Abstract
With the ever-growing number of transgenic mice being used in vision research, a precise knowledge of the cellular organization of the mouse retina is required. As with the cat, rabbit, rat, and primate retinae, as many as 10 cone bipolar types and one rod bipolar type can be expected to exist in the mouse retina; however, they still have to be defined. In the current study, several immunocytochemical markers were applied to sections of mouse retina, and the labeling of bipolar cells was studied using confocal microscopy and electron microscopy. By using antibodies against the neurokinin-3 receptor NK3R; the plasma membrane calcium ATPase1 (PMCA1); and the calcium (Ca)-binding proteins CaB1, CaB5, caldendrin, and recoverin, three different OFF-cone bipolar cells could be identified. One type of ON-cone bipolar cell was identified through its immunoreactivity for CaB5 and PMCA1. Rod bipolar cells, comparable in morphology to those of other mammalian retinae, expressed protein kinase Cα and CaB5. It was also shown that putative OFF-cone bipolar cells receive light signals through flat contacts at the cone pedicle base, whereas ON-cone bipolar signaling involves invaginating contacts. The distribution of the kainate receptor subunit GluR5 was studied by confocal and electron microscopy. GluR5 was expressed at flat bipolar cell contacts; however, it appears to be involved with only certain types of OFF-cone bipolar cells. This suggests that different bipolar cell types receive their light signals through different sets of glutamate receptors.
Indexing terms: mouse retina, bipolar cells, CaB5, NK3R, CaB1, recoverin, glutamate receptors
The mouse retina is becoming an important subject for studies of mammalian retinal organization, and, with the ever-growing number of transgenic mice being used in these studies, a precise knowledge of the cellular organization of the mouse retina is vital. Jeon et al. (1998) have provided a quantitative description of the proportions of different cell types of the mouse retina. Workers in our laboratory have recently performed an immunocytochemical analysis of the mouse retina and have provided markers for individual cell types (Haverkamp and Wässle, 2000). However, in this earlier study, we were not able to classify bipolar cells sufficiently because of a lack of specific markers and because a systematic classification of mouse bipolar cells is still missing.
It is reasonable to expect that, similarly to the case in cat, rabbit, primate, and rat retinae, as many as 10 cone bipolar (CB) cell types and one rod bipolar cell type exist in the mouse retina (Famiglietti, 1981; Cohen and Sterling, 1990a,b; Boycott and Wässle, 1991; Euler and Wässle, 1995; McGillem and Dacheux, 2001). Unfortunately, it has been demonstrated that several markers that recognize specific bipolar cell types in one species, when applied to the retinae of other species, either fail to label the homologous bipolar cell types or appear to be expressed by different bipolar cell types. An example of both these problems is provided by immunoreactivity to the Ca-binding protein calbindin. In the macaque monkey retina, the DB3 bipolar cell, an OFF-CB cell, among other cell types, was found to be immunoreactive for calbindin (Martin and Grünert, 1992; Grünert et al., 1994; Jacoby and Marshak, 2000). In contrast, in the rabbit retina, the calbindin-immunoreactive bipolar cell is an ON-CB cell stratifying close to the ganglion cell layer (Massey and Mills, 1996; McGillem and Dacheux, 2001), whereas, in the rat and mouse retinae, calbindin immunoreactivity was not observed in bipolar cells (Pasteels et al., 1990; Haverkamp and Wässle, 2000). Recoverin is another example of a marker that selects different types of bipolar cells in different species. While in rat and rabbit retinae two types of bipolar cell, an OFF- and an ON-CB cell, appear to be labeled (Milam et al., 1993; Euler and Wässle, 1995; Massey and Mills, 1996), only the OFF-midget bipolar cell is labeled in the macaque monkey retina (Milam et al., 1993; Wässle et al., 1994). Even in closely related species, different bipolar cell types can be selected by the same marker. The antibody against the carbohydrate epitope CD15 (Andressen and Mai, 1997) labels a single population of ON-bipolar cells (DB6) in macaque monkey, whereas DB6 cells and OFF-midget bipolar cells are labeled in marmoset monkeys (Chan et al., 2001a,b).
In our preceding study, various immunocytochemical markers of bipolar cells were applied to the mouse retina (Haverkamp and Wässle, 2000), with the following results. Rod bipolar cells were immunoreactive for protein kinase C (PKCα) and for the Purkinje cell marker L7 (Berrebi et al., 1991; Masu et al., 1995; Soucy et al., 1998). ON-CB cells were preferentially labeled with antibodies against the G-protein Goα (Vardi, 1998) and with the antibody mAb115A10 generated against rabbit olfactory bulb (Mori et al., 1985). Various populations of OFF-CB cells could be immunostained with antibodies against the glutamate transporter GLT-1 (Rauen and Kanner, 1994) and with antibodies against the Ca-binding proteins recoverin (Milam et al., 1993) and caldendrin (Seidenbecher et al., 1998).
