitonin (n/N) | OR (95% CI) | P‐value | Favoured group |
| Annane 2013 | 18/30 | 22/28 | 0.76 (0.54, 1.08) | 0.13 | Procalcitonin |
3.7. Analysis.
Comparison 3 PCT versus non‐PCT ‐ other outcomes of potential interest, Outcome 7 Patients on antibiotics at day 5 (non‐survivors considered as being treated with antibiotic).
| Patients on antibiotics at day 5 (non‐survivors considered as being treated with antibiotic) | |||||
|---|---|---|---|---|---|
| Study | Procalcitonin (n/N) | non‐Procalcitonin (n/N) | OR (95% CI) | P‐value | Favoured group |
| Annane 2013 | 21/30 | 23/28 | 0.85 (0.64, 1.14) | 0.28 | Procalcitonin |
3.8. Analysis.
Comparison 3 PCT versus non‐PCT ‐ other outcomes of potential interest, Outcome 8 Patients on antibiotics at day 1 (among survivals).
| Patients on antibiotics at day 1 (among survivals) | |||||
|---|---|---|---|---|---|
| Study | Procalcitonin (n/N) | non‐Procalcitonin (n/N) | OR (95% CI) | P‐value | Favoured group |
| Annane 2013 | 4/27 | 4/26 | 0.96 (0.27, 3.45) | 0.95 | Procalcitonin |
3.9. Analysis.
Comparison 3 PCT versus non‐PCT ‐ other outcomes of potential interest, Outcome 9 Patients on antibiotics at day 5 (survivors only).
| Patients on antibiotics at day 5 (survivors only) | |||||
|---|---|---|---|---|---|
| Study | Procalcitonin (n/N) | non‐Procalcitonin (n/N) | OR (95% CI) | P‐value | Favoured group |
| Annane 2013 | 18/27 | 21/26 | 0.83 (0.60, 1.14) | 0.25 | Procalcitonin |
3.10. Analysis.
Comparison 3 PCT versus non‐PCT ‐ other outcomes of potential interest, Outcome 10 Therapy withdrawn in hospital.
| Therapy withdrawn in hospital | |||||
|---|---|---|---|---|---|
| Study | Procalcitonin (n/N) | non‐Procalcitonin (n/N) | OR (95% CI) | P‐value | Favoured group |
| Shehabi 2014 | 38/196 | 35/198 | 1.10 (0.72, 1.66) | 0.66 | non‐Procalcitonin |
3.11. Analysis.
Comparison 3 PCT versus non‐PCT ‐ other outcomes of potential interest, Outcome 11 Appropriate empirical antibiotics.
| Appropriate empirical antibiotics | |||||
|---|---|---|---|---|---|
| Study | Procalcitonin (n/N) | non‐Procalcitonin (n/N) | OR (95% CI) | P‐value | Favoured group |
| Dharaniyadewi 2013 | 95/102 | 88/95 | 1.01 (0.93, 1.09) | 0.89 | non‐Procalcitonin |
3.14. Analysis.
Comparison 3 PCT versus non‐PCT ‐ other outcomes of potential interest, Outcome 14 Infection at day 3.
| Infection at day 3 | |||||
|---|---|---|---|---|---|
| Study | Procalcitonin (n/N) | non‐Procalcitonin (n/N) | OR (95% CI) | P‐value | Favoured group |
| Annane 2013 | 1/18 | 1/19 | 1.06 (0.07, 15.64) | 0.97 | non‐Procalcitonin |
3.15. Analysis.
Comparison 3 PCT versus non‐PCT ‐ other outcomes of potential interest, Outcome 15 Infection at day 5.
| Infection at day 5 | |||||
|---|---|---|---|---|---|
| Study | Procalcitonin (n/N) | non‐Procalcitonin (n/N) | OR (95% CI) | P‐value | Favoured group |
| Annane 2013 | 1/18 | 2/19 | 0.53 (0.05, 5.33) | 0.59 | Procalcitonin |
3.16. Analysis.
Comparison 3 PCT versus non‐PCT ‐ other outcomes of potential interest, Outcome 16 Infection at any time point after randomization.
| Infection at any time point after randomization | |||||
|---|---|---|---|---|---|
| Study | Procalcitonin (n/N) | non‐Procalcitonin (n/N) | OR (95% CI) | P‐value | Favoured group |
| Annane 2013 | 2/18 | 3/19 | 0.70 (0.13, 3.73) | 0.68 | Procalcitonin |
3.17. Analysis.
Comparison 3 PCT versus non‐PCT ‐ other outcomes of potential interest, Outcome 17 Nasal swabs positive for methicillin‐resistant Staphylococcus aureus.
| Nasal swabs positive for methicillin‐resistant Staphylococcus aureus | |||||
|---|---|---|---|---|---|
| Study | Procalcitonin (n/N) | non‐Procalcitonin (n/N) | OR (95% CI) | P‐value | Favoured group |
| Annane 2013 | 1/28 | 2/25 | 0.45 (0.04, 4.63) | 0.50 | Procalcitonin |
3.18. Analysis.
Comparison 3 PCT versus non‐PCT ‐ other outcomes of potential interest, Outcome 18 Rectal swabs positive for extended‐spectrum β‐lactamase‐resistant.
| Rectal swabs positive for extended‐spectrum β‐lactamase‐resistant | |||||
|---|---|---|---|---|---|
| Study | Procalcitonin (n/N) | non‐Procalcitonin (n/N) | OR (95% CI) | P‐value | Favoured group |
| Annane 2013 | 1/25 | 0/22 | 2.65 (0.11, 62.00) | 0.50 | non‐Procalcitonin |
3.19. Analysis.
Comparison 3 PCT versus non‐PCT ‐ other outcomes of potential interest, Outcome 19 Rectal swabs positive for Enterobacter, Klebsiella.
| Rectal swabs positive for Enterobacter, Klebsiella | |||||
|---|---|---|---|---|---|
| Study | Procalcitonin (n/N) | non‐Procalcitonin (n/N) | OR (95% CI) | P‐value | Favoured group |
| Annane 2013 | 0/24 | 0/24 | Not estimable | Not estimable | no group |
3.20. Analysis.
Comparison 3 PCT versus non‐PCT ‐ other outcomes of potential interest, Outcome 20 Readmission due to secondary infection.
| Readmission due to secondary infection | |||||
|---|---|---|---|---|---|
| Study | Procalcitonin (n/N) | non‐Procalcitonin (n/N) | OR (95% CI) | P‐value | Favoured group |
| Shehabi 2014 | 6/174 | 12/183 | 0.53 [0.20, 1.37] | 0.19 | Procalcitonin |
3.21. Analysis.
Comparison 3 PCT versus non‐PCT ‐ other outcomes of potential interest, Outcome 21 Isolates with multi‐resistant organisms.
| Isolates with multi‐resistant organisms | |||||
|---|---|---|---|---|---|
| Study | Procalcitonin (n/N) | non‐Procalcitonin (n/N) | OR (95% CI) | P‐value | Favoured group |
| Shehabi 2014 | 45/324 | 43/355 | 1.54 (1.00, 2.36) | 0.05 | non‐Procalcitonin |
3.22. Analysis.
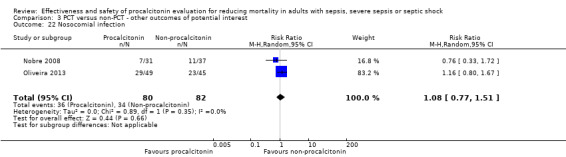
Comparison 3 PCT versus non‐PCT ‐ other outcomes of potential interest, Outcome 22 Nosocomial infection.
3.24. Analysis.
Comparison 3 PCT versus non‐PCT ‐ other outcomes of potential interest, Outcome 24 Antibiotic therapy‐free days (median, IQR) ‐ PCT vs standard care.
| Antibiotic therapy‐free days (median, IQR) ‐ PCT vs standard care | ||||
|---|---|---|---|---|
| Study | Procalcitonin, median (interquartile range [IQR]) | non‐Procalcitonin, median (interquartile range [IQR]) | P‐value | Favoued group |
| Annane 2013 | 0 (0–3) | 0 (0–2) | not available | Procalcitonin |
| Shehabi 2014 | 20 (11‐22) | 17 (7‐22) | 0.18 | Procalcitonin |
3.25. Analysis.
Comparison 3 PCT versus non‐PCT ‐ other outcomes of potential interest, Outcome 25 Antibiotic therapy‐free days ‐ PCT vs CRP.
| Antibiotic therapy‐free days ‐ PCT vs CRP | |||
|---|---|---|---|
| Study | Procalcitonin, median/1,000 live days (interquartile range [IQR]) | C‐Reactive Protein, median/1,000 live days (interquartile range [IQR]) | P‐value |
| Oliveira 2013 | 357.1 (0–541) | 357.14 (33.3–509.2) | 0.998 |
3.26. Analysis.
Comparison 3 PCT versus non‐PCT ‐ other outcomes of potential interest, Outcome 26 Duration of first episode of antibiotic treatment (days)*.
| Duration of first episode of antibiotic treatment (days)* | |||||
|---|---|---|---|---|---|
| Study |
Procalcitonin Mean (SD), N = 49 |
non‐Procalcitonin Mean (SD), N = 45 |
Mean difference (95% CI) | P‐value | Favoured group |
| Oliveira 2013 | 8.1 (3.7) | 7.2 (3.5) | 0.90 (‐0.56, 2.36) | 0.23 | Procalcitonin |
3.27. Analysis.
Comparison 3 PCT versus non‐PCT ‐ other outcomes of potential interest, Outcome 27 Days of antibiotic exposure per 1000 inpatient days.
| Days of antibiotic exposure per 1000 inpatient days | |||||
|---|---|---|---|---|---|
| Study | Log [risk ratio] | Standard error | RR (95% CI) | P‐value | Favoured group |
| Oliveira 2013 | ‐0.079 | 0.059 | 0.92 (0.82, 1.04) | 0.18 | Procalcitonin |
3.31. Analysis.
Comparison 3 PCT versus non‐PCT ‐ other outcomes of potential interest, Outcome 31 Time to antibiotic cessation at day 28.
| Time to antibiotic cessation at day 28 | |||||
|---|---|---|---|---|---|
| Study |
Procalcitonin Median, median (interquartile range [IQR]) (n = 196) |
non‐procalcitonin, median (interquartile range [IQR]) (n = 198) |
Hazard‐ratio (95% CI) | P | Favoured group |
| Shehabi 2014 | 9 [6‐20] | 11 [6‐22] | 1.06 (0.85‐1.33) | 0.59 | Procalcitonin |
3.33. Analysis.
Comparison 3 PCT versus non‐PCT ‐ other outcomes of potential interest, Outcome 33 Antibiotic therapy‐free days according to different subgroups.
| Antibiotic therapy‐free days according to different subgroups | ||||
|---|---|---|---|---|
| Study | Procalcitonin, median (interquartile range [IQR]) | non‐Procalcitonin, median (interquartile range [IQR]) | P‐value | Favoured group |
| Suspected sepsis | ||||
| Shehabi 2014 | 9 (6‐17) | 11 (6‐18) | 0.74 | non‐procalcitonin |
| Suspected septic shock | ||||
| Shehabi 2014 | 9 (6‐22) | 11 (6‐24) | 0.64 | no‐procalcitonin |
| Confirmed positive culture | ||||
| Shehabi 2014 | 13 (7‐27) | 13 (8‐26) | 0.77 | none |
| Negative culture | ||||
| Shehabi 2014 | 8 (4‐12) | 7 (4‐15) | 0.94 | procalcitonin |
| Positive blood culture | ||||
| Shehabi 2014 | 14 (8‐23) | 15 (7‐27) | 0.39 | non‐procalcitonin |
| Positive pulmonary culture | ||||
| Shehabi 2014 | 11 (7‐27) | 15 (8‐27) | 0.33 | non‐procalcitonin |
3.34. Analysis.
Comparison 3 PCT versus non‐PCT ‐ other outcomes of potential interest, Outcome 34 World Health Organization daily defined dose per 100 occupied bed days.
| World Health Organization daily defined dose per 100 occupied bed days | |||||
|---|---|---|---|---|---|
| Study |
Procalcitonin Mean (SD) (N = 196) |
non‐procalcitonin Mean (SD) (N = 198) |
Mean difference (95% CI) | P‐value | Favoured group |
| Shehabi 2014 | 135 (93) | 139 (98) | ‐4.00 (‐22.86, 14.86) | 0.68 | Procalcitonin |
3.35. Analysis.
Comparison 3 PCT versus non‐PCT ‐ other outcomes of potential interest, Outcome 35 Antibiotics maintained for 7 days because of bacteraemia and/or a SOFA score above 10 at inclusion.
| Antibiotics maintained for 7 days because of bacteraemia and/or a SOFA score above 10 at inclusion | |||||
|---|---|---|---|---|---|
| Study | Procalcitonin (n/N) | non‐Procalcitonin (n/N) | RR (95% CI) | P‐value | Favoured group |
| Oliveira 2013 | 17/49 | 8/45 | 1.95 (0.93, 4.08) | 0.08 | non‐Procalcitonin |
3.36. Analysis.
Comparison 3 PCT versus non‐PCT ‐ other outcomes of potential interest, Outcome 36 Antibiotic therapy discontinuation in the first episode of infection*.
| Antibiotic therapy discontinuation in the first episode of infection* | |||
|---|---|---|---|
| Study | Hazard ratio (95% CI) | P‐value | Favoured group |
| Oliveira 2013 | 1.206 (0.774–1.3) | 0.1 | C‐reactive protein |
3.37. Analysis.
Comparison 3 PCT versus non‐PCT ‐ other outcomes of potential interest, Outcome 37 Protocol overruling.
| Protocol overruling | |||||
|---|---|---|---|---|---|
| Study | Procalcitonin (n/N) | non‐Procalcitonin (n/N) | RR (95% CI) | P‐value | Favoured group |
| Oliveira 2013 | 6/49 | 7/45 | 0.79 (0.29, 2.17) | 0.64 | Procalcitonin |
Comparison 4. PCT versus non‐PCT (standard care or CRP: ITT analysis).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality at longest follow‐up (imputing mortality for missing participants) | 10 | 1215 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.73, 1.32] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Annane 2013.
| Methods |
Study design: multi‐centre, parallel, randomized, single‐blind, controlled trial Setting: 8 ICUs in France |
|
| Participants |
Inclusion criteria: all ICU patients with severe or septic shock presenting systemic inflammatory response syndrome, acute dysfunction of at least 1 organ, absence of indisputable clinical infection and negative microbial cultures, all of them for < 48 hours Exclusion criteria: pregnancy, burns over ≥ 15% of body surface area, trauma, outpatient or inpatient cardiac arrest, post‐orthopaedic surgery status drug‐related neutropenia, withdrawal of life‐supportive therapies or a decision to withhold them, indisputable clinical infection or antibiotic exposure ≥ 48 hours during the time shortly before ICU admission |
|
| Interventions |
Group 1 (N = 31): Both initiation and discontinuation of antibiotics were guided by a PCT‐based algorithm applied at 6 hours and on day 3 and day 5 post randomization. Antibiotic therapy was not to be started or was to be halted when PCT was < 0.25 μg/L, was strongly discouraged when PCT was ≥ 0.25 to < 0.5 μg/L, was recommended when PCT was ≥ 0.5 to < 5 μg/L and was strongly recommended when PCT was ≥ 5 μg/L. For participants enrolled in the 48‐hour postoperative period, respective PCT cut‐offs were < 4 μg/L, ≥ 4 to < 9 μg/L and ≥ 9 μg/L. Investigators were strongly asked not to over‐rule the algorithm every day up to study day 5. Group 2 (N = 31): In the control arm, the decision to start or stop antibiotic therapy was made at the discretion of the participant's physician, without knowledge of the participant’s PCT concentrations. |
|
| Outcomes |
|
|
| Conflicts of interest and/or funding | None detected | |
| Notes |
Sample size: Study authors estimated that on day 5, ∼ 85% of control participants would be taking antibiotics. Thus, they calculated that 57 participants in each arm would be needed to detect in a 2‐sided test with an 80% probability and a 0.05 type I error a 25% absolute reduction in the proportion of antibiotic‐treated participants on day 5. They also estimated that 20% of participants would eventually be withdrawn from the study after showing indisputable infection. Thus, 140 participants in total (70 in each arm) would be needed. PCT measures: PCT levels were measured with the BRAHMS PCT‐sensitive KRYPTOR assay |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomized in a 1:1 ratio according to a computer‐generated list and were stratified by centre and according to whether or not participants underwent surgery in the past 48 hours, using permutation blocks. |
| Allocation concealment (selection bias) | Low risk | Randomization was centralized through a secured website and was performed by an independent statistician. The sizes of the strata remained unknown to investigators. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Masking of antibiotic therapy was not feasible in this study, but In the control arm, participants, physicians, nurses, investigators, study co‐ordinators, the statistician and the sponsor remained blinded to PCT levels throughout the study. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | In the control arm, participants, physicians, nurses, investigators, study co‐ordinators, the statistician and the sponsor remained blinded to PCT levels throughout the study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Although 6.45% (4/62) of all participants withdrew after randomization (PCT, n = 1; non‐PCT, n = 3), study authors made available a clear flow of participants, permitting both intention‐to‐treat and available case analyses. |
| Selective reporting (reporting bias) | Low risk | Clinically relevant outcomes were analysed and were previously planned in the clinicaltrials.gov study (NCT01025180). |
| Other bias | Low risk | None was suspected. |
Deliberato 2013.
| Methods |
Study design: parallel‐group, open‐label, randomized controlled trial Setting: ICU of a tertiary care, private hospital in São Paulo, Brazil. This open‐model ICU is a 38‐bed medical‐surgical unit where approximately 2200 patients are admitted each year. |
|
| Participants |
Inclusion criteria: patient with microbiologically confirmed infection (blood, urine, tracheal aspirate or bronchoalveolar lavage fluid cultures) with sepsis, severe sepsis and septic shock (ACCP/SCCM Consensus Conference Committee 1992 criteria). More than 50% of patients had bloodstream infection. Exclusion criteria: (1) start of antibiotic therapy more than 48 hours before the date when cultures were performed; (2) participants younger than 18 years of age; (3) known pregnancy; (4) infection requiring prolonged antibiotic therapy, such as bacterial endocarditis, hepatic or brain abscess, deep abscess, mediastinitis and osteomyelitis; (5) severe infection caused by viruses, parasites, fungi or mycobacteria; (6) chronic localized infection, such as chronic osteomyelitis or chronic prostatitis; (7) patients without indication for ICU admission, as determined by the attending physician; and (8) negative cultures (blood, urine, tracheal aspirate or bronchoalveolar lavage fluid) in participants with suspected sepsis, severe sepsis or septic shock |
|
| Interventions |
Group 1 (N = 42): PCT group had PCT and CRP levels measured at day 0 (bacteraemia), 5 or 7 (if positive blood culture), and then every 48 hours until hospital discharge or death or until antibiotics were stopped. A predefined PCT protocol was used together with the clinical outcome to guide the physician's decision to discontinue antibiotics. The PCT protocol encouraged the physician to discontinue the antibiotics when (1) PCT dropped more than 90% from peak level or (2) an absolute value < 0.5 ng/mL was reached. Investigators did not interfere with the duration of prescribed antibiotic therapy. Group 2 (N = 39): standard care in which all participants received antibiotic therapy based on the possible source of infection and the local susceptibility profile, as prescribed by the attending physician. By our local hospital policy, participants in the ICU cannot receive antibiotic therapy for longer than 14 days unless they have been specified as needing a prolonged duration of antibiotic therapy as the standard of care. Investigators did not interfere with the duration of prescribed antibiotic therapy. |
|
| Outcomes |
|
|
| Conflicts of interest and/or funding | None was detected. | |
| Notes |
Sample size: Study authors estimated that inclusion of 29 participants in each study group would yield 90% power to detect a 40% reduction in exposure to the antibiotic, with a 2‐tailed test of significance set at 0.05. PCT measures: automated test ‐ VIDAS®BRAHMS PCT from bioMérieux (Rhône, France) Questions to study authors How do you define intention‐to‐treat analysis (ITT)? How do you define per‐protocol analysis? Data showed 2 (4.8%) and 4 (10.3%) in‐hospital mortalities by ITT analysis, respectively, in PCT and non‐PCT groups. How did you find these numbers? Did you receive information from excluded participants ? |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Folders were randomly and blindly assigned as “PCT group” or “standard group”. |
| Allocation concealment (selection bias) | High risk | 2 study authors randomly drew 1 folder from a black box containing 100 folders (50 “PCT group” and 50 “control group”). |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | investigators were aware of assignments. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | investigators were aware of assignments. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes were previously planned in the trial registered as NCT01494675. Study authors made available a clear flow of participants within the study. Additionally, they offered both intention‐to‐treat and available case analyses. |
| Selective reporting (reporting bias) | Low risk | Relevant outcomes were evaluated. |
| Other bias | Low risk | None was suspected. |
Dharaniyadewi 2013.