Recently, several new markers of bipolar cells have been described. The Ca-binding proteins CaB1 and CaB5 label several different bipolar cell populations in the mouse retina (Haeseleer et al., 2000). The neurokinin-3 receptor (NK3R) has been found to label OFF-CB cells in rat and mouse retina (Blumauer and Brecha, 1998; Oyamada et al., 1999; Casini et al., 2000; Hirano and Brecha, 2002). The plasma membrane calcium ATPase isoform 1 (PMCA1; Filoteo et al., 1997) has been localized to two distinct bipolar cell types of the mouse retina (Krizaj et al., 2002).
In the present study, we applied antibodies against recoverin, CaB1, CaB5, caldendrin, NK3R, PMCA1, and PKCα to sections of mouse retina and studied the bipolar cell types that were immunolabeled. By using single- and double-labeling immunofluorescence, we were able to identify four types of CB cells and one rod bipolar cell. We also studied the cone contacts of these CB cells using immunoelectron microscopy (EM) and observed both flat (OFF-CB) and invaginating (ON-CB) cone contacts. To identify the ionotropic glutamate receptors (GluRs) expressed at the contacts between cone pedicles and OFF-CB cells, sections were doubly labeled for the GluR5 subunit and the OFF-CB cell marker NK3R.
MATERIALS AND METHODS
Animals and tissue preparation
Adult mice (C57/BL6J), 8–10 weeks of age, were deeply anesthetized with halothane (4% in oxygen) and decapitated. The procedures were approved by the local animal care committees. The eyes were enucleated, the anterior segments removed, and the posterior eyecups immersion fixed in 4% paraformaldehyde in 0.1 M phosphate buffer (PB; pH 7.4) for 10–30 minutes. After fixation, the retinae were dissected from the eyecup and either used as a whole-mount preparation or cryoprotected in graded sucrose solutions (10%, 20%, 30%) and sectioned vertically at 14 μm with a cryostat. For EM, the eyecups were fixed in 4% paraformaldehyde in 0.1 M PB for 30–50 minutes. After cryoprotection, the retina was frozen and thawed, and vertical sections (60 μm) were cut with a vibratome.
Light microscopic immunocytochemistry
The sources and working dilutions of antibodies are listed in Table 1. Immunocytochemical labeling was carried out using the indirect fluorescence method. Retinal sections were blocked for 1 hour in a solution containing 10% normal goat serum (NGS), 1% bovine serum albumin (BSA), and 0.5% Triton X-100 in phosphate-buffered saline (PBS; pH 7.4). The primary antibodies were diluted in 3% NGS, 1% BSA, 0.5% Triton X-100 in PBS and applied overnight at room temperature. After washes in PBS, secondary antibodies were applied for 1 hour. These were conjugated either to Alexa TM 594 (red fluorescence) or Alexa TM 488 (green fluorescence; Molecular Probes, Eugene, OR). In double-labeling experiments, sections were incubated in a mixture of primary antibodies followed by a mixture of secondary antibodies.
TABLE 1.
Sources and Working Dilutions of Antibodies
| Antigen | Antiserum | Source | Working dilution |
|---|---|---|---|
| VGluT1 | Rabbit anti-VGluT1 | R. Jahn, Göttingen, Germany (Takamori et al., 2000) | 1:1,000 |
| CaB5 | Rabbit anti-CaB5 | K. Palczewski, Seattle, WA (Haeseleer et al., 2000) | 1:500, 1:2,000 |
| NK3R | Rabbit anti-NK3R | R. Shigemoto, Okazaki, Japan (Ding et al., 1996) | 1:200, 1:500 |
| PMCA1 | Rabbit anti-PMCA1 | G. Filoteo, Rochester, NY (Filoteo et al., 1997) | 1:300 |
| CaB1 | Rabbit anti-CaB1 | K. Palczewski, Seattle, WA (Haeseleer et al., 2000) | 1:2,000, 1:4,000 |
| Caldendrin | Rabbit anticaldendrin | M. Kreutz, Magdeburg, Germany (Seidenbecher et al., 1998) | 1:1,000, 1:2,000 |
| Glycine | Rat antiglycine | D. Pow, Brisbane, Australia (Pow et al., 1995) | 1:1,000 |
| Recoverin | Rabbit antirecoverin | AM Dizhoor, Detroit, MI (Milam et al., 1993) | 1:1,000 |
| PKCα | Mouse anti-PKCα, clone MC5 | Amersham, Arlington Heights, IL | 1:100 |
| PSD95 | Mouse anti-PSD95, clone K28/43 | Upstate Biotechnology, Lake Placid, NY | 1:1,000 |
| GluR5 | Goat anti-GluR5 (C-18) | Santa Cruz Biotechnology, Santa Cruz, CA | 1:100 |
| GluR1 | Rabbit anti-GluR1 | Chemicon International Inc., Temecula, CA | 1:100 |
For double labeling with two rabbit polyclonal antibodies, sections were incubated first with one primary antibody, which was diluted tenfold compared with the normal working dilution. The staining was then visualized by using a secondary antibody raised in goat and was intensified by following this with a tertiary donkey anti-goat antibody carrying the same fluorophore. The next step was incubation with the second primary antibody using the normal working dilution, followed by incubation in the secondary antibody.
Fluorescent specimens were viewed using a Zeiss Axiophot microscope. Digital images were taken with a cooled CCD camera (Spot 2; Diagnostic Instruments, Sterling Heights, MI). Confocal micrographs were taken using a Zeiss LSM5 Pascal confocal microscope equipped with an argon (Ar) laser and a heliumneon (HeNe) laser. High-resolution scanning was performed with a Plan-Apochromat 63×/1.4 objective and at a resolution of 1,024 × 1,024 or 2,048 × 2,048 pixels. The brightness and the contrast of the final images were adjusted using Adobe Photoshop 5.5.
Preembedding immuno-EM
After blocking, vibratome sections were incubated for 4 days at 4°C in a primary incubation solution as used for light microscopy (LM) but without Triton X-100. Detection of the immunostaining and microscopic analysis were performed as described previously (Sassoè-Pognetto et al., 1994). Ultrathin sections were viewed with a Zeiss EM10 electron microscope (Zeiss, Oberkochen, Germany).
RESULTS
In both the primate and the rat retinae, the shape and stratification of bipolar cell axons in the inner plexiform layer (IPL) are the major distinguishing features of the different bipolar cell types (Boycott and Wässle, 1991; Euler and Wässle, 1995). Physiological recordings have demonstrated that bipolar cells with axons terminating in the outer IPL are OFF-bipolar cells, whereas those with axons terminating in the inner IPL are ON-bipolar cells (Euler et al., 1996; Hartveit, 1997). These criteria are also applied in the following classification of mouse bipolar cells.
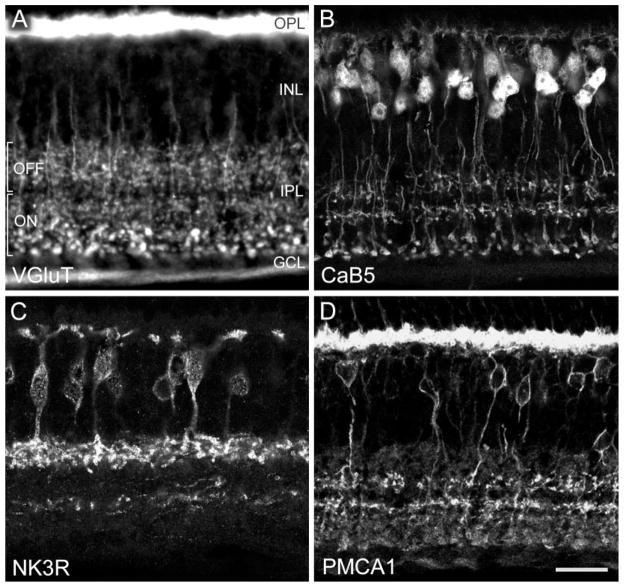
Figure 1A–D shows immunofluorescence micrographs depicting the labeling of different types of bipolar cells by the markers applied in the present study. Bipolar cells release glutamate at their synaptic terminals in the IPL. These terminals are filled with vesicles that accumulate glutamate through the vesicular glutamate transporter 1 (VGluT1; Takamori et al., 2000). Figure 1A shows a section through the mouse retina immunolabeled for VGluT1. The photoreceptor terminals (rod spherules and cone pedicles) in the outer plexiform layer (OPL) are intensely labeled. Bipolar cell axon terminals are labeled throughout the IPL, and the optic nerve fibres also express VGluT1. The lobular axon terminals of putative rod bipolar cells close to the ganglion cell layer can be recognized on the basis of their larger size (bottom of Fig. 1A). The IPL can be subdivided into an outer (upper) and inner (lower) half, with an area of reduced labeling in between, which we interpret, in accordance with findings in the rat retina, as the layers of OFF- and ON-bipolar cell axon terminals, respectively (Euler et al., 1996; Hartveit, 1997). VGluT1 immunofluorescence is found only in bipolar cell axon terminals, whereas their parent cell bodies are not labeled. However, the VGlut1 labeling demonstrates the dense packing of the IPL with bipolar cell axon terminals, and the comparison with the other bipolar cell markers applied in the present study will show that the latter reveal only small subsets of bipolar cells.