| Methods |
Study design: parallel‐group, randomized controlled trial Setting: emergency department in internal medicine (Cipto Mangunkusomo Hospital, Indonesia) |
|
| Participants |
Inclusion criteria: septic participants (with at least 2 concomitant systemic inflammatory response syndrome criteria) older than 18 years with and without signs of organ hypoperfusion or dysfunction Exclusion criteria: not informed by study authors |
|
| Interventions |
Group 1 (N = 100): semiquantitative PCT‐examined patients Group 2 (N = 105): standard care: Semi‐quantitative PCT test results will be informed to physicians taking care of participants |
|
| Outcomes |
|
|
| Conflicts of interest and/or funding | None was detected. | |
| Notes |
Sample size: not informed PCT measures: not informed A tropical infection consultant assessed the appropriateness of empirical antibiotics on the basis of Pedoman Umum Penggunaan Antibiotik Departemen Kesehatan Republik Indonesia. We had no success in contacting study authors. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information was provided by study authors. |
| Allocation concealment (selection bias) | Unclear risk | No information was provided by study authors. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information was provided by study authors. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information was provided by study authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study authors made clear the flow diagram of participants within the study (per‐protocol analysis). Outcomes were previously planned in the trial registered as NCT01862185. |
| Selective reporting (reporting bias) | High risk | Relevant outcomes were evaluated. |
| Other bias | High risk | Study authors did not provide detailed PCT algorithm for dealing with antimicrobial therapies. |
Hochreiter 2009.
| Methods |
Study design: parallel‐group, randomized controlled trial Setting: surgical intensive care ward at the West Coast Hospital Heide (Germany) |
|
| Participants |
Inclusion criteria: participants requiring antibiotic therapy on the basis of confirmed or highly suspected bacterial infection and at least 2 concomitant SIRS criteria Exclusion criteria: not informed by study authors |
|
| Interventions |
Group 1 (N = 57): PCT‐guided antibiotic regimen based on confirmed or highly suspected bacterial infection. Antibiotic therapy was discontinued if clinical signs and symptoms of infection improved and PCT decreased to less than 1 ng/mL, or if the PCT value was greater than 1 ng/m: but had dropped to 25% to 35% of the initial value over 3 days. The physician in charge had the option to proceed with or adjust the antibiotic treatment if he or she had clinical reasons to do so, at any time point. Group 2 (N = 53): standard antibiotic regimen also based on confirmed or highly suspected bacterial infection. Antibiotic treatment was applied as standard regimen over 8 days. Also, the physician in charge had the option to proceed with or adjust the antibiotic treatment if he or she had clinical reasons to do so, at any time point. |
|
| Outcomes |
|
|
| Conflicts of interest and/or funding | SS has served as consultant and has received payments from BRAHMS AG for speaking engagements. | |
| Notes |
Sample size: not informed PCT measures: by BRAHMS PCT LIA® (BRAHMS Aktiengesellschaft, Hennigsdorf, Germany) We had no success in contacting study authors. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not informed |
| Allocation concealment (selection bias) | High risk | An open‐label study as reported by study authors |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | An open‐label study as reported by study authors |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | An open‐label study as reported by study authors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study authors made available a clear flow of participants within the study (all participants were analysed). |
| Selective reporting (reporting bias) | Low risk | Relevant outcomes were evaluated. |
| Other bias | Low risk | None was suspected. |
Liu 2013.
| Methods |
Study design: parallel‐group, randomized controlled trial Setting: 1 University ICU (First Hospital of Jilin Universit, China) |
|
| Participants |
Inclusion criteria: participants aged ≥ 18 years with suspected bacterial sepsis, severe sepsis and septic shock, according to ACCP/SCCM Consensus Conference Committee 1992) criteria Exclusion criteria: bacterial culture results for Pseudomonas aeruginosa, Aeromonas, Acinetobacter baumannii, Mycobacterium tuberculosis or fungal infection; suspected virus or parasite infection; chronic localized infection; more than 48‐hour antimicrobial drug treatment; immunodeficiency (HIV, white blood disease); patients with cancer |
|
| Interventions |
Group 1 (N = 42): Antibiotic therapy was guided by PCT results on a daily basis. When no active symptoms of infection were shown; acute physiology and APACHE Ⅱ scores declined; and PCT values decreased by more than 90% or PCT value was lower than 0.25 µg/L ‐ selected as drug withdrawal Group 2 (N = 40): regular antimicrobial therapy |
|
| Outcomes |
|
|
| Conflicts of interest and/or funding | None was detected. | |
| Notes |
Sample size: No information was provided. PCT measures: Study authors followed‐up discharged participants by telephone. We had no success in contacting study authors. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not informed |
| Allocation concealment (selection bias) | Unclear risk | Not informed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not informed |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not informed |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No clear information was available about the flow of participants (all participants informed in the study were analysed). |
| Selective reporting (reporting bias) | Low risk | Relevant outcomes were evaluated. |
| Other bias | Low risk | None was suspected. |
Nobre 2008.
| Methods |
Study design: parallel‐group, open‐label, randomized controlled trial Setting: tertiary care, University Hospitals of Geneva (Switzerland) |
|
| Participants |
Inclusion criteria: all patients with suspected severe sepsis or septic shock admitted to the ICU (32‐bed, mixed medical and surgical adult patients, with 3200 admissions per year). Patients developing severe sepsis or septic shock during their ICU stay were also considered for enrolment. Study authors used Bone 1992 criteria and reported 42% severe sepsis and 43% septic shock. Exclusion criteria: (1) microbiologically documented infection caused by Pseudomonas aeruginosa, Acinetobacter baumanni, Listeria spp.,Legionella pneumophila, Pneumocystis jiroveci or Mycobacterium tuberculosis, for which a prolonged duration of antibiotic therapy is standard of care (17); (2) severe infection due to viruses or parasites (e.g. haemorrhagic fever, malaria); (3) infectious condition requiring prolonged antibiotic therapy (e.g. bacterial endocarditis, brain abscess, deep abscesses); (4) antibiotic therapy started 48 hours or longer before enrolment; (5) chronic localized infection (e.g. chronic osteomyelitis); (6) severely immunocompromised patients, such as those infected with human immunodeficiency virus and with a CD4 count less than 200 cells/mm3, neutropenic patients (500 neutrophils/mm3)or patients receiving immunosuppressive therapy after solid organ transplantation; (7) withholding of life support; (8) absence of antimicrobial treatment despite clinical suspicion of sepsis |
|
| Interventions |
Group 1 (N = 39): For participants with a favourable clinical course, investigators used predefined ‘‘stopping rules’’ based on circulating PCT levels to encourage caregivers to discontinue antibiotics. Participants with baseline PCT level greater than or equal to 1 mg/L were reevaluated at day 5. Investigators encouraged treating physicians to discontinue antibiotics when (1) PCT dropped by more than 90% from the baseline peak level, or (2) an absolute value below 0.25 mg/L was reached. Participants with PCT levels below 1 mg/L at baseline were reevaluated at day 3; treating physicians were encouraged to discontinue antibiotics when the PCT level was below 0.1 mg/L and careful clinical evaluation ruled out severe infection. However, the final decision concerning antibiotic therapy duration was always left to the discretion of the physician in charge. Group 2 (N = 40): standard practice, whereby participants received initial antibiotic therapy based on local guidelines and susceptibility patterns, according to the decision of the treating physician, who was unaware of the participant’s initial PCT levels; the final decision concerning antibiotic therapy duration was always left to the discretion of the physician in charge. |
|
| Outcomes |
|
|
| Conflicts of interest and/or funding | SH and JP received speaker honoraria from BRAHMS AG. | |
| Notes |
Sample size: The trial was designed to enrol at least 66 participants, to obtain power of 90% to detect a 33% (4 day) difference in duration of antibiotic therapy for the initial infection between the 2 groups based on an estimated baseline duration of 12 days. We assumed a standard deviation (SD) of 5 days in both groups and an a error of 0.05. PCT measures: Kryptor‐PCT (Brahms Diagnostica, Hennigsdorf, Germany) Other notes:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐based random number generation |
| Allocation concealment (selection bias) | Low risk | Allocation was issued by using opaque, sealed, numbered envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label RCT |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label RCT |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Primary endpoints were first analysed on the basis of an intention‐to‐treat analysis, including all randomized participants. Additionally, study authors made available a clear flow of participants within the study. |
| Selective reporting (reporting bias) | Low risk | Outcomes were previously planned in clinicaltrials.gov NCT 00250666. Relevant outcomes were evaluated. |
| Other bias | Low risk | None was suspected. |
Oliveira 2013.
| Methods |
Study design: parallel‐group, open‐label, randomized controlled trial Setting: 2 teaching ICUs (Brazil) |
|
| Participants |
Inclusion criteria: all adult patients 18 years of age or older with suspected severe sepsis or septic shock (according to Bone 1992; Levy 2003 criteria). Study authors reported 63% severe sepsis and 55.6% septic shock. Exclusion criteria: (1) confirmed microbiological infection by Pseudomonas aeruginosa, Acinetobacter baumannii,Listeria species,Mycobacterium tuberculosis or fungi; (2) Staphylococcus aureus bacteraemia; (3) suspected or confirmed severe infection caused by virus or parasite; (4) infection that required long‐term treatment, regardless of the etiologic agent (e.g. bacterial endocarditis); (5) localized chronic infection (e.g. chronic osteomyelitis); (6) more than 48 hours of antibiotic treatment; (7) immunosuppressed patients (such as those diagnosed with HIV), patients with neutropenia (less than 500 neutrophils/mm3), patients post solid organ transplant, patients receiving immunosuppressive therapy and patients who received more than 1 mg/kg of prednisone or equivalent; (8) patients under palliative care; (9) patients who suffered multiple trauma, burns or major surgery in the previous 5 days; (10) patients given a diagnosis of pulmonary neoplasia, carcinoid tumour or medullary tumour of the thyroid; and (11) patients who remained in the ICU for no longer than 24 hours |
|
| Interventions |
Group 1 (N = 50): protocol based on serum PCT levels. Daily measurements, every 48 hours for 2 measurements in participants remaining in the ICU, then every 5 days. The duration of antibiotic therapy was based on circulating PCT levels. Investigators proposed interruption of antibiotics if a relative reduction of 90% in baseline PCT levels, or if an absolute value lower than 0.1 ng/mL, was reached. Group 2 (N = 47): protocol based on serum CRP levels. Daily measurements, every 48 hours for 2 measurements in participants remaining in the ICU, then every 5 days. The duration of antibiotic therapy was based on circulating CRP levels. Investigators proposed interruption of antibiotics if a relative reduction of 50% in baseline CRP levels, or if a value lower than 25 mg/dL, was reached. |
|
| Outcomes |
Participants were followed up for 28 days, or until death or hospital transference, whichever came first. |
|
| Conflicts of interest and/or funding | Dr Nobre was paid for lectures by bioMérieux. | |
| Notes |
Sample size: based on duration of antibiotic therapy in participants treated with a PCT‐guided protocol; would be at least 25% shorter than the duration observed in participants treated according to a protocol based on serum CRP levels, resulting in 58 participants per group ‐ totalling 116 individuals (power of 80% and alpha error of 5%) PCT measures: Vidas BRAHMS PCT (bioMérieux, Lyon, France) CRP measures: Reactive test VITROS (Johnson & Johnson Clinical Diagnostics, Inc., Rochester, NY) was used to quantitatively measure the concentration of serum or plasma CRP. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random computer‐generated numbers. Sealed opaque envelopes were used for randomization. |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes were used for randomization. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label RCT |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label RCT |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study authors made available a clear flow of participants within the study (per‐protocol analysis). |
| Selective reporting (reporting bias) | Low risk | Relevant outcomes were evaluated. Protocol was registered at ClinicalTrials.org (NCT00934011). |
| Other bias | Low risk | None was suspected. |
Schroeder 2009.
| Methods |
Study design: parallel‐group, open‐label, randomized controlled trial Setting: ICU of the Department of Anesthesiology and Intensive Care Medicine at Westküstenklinikum Heide (Germany) |
|
| Participants |
Inclusion criteria: patients after abdominal surgery and after the start of antibiotic treatment with the diagnosis of severe sepsis (according to ACCP/SCCM Consensus Conference Committee 1992 criteria) Exclusion criteria: patients who did not meet the inclusion criteria, who refused informed consent or who already had received antibiotic treatment before admission to the ICU |
|
| Interventions |
Group 1 (n = 14): Antibiotic therapy was discontinued if clinical signs and symptoms of sepsis improved and PCT values decreased to 1 ng/mL or less or dropped to 25% to 35% of the initial PCT concentration over 3 consecutive days, but the physician in charge was always free to decide whether to continue or change the antibiotic regimen upon clinical judgement. Daily standard routine laboratory analysis including C‐reactive protein (CRP) was performed. Participants were also subjected to daily standard routine laboratory analysis, including CRP. Group 2 (n = 13): Antibiotic treatment was discontinued according to clinical signs and empirical rules, but the physician in charge was always free to decide whether to continue or change the antibiotic regimen upon clinical judgement. Participants were also subjected to daily standard routine laboratory analysis, including CRP, in the same way as those in the PCT group. |
|
| Outcomes |
|
|
| Conflicts of interest and/or funding | The corresponding author declared speaking engagements for BRAHMS AG. | |
| Notes |
Sample size: Duration of antibiotic therapy for participants treated with a PCT‐guided protocol would be at least 25% shorter than the duration observed in participants treated according to a protocol based on serum CRP levels, resulting in 58 participants per group ‐ totalling 116 individuals (power of 80% and alpha error of 5%). PCT measures: BRAHMS PCT LIA® ‐ B.R.A.H.M.S. Aktiengesellschaft, Hennigsdorf (Germany) We had no success in contacting study authors. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not informed |
| Allocation concealment (selection bias) | Unclear risk | Not informed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not informed |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not informed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study authors made available a clear flow of participants within the study (all participants were analysed). |
| Selective reporting (reporting bias) | Low risk | Relevant outcomes were evaluated. |
| Other bias | Low risk | None was suspected. |
Shehabi 2014.
| Methods |
Study design: parallel‐group, randomized controlled trial Setting: 11 general academic and non‐academic ICUs (Australia) |
|
| Participants |
Inclusion criteria: patients >18 years of age, admitted to ICU within previous 72 hours, receiving parenteral and/or enteral antibiotics for a suspected bacterial infection (2) (with 2 or more systemic inflammatory response syndrome criteria) and expected to remain in the ICU for longer than 24 hours Exclusion criteria: patients receiving antibiotics for surgical prophylaxis or with proven bacterial infection requiring more than 3 weeks of antibiotic therapy, with isolated systemic fungal or systemic viral infection in the absence of bacterial infection, with neutropenia with a neutrophil count less than 1000 cells/mm3, receiving immunosuppressive agents, undergoing cardiac surgery or trauma or heat stroke within 48 hours, with medullary thyroid or small cell lung cancer, not expected to survive to hospital discharge or with known pregnancy |
|
| Interventions |
Group 1 (N = 200): Clinicians could order additional PCT levels after day 7 at their discretion. Daily PCT results were made available to the treating clinician. Antibiotics were prescribed according to Australian Antibiotics Therapeutic Guidelines (24) and antimicrobial stewardship (implemented by infectious diseases twice‐weekly rounds and on the basis of need consultations). Physicians were recommended to cease antibiotics if initial or any subsequent PCT was negative at level < 0.10 ng/mL; if initial or any subsequent PCT was borderline ‐ level 0.10 to 0.25 ng/mL ‐ and infection was highly unlikely; or if subsequent PCT level declined by more than 90% from baseline. Investigators sought to assess antibiotic appropriateness and/or adequacy of source control if PCT level at 48 hours was greater than 70% of baseline value. Group 2 (N = 200): standard care, with clinicians blinded to PCT levels; results were faxed directly to the Clinical Informatics and Data Management Unit, Department of Epidemiology and Preventive Medicine. Antibiotics were prescribed according to the Australian Antibiotics Therapeutic Guidelines and antimicrobial stewardship (implemented by infectious diseases twice‐weekly rounds and on the basis of need consultations). |
|
| Outcomes |
|
|
| Conflicts of interest and/or funding | Material support was provided by Roche Diagnostics, Thermo Fisher Scientific and BioMérieux. Roche Diagnostics and Thermo Fisher Scientific provided additional unrestricted grant funding. | |
| Notes | Sample size: Study authors assumed a 25% (2.3 days) reduction in antibiotic treatment from baseline of 9 days. To further account for potential dropout or loss to follow‐up (anticipated to be < 5%), a total of 400 participants were recruited (sample power of 90%). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization (via a secured central study website) by block (1:1 ratio) and stratified according to the presence of septic shock (defined by receipt of inotrope and/or any vasopressors within the previous 24 hours) |
| Allocation concealment (selection bias) | Low risk | Randomization via a secured central study website |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Physicians who treated participants were aware of the assignments. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Although data management was conducted by a central body with a blinded statistician, physicians who collected data were aware of the assignments. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study authors made available a clear flow of participants within the study (per‐protocol analysis). |
| Selective reporting (reporting bias) | Low risk | Relevant outcomes were evaluated. Protocol was registered in the ANZRC 01111‐1117‐1760 (ACTRN12610000809033). |
| Other bias | Low risk | None was suspected. |
Svoboda 2007.
| Methods |
Study design: parallel‐group, randomized controlled trial Setting: 04 traumatological ICUs (Czech) |
|
| Participants |
Inclusion criteria: multiple trauma participants aged ≥ 18 years who developed sepsis (according to Bone 1992 criteria). Study authors reported septic shock in 71% and 68% in PCT vs non‐PCT comparison groups, respectively. Exclusion criteria: patients with chemical or burn trauma; with death perceived to be imminent; or who had been designated as "not full support" or "do not resuscitate" |
|
| Interventions |
Group 1 (N = 38): treatment decision according to PCT level. For severe sepsis with PCT > 2 ng/mL and signalized bacteraemia, clinicians were motivated to change antibiotics and catheters; participants with PCT ≤ 2 ng/mL and confirmed localized infection were subjected to ultrasonography and/or CT, followed by repeated surgical treatment (drainage, reoperation). Group 2 (N = 34): standard evaluation of all parameters by consultant surgeon according to treatment protocol of the health service. Standard supportive care, broad‐spectrum antibiotics and change of intravascular catheters were provided to all septic patients according to evidence‐base guidelines. |
|
| Outcomes |
|
|
| Conflicts of interest and/or funding | None was suspected. | |
| Notes |
Sample size: not informed PCT measures: PCT‐Q, B.R.A.H.M.S., Hennigsdorf (Germany) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number table |
| Allocation concealment (selection bias) | Low risk | Study authors used opaque sealed numbered envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not informed |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not informed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study authors made available a clear flow of participants within the study (all participants were analysed). |
| Selective reporting (reporting bias) | Low risk | Relevant outcomes were evaluated. |
| Other bias | Low risk | None was suspected. |
ACCP: American College of Chest Physicians.
APACHE: Acute Physiology and Chronic Health Evaluation.
CD4: cluster of differentiation 4.
CRP: C‐reactive protein.
DDD: daily defined doses.
HIV: human immunodeficiency virus.
ICU: intensive care unit.
IL: interleukin.
IRR: incidence rate ratio.
ITT: intention‐to‐treat analysis.
mg/dL: milligrams per decilitre.
MIC: minimum inhibitory concentration.
mL: millilitre.
N: total number of participants.
ng/mL: nanogram per millilitre.
PCT: procalcitonin.
pg/mL: picogram/ millilitre.
PO2: partial pressure of oxygen.
RCT: randomized controlled trial.
SCCM: Society of Critical Care Medicine.
SD: standard deviation.
SIRS: systemic inflammatory response syndrome.
SOFA: sequential organ failure assessment.
TNF: tumour necrosis factor.
μg/L: microgram per litre.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bodmann 2016 | Retrospective study design |
| Bouadma 2010 | RCT. Inclusion criteria not specific for sepsis, severe sepsis and/or septic shock (participants with suspected bacterial infection, with no clear definition for diagnosis of sepsis) |
| Bréchot 2015 | Economic analysis |
| Harrison 2015 | Economic analysis |
| Jensen 2011 | RCT with critically ill participants. Inclusion criteria not specific for sepsis, severe sepsis and/or septic shock |
| Kiehntopf 2011 | Retrospective study design |
| Kip 2015 | Economic evaluation |
| Kolici 2013 | RCT in neonates |
| Kopterides 2010 | Systematic review |
| Layios 2012 | RCT. Inclusion criteria not specific for sepsis, severe sepsis and/or septic shock |
| Lima 2016 | RCT. Inclusion criteria strict for febrile neutropenia |
| Mann 2011 | Systematic review |
| Pantelidou 2015 | Narrative review |
| Prkno 2013 | Systematic review |
| Sandifer 2012 | Systematic review |
| Schuetz 2011 | Systematic review |
| Schuetz 2013 | Narrative review |
| Soni 2013 | Systematic review |
| Stocker 2010a | RCT in neonates |
| Stocker 2010b | RCT in neonates |
| Ternhag 2010 | Narrative review for chronic obstructive pulmonary disease |
| Westwood 2015 | Economic evaluation |
RCT: randomized controlled trial.