Fig. 1.
Fluorescence photomicrographs of vertical frozen sections through mouse retina that were labeled immunocytochemically. The retinal layers are indicated. OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer. A: Conventional photomicrograph showing immunolabeling for the vesicular glutamate transporter 1 (VGluT1). Photoreceptor terminals in the OPL and bipolar cell axon terminals in the IPL are labeled. In the outer half of the IPL, putative OFF-cone bipolar cells terminate; in the inner half of the IPL, putative ON-cone bipolar cells and rod bipolar cells terminate. B: Confocal photomicrograph showing immunolabeling for the calcium-binding protein CaB5. Dendrites of bipolar cells in the OPL, their perikarya in the INL, and their axons terminating in three bands in the IPL are labeled. C: Confocal micrograph of a section immunolabeled for the neurokinin-3 receptor NK3R. Bipolar cells and their axons terminating in the outer IPL are labeled. The processes labeled in the inner IPL are amacrine cell dendrites. D: Confocal micrograph showing immunolabeling for the plasma membrane calcuim ATPase 1 (PMCA1). Photoreceptor terminals in the OPL, bipolar cell bodies in the INL, and bipolar cell axon terminals in the IPL are labeled. Scale bar = 19 μm for A, 20 μm for B,D, 17.7 μm for C.
Antibodies against CaB5 (Fig. 1B) exclusively label bipolar cells, and their cell bodies are found in the outer half of the inner nuclear layer (INL). Their axons descend into the IPL and terminate in three distinct layers (Haeseleer et al., 2000), suggesting labeling of three bipolar cell types. The neurokinin-3 receptor NK3R (Fig. 1C) is expressed in bipolar cell bodies located toward the middle of the INL, and their axon terminals are found in the outermost part of the IPL (Blumauer and Brecha, 1998; Oyamada et al., 1999; Casini et al., 2000; Hirano and Brecha, 2002). There are also some amacrine cells expressing NK3R immunoreactivity (see arrow in Fig. 2A), and their processes form the labeled band in the inner IPL in Figure 1C. The antiserum against the plasma membrane calcium AT-Pase1 (PMCA1; Fig. 1D) binds prominently to rod spherules and cone pedicles in the OPL. In the INL, the perikaryal membrane of a restricted population of bipolar cells is labeled, and their axons can be observed descending into the IPL, where they terminate in two discrete layers (Krizaj et al., 2002). In addition, varicose, putative axon terminals of other bipolar cells appear to be weakly labeled throughout the IPL (Fig. 1D).
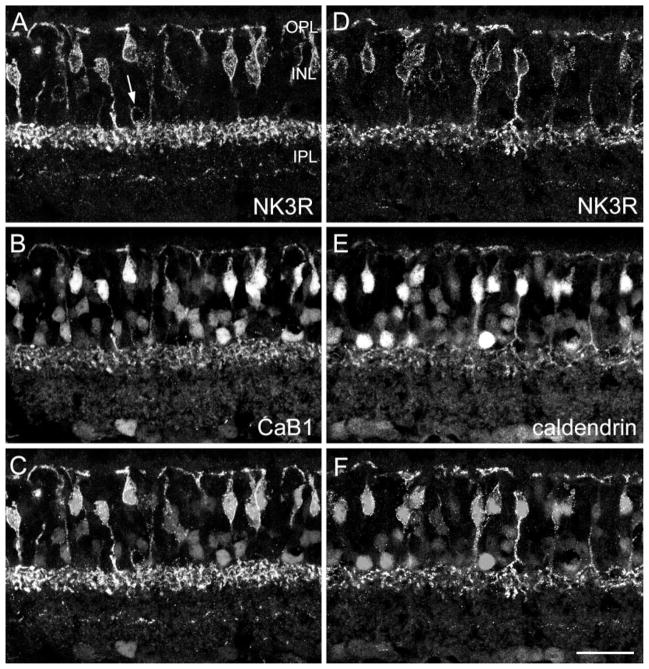
Fig. 2.