Characteristics of studies awaiting assessment [ordered by study ID]
Bloos 2016.
| Methods |
Study design: multi‐centre, randomized, placebo‐controlled trial Setting: ICUs from 35 hospitals in Germany |
| Participants |
Inclusion criteria: severe sepsis/septic shock (ACCP/SCCM criteria), onset < 24 hours), ≥ 18 years of age Exclusion criteria: pregnancy or breast‐feeding, fertile female women not using contraceptive treatment, concomitant participation in any study (last 30 days), previous participation in this trial, selenium intoxication, no compliance with instructions of the study, imminent possibility of death due to coexisting disease(s), relationship of the participant to one or more members of the study team, long‐lasting duration of antimicrobial therapy due to infectious diseases (i.e. endocarditis, tuberculosis, malaria, etc), immunocompromised participants |
| Interventions |
Group 1 (N = 552): PCT‐based algorithm, both with and without sodium‐selenite Group 2 (N = 537): no PCT‐based algorithm (antimicrobial therapy according to the discretion of the treating physician), both with and without sodium‐selenite Sodium‐selenite 1000 µg/d until the end of ICU treatment or for ≤ 21 days PCT was measured at randomization and at days 4, 7, 10 and 14. |
| Outcomes |
|
| Notes |
Sample size: not available PCT measures: (BRAHMS, Germany; PCT‐sensitive KRYPTOR Compact) Financial support/funding: Kompetenznetz Sepsis, Biosyn, Brahms AG |
de Jong 2016.
| Methods |
Study design: multi‐centre, randomized, open‐label, controlled trial Setting: ICUs from 15 hospitals in the Netherlands. |
| Participants |
Inclusion criteria: > 18 years, antibiotics initiated for suspected or proven infection on admission or during ICU admission Exclusion criteria: > 3 weeks of prolonged antibiotic therapy for infectious clinical conditions such as endocarditis and cerebral/hepatic abscess;severe infections due to viruses, parasites or tuberculosis; ICU admission for exclusive short‐term postoperative follow‐up; anticipated length of stay < 24 hours; cystic fibrosis; severely immunocompromised (e.g. HIV and CD4 count < 200 cells/mm, neutropenic patients (< 500 neutrophils/mL)); solid organ transplantation; moribund participants |
| Interventions |
Group 1 (N = 761): to discontinue antibiotics when procalcitonin concentration had decreased by at least 80% or was ≤ 0·5 μg/L Group 1 (N = 785): local antibiotic protocols |
| Outcomes |
|
| Notes |
Sample size: Study authors estimated 1816 participants, based on a mean baseline antibiotic duration of 8 days, type I error = 5%; type II error = 90% and drop‐out rate of 20%. PCT measures: automated Kryptor platform (Thermo Fisher Scientific, Hennigsdorf, Germany), Roche Elecsys Thermo Fisher Scientific PCT assay, Siemens Centaur Thermo Fisher Scientific PCT assay or BioMerieux Vidas Thermo Fisher Scientific PCT assay Financial support/funding: Thermo Fisher Scientific |
Najafi 2015.
| Methods |
Study design: single‐centre, randomized, single‐blind, controlled trial Setting: 1 ICU, Tehran |
| Participants |
Inclusion criteria: Patients with SIRS, according to at least 2 of the following criteria: body temperature > 38° C or < 36° C, tachycardia > 90/min, tachypnoea > 20/min and leucocytosis > 12 × 109/L or a leftward shift with more than 10% band cells or leukopenia < 4 × 109/L Exclusion criteria: documented infection, pus from wound or abscess, empyema, thrombophlebitis, infection due to virus or parasites, hypoxaemia (PO2 < 60 mmHg), oliguria (urine output < 30 mL/h), Glasgow Coma Scale score of 3 without sedation, parenteral antibiotic usage (24 hours before admission to ICU), hospitalization (48 hours before enrolment), conditions requiring prolonged antibiotic therapy (e.g. endocarditis, chronic localized infection such as osteomyelitis), severely immunocompromised patients |
| Interventions |
Group 1 (N = 30): antibiotic treatment based on serum level of PCT (measured during 4‐6 hours). PCT < 0.5 ng/mL (no antimicrobial treatment, new measurement after 12 hours); PCT 0.5‐2.0 ng/mL (no antimicrobial treatment, new measurement after 8 hours); and PCT ≥ 2 ng/mL (antimicrobial treatment recommended) Group 2 (N = 30): antibiotic empirical therapy |
| Outcomes |
|
| Notes |
Sample size: Study authors estimated 60 participants, based on 95% power to detect a 30% reduction in the use of antibacterial agents. PCT measures: PCT levels were measured with time‐resolved amplified cryptate emission (TRACE) assay (BRAHMS, Germany; PCT‐sensitive KRYPTOR Compact) Financial support/funding: not reported |
Differences between protocol and review
We selected studies and extracted data in an unblinded and dependent fashion (not independently, as previously stated in the protocol) (Silva 2014).
Primary outcomes: We included mortality at longest follow‐up as an additional primary outcome because absence of evidence on mortality often results from insufficient power, as well as from clinical and methodological heterogeneity associated with primary studies, although the latter was not the specific case for this systematic review.
We performed a post hoc sensitivity analysis to test the estimate of effect of the intervention by removing a study that made a singular comparison: procalcitonin versus C‐reactive protein.
We changed the "Synthesis of the quality of the body of evidence" section from:
"We will use the principles of the GRADE system (Guyatt 2008) to assess the quality of the body of evidence associated with mortality, hospital and intensive care unit length of stay, clinical severity, new infection/reinfection and use of antimicrobial agents. We will also construct a 'Summary of findings' (SoF) table using the GRADE software. The GRADE approach appraises the quality of a body of evidence based on the extent to which one can be confident that an estimate of effect or association reflects the item being assessed. The quality of a body of evidence reflects within‐study risk of bias (methodological quality), directness of evidence, heterogeneity of data, precision of effect estimates and risk of publication bias" to:
"We used the principles of the GRADE approach (Guyatt 2008) to assess the quality of the body of evidence for our primary outcomes of mortality at 28 days, mortality at ICU discharge, mortality at hospital discharge and time receiving antimicrobial therapy. We imported effect estimates from RevMan 5.3 to GRADE profiler (GRADEpro 2014) to create Table 1. This table provides outcome‐specific information concerning the overall quality of evidence from studies included in the comparison, the magnitude of effect of interventions examined and the sum of available data on outcomes that we considered. The GRADE approach appraises the quality of a body of evidence according to the extent to which one can be confident that an estimate of effect or association reflects the item assessed. The quality of a body of evidence is based on different items, which reflect within‐study risk of bias (methodological quality), directness of evidence, heterogeneity of data, precision of effect estimates and risk of publication bias. Thus, we considered each of these items as having 'no limitation', 'serious limitation' or 'very serious limitation' (by downgrading them respectively for one or two levels), resulting in one of the following four overall qualities of evidence for each outcome: 'high', 'moderate', 'low' or 'very low' quality."
Contributions of authors
Brenda NG Andriolo (BNGA), Régis B Andriolo (RBA), Reinaldo Salomão (RS), Álvaro N Atallah (ÁNA).
Conceiving of the review: BNGA, RBA, RS, ÁNA.
Co‐ordinating the review: BNGA.
Undertaking manual searches: BNGA, RBA.
Screening search results: BNGA, RBA.
Organizing retrieval of papers: BNGA, RBA.
Screening retrieved papers against inclusion criteria: BNGA, RBA, RS.
Appraising the quality of papers: BNGA, RBA, RS, ÁNA.
Abstracting data from papers: BNGA, RBA, RS.
Writing to authors of papers to ask for additional information: BNGA.
Providing additional data about papers: BNGA, RBA, RS, ÁNA.
Obtaining and screening data on unpublished studies: BNGA, RBA.
Managing data for the review: BNGA, RBA, RS, ÁNA.
Entering data into Review Manager (RevMan 5.3): BNGA, RBA.
Handling RevMan statistical data: BNGA, RBA.
Performing other statistical analysis not using RevMan: BNGA, RBA.
Interpreting data: BNGA, RBA, RS, ANA.
Making statistical inferences: BNGA, RBA.
Writing the review: BNGA, RBA.
Performing previous work that was the foundation of the present study: BNGA, RBA, RS.
Serving as guarantor for the review (one review author): BNGA.
Taking responsibility for reading and checking the review before submission: BNGA, RBA, RS, ÁNA.
Sources of support
Internal sources
-
No source of support, Other.
Own sources
External sources
-
No source of support, Other.
Own sources
Declarations of interest
Brenda NG Andriolo: none known.
Régis B Andriolo: none known.
Reinaldo Salomão: none known.
Álvaro N Atallah: none known.
Edited (no change to conclusions)
References
References to studies included in this review
Annane 2013 {published data only}
- Annane D, Maxime V, Faller JP, Mezher C, Clec'h C, Martel P, et al. Procalcitonin levels to guide antibiotic therapy in adults with non‐microbiologically proven apparent severe sepsis: a randomised controlled trial. BMJ Open 2013;3(2):e002186. [PUBMED: 23418298] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deliberato 2013 {published data only}
- Deliberato RO, Marra AR, Sanches PR, Martino MD, Ferreira CE, Pasternak J, et al. Clinical and economic impact of procalcitonin to shorten antimicrobial therapy in septic patients with proven bacterial infection in an intensive care setting. Diagnostic Microbiology and Infectious Disease 2013;76(3):266‐71. [PUBMED: 23711530] [DOI] [PubMed] [Google Scholar]
Dharaniyadewi 2013 {published data only}
- Dharaniyadewi D, Lie KC, Sukmana N, Rumende CM. Effect of semi‐quantitative procalcitonin assay on the adequacy of empirical antibiotics and mortality in septic patients. Critical Care 2013;17:10. [DOI: 10.1186/cc12915] [DOI] [Google Scholar]
Hochreiter 2009 {published data only}
- Hochreiter M, Köhler T, Schweiger AM, Keck FS, Bein B, Spiegel T, et al. Procalcitonin to guide duration of antibiotic therapy in intensive care patients: a randomized prospective controlled trial. Critical Care (London, England) 2009;13(3):R83. [PUBMED: 19493352] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2013 {published data only}
- Liu BH, Li HF, Lei Y, Zhao SX, Sun ML. Clinical significance of dynamic monitoring of procalcitonin in guiding the use of antibiotics in patients with sepsis in ICU [动态监测降钙素原对 ICU 脓毒症患者 抗菌药物使用的临床意义]. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2013;25(11):690‐3. [PUBMED: 24225216] [DOI] [PubMed] [Google Scholar]
Nobre 2008 {published data only}
- Nobre V, Harbarth S, Graf JD, Rohner P, Pugin J. Use of procalcitonin to shorten antibiotic treatment duration in septic patients: a randomized trial. American Journal of Respiratory and Critical Care Medicine 2008;177(5):498‐505. [PUBMED: 18096708] [DOI] [PubMed] [Google Scholar]
Oliveira 2013 {published data only}
- Oliveira CF, Botoni FA, Oliveira CA, Pereira HAC, Padua M, Nobre V. The usefulness of C‐reactive protein (CRP) compared to procalcitonin (PCT) to reduce antibiotic exposure in critically ill patients with sepsis: a pilot study. Intensive Care Medicine. 2011; Vol. 37:S200. [CN‐01035397]
- Oliveira CF, Botoni FA, Oliveira CR, Silva CB, Pereira HA, Serufo JC, et al. Procalcitonin versus C‐reactive protein for guiding antibiotic therapy in sepsis: a randomized trial. Critical Care Medicine 2013;41(10):2336‐43. [PUBMED: 23921272] [DOI] [PubMed] [Google Scholar]
Schroeder 2009 {published data only}
- Schroeder S, Hochreiter M, Koehler T, Schweiger AM, Bein B, Keck FS, et al. Procalcitonin (PCT)‐guided algorithm reduces length of antibiotic treatment in surgical intensive care patients with severe sepsis: results of a prospective randomized study. Langenbeck's Archives of Surgery 2009;394(2):221‐6. [PUBMED: 19034493] [DOI] [PubMed] [Google Scholar]
Shehabi 2014 {published data only}
- Shehabi Y, Rachakonda KS, Bailey MJ, Peake S. Reply. No significant reduction in antibiotic treatment using a procalcitonin algorithm with low cutoff value in the intensive care unit?. American Journal of Respiratory and Critical Care Medicine 2015;191(7):859‐60. [DOI] [PubMed] [Google Scholar]
- Shehabi Y, Sterba M, Garrett PM, Rachakonda KS, Stephens D, Harrigan P, et al. Procalcitonin algorithm in critically ill adults with undifferentiated infection or suspected sepsis. A randomized controlled trial. American Journal of Respiratory and Critical Care Medicine 2014;190(10):1102‐10. [PUBMED: 25295709] [DOI] [PubMed] [Google Scholar]
Svoboda 2007 {published data only}
- Svoboda P, Kantorová I, Scheer P, Radvanova J, Radvan M. Can procalcitonin help us in timing of re‐intervention in septic patients after multiple trauma or major surgery?. Hepatogastroenterology 2007;54(74):359‐63. [PUBMED: 17523274] [PubMed] [Google Scholar]
References to studies excluded from this review
Bodmann 2016 {published data only}
- Bodmann KF, Schenker M, Heinlein W, Wilke MH. Procalcitonin as a tool for the assessment of successful therapy of severe sepsis: an analysis using clinical routine data [Eine Untersuchung mit klinischen Routinedaten]. Medizinische Klinik, Intensivmedizin und Notfallmedizin 2016;Epub ahead of print:1‐8. [PUBMED: 27376540] [DOI] [PubMed] [Google Scholar]
Bouadma 2010 {published data only}
- Bouadma L, Luyt CE, Tubach F, Cracco C, Alvarez A, Schwebel C, et al. Use of procalcitonin to reduce patients' exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. The Lancet 2010;375(9713):463‐74. [PUBMED: 20097417] [DOI] [PubMed] [Google Scholar]
Bréchot 2015 {published data only}
- Bréchot N, Hékimian G, Chastre J, Luyt CE. Procalcitonin to guide antibiotic therapy in the ICU. International Journal of Antimicrobial Agents 2015;46(Suppl 1):S19‐24. [PUBMED: 26607343] [DOI] [PubMed] [Google Scholar]
Harrison 2015 {published data only}
- Harrison M, Collins CD. Is procalcitonin‐guided antimicrobial use cost‐effective in adult patients with suspected bacterial infection and sepsis? Infection control and hospital epidemiology. Infection Control and Hospital Epidemiology 2015;36(3):265‐72. [DOI: 10.1017/ice.2014.60] [DOI] [PubMed] [Google Scholar]
Jensen 2011 {published data only}
- Jensen JU, Hein L, Lundgren B, Bestle MH, Mohr T, Andersen MH, et al. Kidney failure related to broad‐spectrum antibiotics in critically ill patients: secondary end point results from a 1200 patient randomised trial. BMJ Open 2012;2(2):e000635. [PUBMED: 22411933] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen JU, Hein L, Lundgren B, Bestle MH, Mohr TT, Andersen MH, et al. Procalcitonin‐guided interventions against infections to increase early appropriate antibiotics and improve survival in the intensive care unit: a randomized trial. Critical Care Medicine 2011;39(9):2048‐58. [PUBMED: 21572328] [DOI] [PubMed] [Google Scholar]
- Jensen JU, Lundgren B, Hein L, Mohr T, Petersen PL, Andersen LH, et al. The Procalcitonin And Survival Study (PASS) ‐ a randomised multi‐center investigator‐initiated trial to investigate whether daily measurements biomarker Procalcitonin and pro‐active diagnostic and therapeutic responses to abnormal Procalcitonin levels, can improve survival in intensive care unit patients. Calculated sample size (target population): 1000 patients. BMC Infectious Diseases 2008;8:91. [PUBMED: 18620598] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen ME, Jensen JU, Bestle MH, Hein L, Lauritsen AØ, Tousi H, et al. The potential of antimicrobials to induce thrombocytopenia in critically ill patients: data from a randomized controlled trial. Public Library of Science PLoS ONE 2013;8(11):e81477. [PUBMED: 24312305] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kiehntopf 2011 {published data only}
- Kiehntopf M, Hutagalung R, Bloos F, Oppert M, Grundling M, Weiler N, et al. Prognostic impact of procalcitonin in severe sepsis and septic shock: results of the VISEP study. Infection 2011;39(Suppl 2):S114‐5. [CENTRAL: CN‐01005880] [Google Scholar]
Kip 2015 {published data only}
- Kip MM, Kusters R, IJzerman MJ, Steuten LM. A PCT algorithm for discontinuation of antibiotic therapy is a cost‐effective way to reduce antibiotic exposure in adult intensive care patients with sepsis. Journal of Medical Economics 2015;18(11):944‐53. [PUBMED: 26105574] [DOI] [PubMed] [Google Scholar]
Kolici 2013 {published data only}
- Kolici N, Tushe E, Dedej G, Kolici E, Foto E. Role of semi‐quantitative procalcitonin test kit versus CRP to "rule out" neonatal sepsis. Intensive Care Medicine 2013;41(1):S189‐90. [CENTRAL: CN‐01060306; DOI: 10.1007/s00134-013-2950-8] [DOI] [Google Scholar]
Kopterides 2010 {published data only}
- Kopterides P, Siempos II, Tsangaris I, Tsantes A, Armaganidis A. Procalcitonin‐guided algorithms of antibiotic therapy in the intensive care unit: a systematic review and meta‐analysis of randomized controlled trials. Critical Care Medicine 2010;38(11):2229‐41. [PUBMED: 20729729] [DOI] [PubMed] [Google Scholar]
Layios 2012 {published data only}
- Layios N, Lambermont B, Canivet JL, Morimont P, Preiser JC, Garweg C, et al. Procalcitonin usefulness for the initiation of antibiotic treatment in intensive care unit patients. Critical Care Medicine 2012;40(8):2304‐9. [PUBMED: 22809906] [DOI] [PubMed] [Google Scholar]
Lima 2016 {published data only}
- Lima SS, Nobre V, Castro Romanelli RM, Clemente WT, Silva BittencourtHN, Melo AC, et al. Procalcitonin‐guided protocol is not useful to manage antibiotic therapy in febrile neutropenia: a randomized controlled trial. Annals of Hematology 2016;95(7):1169‐76. [PUBMED: 27118539] [DOI] [PubMed] [Google Scholar]
Mann 2011 {published data only}
- Mann EA, Wood GL, Wade CE. Use of procalcitonin for the detection of sepsis in the critically ill burn patient: a systematic review of the literature. Burns 2011;37(4):549‐58. [PUBMED: 20537467] [DOI] [PubMed] [Google Scholar]
Pantelidou 2015 {published data only}
- Pantelidou IM, Giamarellos‐Bourboulis EJ. Can procalcitonin monitoring reduce the length of antibiotic treatment in bloodstream infections?. International Journal of Antimicrobial Agents 2015;48(Suppl 1):S10‐2. [PUBMED: 26686272] [DOI] [PubMed] [Google Scholar]
Prkno 2013 {published data only}
- Prkno A, Wacker C, Brunkhorst FM, Schlattmann P. Procalcitonin‐guided therapy in intensive care unit patients with severe sepsis and septic shock: a systematic review and meta‐analysis. Critical Care (London, England) 2013;17(6):R291. [PUBMED: 24330744] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sandifer 2012 {published data only}
- Sandifer JP, Jones AE. Can procalcitonin levels guide antibiotic therapy in bacterial infections and reduce antibiotic over consumption without having a negative effect on clinical outcomes?. Annals of Emergency Medicine 2012;60(3):370‐1. [PUBMED: 22305332] [DOI] [PubMed] [Google Scholar]
Schuetz 2011 {published data only}
- Schuetz P, Chiappa V, Briel M, Greenwald JL. Procalcitonin algorithms for antibiotic therapy decisions: a systematic review of randomized controlled trials and recommendations for clinical algorithms. Archives of Internal Medicine 2011;171(15):1322‐31. [PUBMED: 21824946] [DOI] [PubMed] [Google Scholar]
Schuetz 2013 {published data only}
- Schuetz P, Raad I, Amin DN. Using procalcitonin‐guided algorithms to improve antimicrobial therapy in ICU patients with respiratory infections and sepsis. Current Opinion in Critical Care 2013;19(5):453‐60. [PUBMED: 23817026] [DOI] [PubMed] [Google Scholar]
Soni 2013 {published data only}
- Soni NJ, Samson DJ, Galaydick JL, Vats V, Huang ES, Aronson N, et al. Procalcitonin‐guided antibiotic therapy: a systematic review and meta‐analysis. Journal of Hospital Medicine 2013;8(9):530‐40. [PUBMED: 23955852] [DOI] [PubMed] [Google Scholar]
Stocker 2010a {published data only}
- Stocker M, Hop WC, Rossum AM. Neonatal Procalcitonin Intervention Study (NeoPInS): effect of procalcitonin‐guided decision making on duration of antibiotic therapy in suspected neonatal early‐onset sepsis: a multi‐centre randomized superiority and non‐inferiority intervention study. BioMed Central Pediatrics 2010;10:89. [PUBMED: 21143869] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stocker 2010b {published data only}
- Stocker M, Fontana M, Helou S, Wegscheider K, Berger TM. Use of procalcitonin‐guided decision‐making to shorten antibiotic therapy in suspected neonatal early‐onset sepsis: prospective randomized intervention trial. Neonatology 2010;97(2):165‐74. [PUBMED: 19776651] [DOI] [PubMed] [Google Scholar]
Ternhag 2010 {published data only}
- Ternhag A, Cars O, Norman C, Struwe J. Antibiotics or not ‐ procalcitonin can guide therapeutic choices. At least at the emergency department for adults with lower respiratory tract infection [Antibiotika eller inte – prokalcitonin kan vägleda behandlingsval]. Lakartidningen 2010;107(15):98. [MEDLINE: ] [PubMed] [Google Scholar]
Westwood 2015 {published data only}
- Westwood M, Ramaekers B, Whiting P, Tomini F, Joore M, Armstrong N, et al. Procalcitonin testing to guide antibiotic therapy for the treatment of sepsis in intensive care settings and for suspected bacterial infection in emergency department settings: a systematic review and cost‐effectiveness analysis. Health Technology Assessment (Winchester, England) 2015;19(96):v‐xxv, 1‐236. [PUBMED: 26569153] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Bloos 2016 {published data only}
- Bloos F, Trips E, Nierhaus A, Briegel J, Heyland DK, Jaschinski U, et al. Effect of sodium selenite administration and procalcitonin‐guided therapy on mortality in patients with severe sepsis or septic shock: a randomized clinical trial. JAMA Internal Medicine 2016;176(9):1266‐76. [PUBMED: 27428731] [DOI] [PubMed] [Google Scholar]
de Jong 2016 {published data only}
- Assink‐de Jong E, Lange DW, Oers JA, Nijsten MW, Twisk JW, Beishuizen A. Stop Antibiotics on guidance of Procalcitonin Study (SAPS): a randomised prospective multicenter investigator‐initiated trial to analyse whether daily measurements of procalcitonin versus a standard‐of‐care approach can safely shorten antibiotic duration in intensive care unit patients ‐ calculated sample size: 1816 patients. BioMed Central Infectious Diseases 2013;13:178. [PUBMED: 23590389] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jong E, Oers JA, Beishuizen A, Vos P, Vermeijden WJ, Haas LE, et al. Efficacy and safety of procalcitonin guidance in reducing the duration of antibiotic treatment in critically ill patients: a randomised, controlled, open‐label trial. The Lancet Infectious Diseases 2016;16(7):819‐27. [PUBMED: 26947523] [DOI] [PubMed] [Google Scholar]
Najafi 2015 {published data only}
- Najafi A, Khodadadian A, Sanatkar M, Moharari RS, Etezadi F, Ahmadi A, et al. The comparison of procalcitonin guidance administer antibiotics with empiric antibiotic therapy in critically ill patients admitted in intensive care unit. Acta Medica Iranica 2015;53(9):562‐7. [PUBMED: 26553084] [PubMed] [Google Scholar]
Additional references
ACCP/SCCM Consensus Conference Committee 1992
- American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Critical Care Medicine 1992;20(6):864‐74. [PUBMED: 1597042] [PubMed] [Google Scholar]
Adhikari 2010
- Adhikari NK, Fowler RA, Bhagwanjee S, Rubenfeld GD. Critical care and the global burden of critical illness in adults. The Lancet 2010;376(9749):1339‐46. [PUBMED: 20934212] [DOI] [PMC free article] [PubMed] [Google Scholar]
Assunção 2010
- Assunção M, Akamine N, Cardoso GS, Mello PV, Teles JM, Nunes AL, et al. Survey on physicians' knowledge of sepsis: do they recognize it promptly?. Journal of Critical Care 2010;25(4):545‐52. [PUBMED: 20646902] [DOI] [PubMed] [Google Scholar]
Azevedo 2012
- Azevedo JR, Torres OJ, Czeczko NG, Tuon FF, Nassif PA, Souza GD. Procalcitonin as a prognostic biomarker of severe sepsis and septic shock. Revista do Colégio Brasileiro de Cirurgiões 2012;39(6):456‐61. [PUBMED: 23348640] [DOI] [PubMed] [Google Scholar]
Becker 2008
- Becker KL, Snider R, Nylen ES. Procalcitonin assay in systemic inflammation, infection, and sepsis: clinical utility and limitations. Critical Care Medicine 2008;36(3):941‐52. [PUBMED: 18431284] [DOI] [PubMed] [Google Scholar]
Bone 1992
- Bone RC, Balk RA, Cerra FB. Definition for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992;101(6):1644‐55. [PUBMED: 1303622] [DOI] [PubMed] [Google Scholar]
Bone 2009
- Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 2009;136(5 Suppl):e28. [PUBMED: 20162763] [DOI] [PubMed] [Google Scholar]
Brok 2009
- Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta‐analyses may be inconclusive ‐ trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta‐analyses. International Journal of Epidemiology 2009;38(1):287‐98. [PUBMED: 18824466] [DOI] [PubMed] [Google Scholar]
Chalupa 2011
- Chalupa P, Beran O, Herwald H, Kaspříková N, Holub M. Evaluation of potential biomarkers for the discrimination of bacterial and viral infections. Infection 2011;39(5):411‐7. [PUBMED: 21720792] [DOI] [PubMed] [Google Scholar]
Christensen 2006
- Christensen PM, Kristiansen IS. Number‐needed‐to‐treat (NNT) ‐ needs treatment with care. Basic & Clinical Pharmacology & Toxicology 2006;99(1):12‐6. [PUBMED: 16867164] [DOI] [PubMed] [Google Scholar]
Cuthbertson 2013
- Cuthbertson BH, Elders A, Hall S, Taylor J, Maclennan G, McKirdy F, et al. Mortality and quality of life in the five years after severe sepsis. Critical Care 2013;17(2):R70. [PUBMED: 23587132] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dellinger 2013
- Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock 2012. Intensive Care Medicine 2013;39(2):165‐228. [PUBMED: 23361625] [DOI] [PMC free article] [PubMed] [Google Scholar]
García de Guadiana‐Romualdo 2015
- García de Guadiana‐Romualdo LM, Rebollo‐Acebes S, Esteban‐Torrella P, Jiménez‐Sánchez R, Hernando‐Holgado A, Ortín‐Freire A, et al. Prognostic value of lipopolysaccharide binding protein and procalcitonin in patients with severe sepsis and septic shock admitted to intensive care [Valor pronóstico de la proteína fijadora de lipopolisácaridos y la procalcitonina en pacientes con sepsis grave y shock séptico ingresados en una unidad de cuidados intensivos]. Medicina Intensiva 2015;39(4):207‐12. [PUBMED: 24953001] [DOI] [PubMed] [Google Scholar]
Garnacho‐Montero 2014
- Garnacho‐Montero J, Huici‐Moreno MJ, Gutiérrez‐Pizarraya A, López I, Márquez‐Vácaro JA, Macher H, et al. Prognostic and diagnostic value of eosinopenia, C‐reactive protein, procalcitonin, and circulating cell‐free DNA in critically ill patients admitted with suspicion of sepsis. Critical Care 2014;18(3):R116. [PUBMED: 24903083] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gendrel 1999
- Gendrel D, Raymond J, Coste J, Moulin F, Lorrot M, Guérin S, et al. Comparison of procalcitonin with C‐reactive protein, interleukin 6 and interferon‐alpha for differentiation of bacterial vs. viral infections. The Pediatric Infectious Disease Journal 1999;18(10):875‐81. [PUBMED: 10530583] [DOI] [PubMed] [Google Scholar]
GRADEpro 2014 [Computer program]
- McMaster University. GRADEpro. McMaster University, 2014.
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schunemann HJ. What is "quality of evidence" and why is it important to clinicians. BMJ 2008;336(7651):995‐8. [PUBMED: 18456631] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Hingorani 2013
- Hingorani AD, Windt DA, Riley RD, Abrams K, Moons KG, Steyerberg EW, et al. Prognosis research strategy (PROGRESS) 4: stratified medicine research. BMJ 2013;346:e5793. [PUBMED: 23386361] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hoeboer 2015
- Hoeboer SH, Geest PJ, Nieboer D, Groeneveld AB. The diagnostic accuracy of procalcitonin for bacteraemia: a systematic review and meta‐analysis. Clinical Microbiology and Infection 2015;21(5):474‐81. [PUBMED: 25726038] [DOI] [PubMed] [Google Scholar]
Hohn 2013
- Hohn A, Schroeder S, Gehrt A, Bernhardt K, Bein B, Wegscheider K, et al. Procalcitonin‐guided algorithm to reduce length of antibiotic therapy in patients with severe sepsis and septic shock. BioMed Central Infectious Diseases 2013;13:158. [PUBMED: 23547790] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hohn 2015
- Hohn A, Heising B, Hertel S, Baumgarten G, Hochreiter M, Schroeder S. Antibiotic consumption after implementation of a procalcitonin‐guided antimicrobial stewardship programme in surgical patients admitted to an intensive care unit: a retrospective before‐and‐after analysis. Infection 2015;43(4):405‐12. [PUBMED: 25588968] [DOI] [PubMed] [Google Scholar]
Hosein 2011
- Hosein S, Udy AA, Lipman J. Physiological changes in the critically ill patient with sepsis. Current Pharmaceutical Biotechnology 2011;12(12):1991‐5. [PUBMED: 21554219] [DOI] [PubMed] [Google Scholar]
Jawad 2012
- Jawad I, Lukšić I, Rafnsson SB. Assessing available information on the burden of sepsis: global estimates of incidence, prevalence and mortality. Journal of Global Health 2012;2(1):10404. [PUBMED: 23198133] [DOI] [PMC free article] [PubMed] [Google Scholar]
Karlsson 2009
- Karlsson S, Ruokonen E, Varpula T, Ala‐Kokko TI, Pettilä V, Finnsepsis Study Group. Long‐term outcome and quality‐adjusted life years after severe sepsis. Critical Care Medicine 2009;37(4):1268‐74. [PUBMED: 19242321] [DOI] [PubMed] [Google Scholar]
Kenzaka 2012
- Kenzaka T, Okayama M, Kuroki S, Fukui M, Yahata S, Hayashi H, et al. Use of a semiquantitative procalcitonin kit for evaluating severity and predicting mortality in patients with sepsis. International Journal of General Medicine 2012;5:483–8. [PUBMED: 22701089] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kicinski 2013
- Kicinski M. Publication bias in recent meta‐analyses. PLoS ONE 2013;8(11):e81823. [DOI: 10.1371/journal.pone.0081823] [DOI] [PMC free article] [PubMed] [Google Scholar]
Klein 2012
- Klein Klouwenberg PM, Ong DS, Bonten MJ, Cremer OL. Classification of sepsis, severe sepsis and septic shock: the impact of minor variations in data capture and definition of SIRS criteria. Intensive Care Medicine 2012;38(5):811‐9. [PUBMED: 22476449] [DOI] [PubMed] [Google Scholar]
Kuehn 2013
- Kuehn BM. Guideline promotes early, aggressive sepsis treatment to boost survival. JAMA 2013;309(10):969‐70. [PUBMED: 23483148] [DOI] [PubMed] [Google Scholar]
Kumar 2010
- Kumar A. Early antimicrobial therapy in severe sepsis and septic shock. Current Infectious Disease Reports 2010;12(5):336‐44. [PUBMED: 21308515] [DOI] [PubMed] [Google Scholar]
Lagu 2012
- Lagu T, Rothberg MB, Shieh MS, Pekow PS, Steingrub JS, Lindenauer PK. Hospitalizations, costs, and outcomes of severe sepsis in the United States 2003 to 2007. Critical Care Medicine 2012;40(3):754‐61. [PUBMED: 21963582] [DOI] [PubMed] [Google Scholar]
Leli 2014
- Leli C, Cardaccia A, Ferranti M, Cesarini A, D'Alò F, Ferri C, et al. Procalcitonin better than C‐reactive protein, erythrocyte sedimentation rate, and white blood cell count in predicting DNAemia in patients with sepsis. Scandinavian Journal of Infectious Diseases 2014;46(11):745‐52. [PUBMED: 25195647] [DOI] [PubMed] [Google Scholar]
Levy 2003
- Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Medicine 2003;29(4):530‐8. [PUBMED: 12664219] [DOI] [PubMed] [Google Scholar]
Lichtenstern 2012
- Lichtenstern C, Brenner T, Bardenheuer HJ, Weigand MA. Predictors of survival in sepsis: what is the best inflammatory marker to measure?. Current Opinion in Infectious Diseases 2012;25(3):328‐36. [PUBMED: 22421751] [DOI] [PubMed] [Google Scholar]
Liu 2015
- Liu D, Su L, Han G, Yan P, Xie L. Prognostic value of procalcitonin in adult patients with sepsis: a systematic review and meta‐analysis. PLoS ONE 2015;10(6):e0129450. [PUBMED: 26076027] [DOI] [PMC free article] [PubMed] [Google Scholar]
Machado 2013
- Machado FR, Salomão R, Rigato O, Ferreira EM, Schettino G, Mohovic T, et al. Late recognition and illness severity are determinants of early death in severe septic patients. Clinics (Sao Paulo) 2013;68(5):586‐91. [PUBMED: 23778420] [DOI] [PMC free article] [PubMed] [Google Scholar]
Maravić‐Stojković 2011
- Maravić‐Stojković V, Lausević‐Vuk L, Jović M, Ranković A, Borzanović M, Marinković J. Procalcitonin‐based therapeutic strategy to reduce antibiotic use in patients after cardiac surgery: a randomized controlled trial. Srpski Arhiv za Celokupno Lekarstvo 2011;139(11‐12):736‐42. [PUBMED: 22338468] [PubMed] [Google Scholar]
Maseda 2015
- Maseda E, Suarez‐de‐la‐Rica A, Anillo V, Tamayo E, García‐Bernedo CA, Ramasco F, et al. Procalcitonin‐guided therapy may reduce length of antibiotic treatment in intensive care unit patients with secondary peritonitis: a multicenter retrospective study. Journal of Critical Care 2015;30(3):537‐42. [PUBMED: 25600574] [DOI] [PubMed] [Google Scholar]
Matthaiou 2012
- Matthaiou DK, Ntani G, Kontogiorgi M, Poulakou G, Armaganidis A, Dimopoulos G. An ESICM systematic review and meta‐analysis of procalcitonin‐guided antibiotic therapy algorithms in adult critically ill patients. Intensive Care Medicine 2012;38(6):940‐9. [PUBMED: 22538461] [DOI] [PubMed] [Google Scholar]
McCann 2012
- McCann FJ, Chapman SJ, Yu WC, Maskell NA, Davies RJ, Lee YC. Ability of procalcitonin to discriminate infection from non‐infective inflammation using two pleural disease settings. PLoS ONE 2012;7(12):e49894. [PUBMED: 23251353] [DOI] [PMC free article] [PubMed] [Google Scholar]
Morgenthaler 2003
- Morgenthaler NG, Struck J, Chancerelle Y, Weglöhner W, Agay D, Bohuon C, et al. Production of procalcitonin (PCT) in non‐thyroidal tissue after LPS injection. Hormone and Metabolic Research 2003;35(5):290‐5. [PUBMED: 12915998] [DOI] [PubMed] [Google Scholar]
Nargis 2014
- Nargis W, Ibrahim M, Ahamed BU. Procalcitonin versus C‐reactive protein: usefulness as biomarker of sepsis in ICU patient. International Journal of Critical Illness and Injury Science 2014;4(3):195‐9. [PUBMED: 25337480] [DOI] [PMC free article] [PubMed] [Google Scholar]
Redl 2000
- Redl H, Schlag G, Tögel E, Assicot M, Bohuon C. Procalcitonin release patterns in a baboon model of trauma and sepsis: relationship to cytokines and neopterin. Critical Care Medicine 2000;28(11):3659‐63. [PUBMED: 11098970] [DOI] [PubMed] [Google Scholar]
RevMan 5.3 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rivers 2012
- Rivers EP, Katranji M, Jaehne KA, Brown S, Abou Dagher G, Cannon C, et al. Early interventions in severe sepsis and septic shock: a review of the evidence one decade later. Minerva Anestesiologica 2012;78(6):712‐24. [PUBMED: 22447123] [PubMed] [Google Scholar]
Rodger 2012
- Rodger M, Ramsay T, Fergusson D. Diagnostic randomized controlled trials: the final frontier. Trials Journal 2012;13:137. [PUBMED: 22897974] [DOI] [PMC free article] [PubMed] [Google Scholar]
Salluh 2011
- Salluh JI, Lisboa T. C‐reactive protein in community‐acquired sepsis: you can teach new tricks to an old dog. Critical Care 2011;15(5):186. [PUBMED: 21955725] [DOI] [PMC free article] [PubMed] [Google Scholar]
Salvo 1995
- Salvo I, Cian W, Musicco M, Langer M, Piadena R, Wolfler A, et al. The Italian SEPSIS study: preliminary results on the incidence and evolution of SIRS, sepsis, severe sepsis and septic shock. Intensive Care Medicine 1995;Suppl 2:S244‐9. [PUBMED: 8636531] [DOI] [PubMed] [Google Scholar]
Schuetz 2012
- Schuetz P, Müller B, Christ‐Crain M, Stolz D, Tamm M, Bouadma L, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD007498.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shaikh 2011
- Shaikh N, Evron J, Leeflang MM. Procalcitonin, C‐reactive protein, and erythrocyte sedimentation rate for the diagnosis of acute pyelonephritis in children. Cochrane Database of Systematic Reviews 2011, Issue 6. [DOI: 10.1002/14651858.CD009185] [DOI] [PMC free article] [PubMed] [Google Scholar]
Silva 2004
- Silva E, Pedro Mde A, Sogayar AC, Mohovic T, Silva CL, Janiszewski M, et al. Brazilian Sepsis Epidemiological Study (BASES study). Critical Care 2004;8(4):R251‐60. [DOI: 10.1186/cc2892; PUBMED: 15312226] [DOI] [PMC free article] [PubMed] [Google Scholar]
Suresh 2013
- Suresh G, Soll R. The diagnostic accuracy of C‐reactive protein and procalcitonin for neonatal sepsis (registered Cochrane title). http://www.archie.cochrane.org/search.jsp (accessed 31 January 2014).
Tang 2007
- Tang BM, Eslick GD, Craig JC, McLean AS. Accuracy of procalcitonin for sepsis diagnosis in critically ill patients: systematic review and meta‐analysis. The Lancet Infectious Diseases 2007;7(3):210‐7. [PUBMED: 17317602] [DOI] [PubMed] [Google Scholar]
Tang 2009
- Tang H, Huang T, Jing J, Shen H, Cui W. Effect of procalcitonin‐guided treatment in patients with infections: a systematic review and meta‐analysis. Infection 2009;37(6):497‐507. [PUBMED: 19826761] [DOI] [PubMed] [Google Scholar]
Tasdelen 2010
- Tasdelen Fisgin N, Aliyazicioglu Y, Tanyel E, Coban AY, Ulger F, Zivalioglu M, et al. The value of neopterin and procalcitonin in patients with sepsis. Southern Medical Journal 2010;103(3):216‐9. [PUBMED: 20134373] [DOI] [PubMed] [Google Scholar]
te Witt 2012
- Witt R, Hassing RJ, Petit PP, Belkum A, Genderen PJ. Procalcitonin and neopterin levels do not accurately distinguish bacterial from viral infections in ill‐returned travellers with fever. Transactions of the Royal Society of Tropical Medicine and Hygiene 2012;106(4):264‐6. [PUBMED: 22357400] [DOI] [PubMed] [Google Scholar]
Tsalik 2012
- Tsalik EL, Jaggers LB, Glickman SW, Langley RJ, Velkinburgh JC, Park LP, et al. Discriminative value of inflammatory biomarkers for suspected sepsis. Journal of Emergency Medicine 2012;43(1):97‐106. [PUBMED: 22056545] [DOI] [PMC free article] [PubMed] [Google Scholar]
Uusitalo‐Seppälä 2011
- Uusitalo‐Seppälä R, Koskinen P, Leino A, Peuravuori H, Vahlberg T, Rintala EM. Early detection of severe sepsis in the emergency room: diagnostic value of plasma C‐reactive protein, procalcitonin, and interleukin‐6. Scandinavian Journal of Infectious Diseases 2011;43(11‐12):883‐90. [PUBMED: 21892899] [DOI] [PubMed] [Google Scholar]
Vaughan‐Sarrazin 2011
- Vaughan‐Sarrazin MS, Bayman L, Cullen JJ. Costs of postoperative sepsis: the business case for quality improvement to reduce postoperative sepsis in Veterans Affairs hospitals. Archives of Surgery 2011;146(8):944‐51. [PUBMED: 21502443] [DOI] [PubMed] [Google Scholar]
Wacker 2013
- Wacker C, Prkno A, Brunkhorst FM, Schlattmann P. Procalcitonin as a diagnostic marker for sepsis: a systematic review and meta‐analysis. The Lancet Infectious Diseases 2013;13(5):426‐35. [PUBMED: 23375419] [DOI] [PubMed] [Google Scholar]
Wetterslev 2008
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. [PUBMED: 18083463] [DOI] [PubMed] [Google Scholar]
Zannoni 2012
- Zannoni A, Giunti M, Bernardini C, Gentilini F, Zaniboni A, Bacci ML, et al. Procalcitonin gene expression after LPS stimulation in the porcine animal model. Research in Veterinary Science 2012;93(2):921‐7. [PUBMED: 22001598] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Silva 2014
- Silva BNG, Andriolo RB, Salomão R, Atallah ÁN. Effectiveness and safety of procalcitonin evaluation for reducing mortality in adult patients with sepsis, severe sepsis and septic shock. Cochrane Database of Systematic Reviews 2014, Issue 2. [DOI: 10.1002/14651858.CD010959] [DOI] [PMC free article] [PubMed] [Google Scholar]