Confocal micrographs of vertical frozen sections through mouse retina that were doubly labeled for NK3R and for the calcium-binding proteins CaB1 (A–C) and caldendrin (D–F). A: NK3R immunofluorescence. Many bipolar cells are labeled. The arrow indicates an amacrine cell body. B: CaB1 immunolabeling can be observed in many bipolar cells, amacrine cells, and ganglion cells. C: Superimposition of A and B shows that the NK3R-labeled bipolar cells from A and the strongly CaB1-immunoreactive bipolar cells from B correspond to the same cells. D–F: NK3R-labeled bipolar cells also express caldendrin immunoreactivity. Scale bar = 25 μm.
NK3R-immunoreactive bipolar cells
NK3R immunoreactivity (Fig. 1C) is present in bipolar cells with axons terminating in the outermost IPL. Antibodies against the two closely related Ca-binding proteins CaB1 (Haeseleer et al., 2000) and caldendrin (Seidenbecher et al., 1998; Haverkamp and Wässle, 2000) have been found to label bipolar cells similar to those labeled with antibodies against NK3R. We wanted to determine whether NK3R and CaB1 label the same bipolar cell types, so we double labeled the sections accordingly (Fig. 2A–C). Although both antisera were raised in the same species (rabbit), we could successfully separate the two labels, because NK3R labeling was restricted to the plasma membrane, whereas CaB1 labeling filled the entire cell body. Comparison of Figure 2A–C shows that all NK3R-immunoreactive bipolar cell bodies (Fig. 2A,C) are also strongly immunoreactive for CaB1 (Fig. 2B,C). The same holds true for their axon terminals in the IPL.
Next, we double labeled sections for NK3R and caldendrin (Fig. 2D–F). The NK3R-immunoreactive bipolar cells (Fig. 2D) and the strongly caldendrin-immunoreactive bipolar cells (Fig. 2E) appear to make up the same population (Fig. 2F).
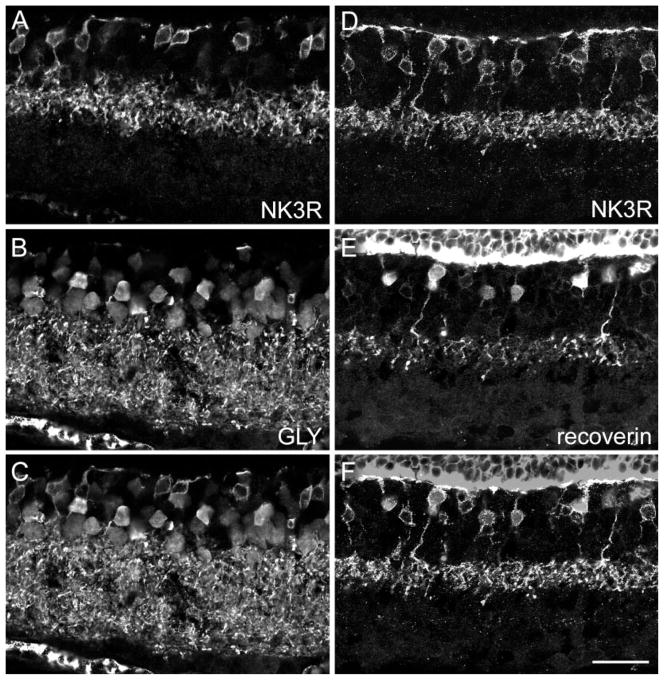
It has been shown for different mammalian retinae that ON-CB cells are electrically coupled via gap junctions to AII amacrine cells (for review see Mills and Massey, 2000; Trexler et al., 2001). Glycine, the AII cell transmitter, enters ON-CB cells through these gap junctions, and consequently ON-CB cells exhibit glycine immunoreactivity (Vaney et al. 1998). Figure 3A–C shows a section that was doubly labeled for NK3R (Fig. 3A) and for glycine (Fig. 3B). Comparison of Figure 3A and B and the superimposition in Figure 3C shows that NK3R-immunoreactive bipolar cells are not labeled for glycine. They are not, therefore, ON-CB cells but are, as expected from their level of axon termination in the outer IPL, OFF-CB cells.
Fig. 3.
Confocal micrographs of vertical frozen sections through mouse retina that were doubly labeled for NK3R and glycine (A–C) or for NK3R and recoverin (D–F). A: NK3R immunofluorescence is found in eight bipolar cell perikarya and in their axon terminals in the IPL. B: Bipolar cells, amacrine cells, and their processes in the IPL express glycine immunoreactivity. C: Superimposition of A and B shows that none of the eight NK3R-labeled bipolar cell perikarya exhibits glycine immunofluorescence. D: NK3R-labeled bipolar cells and their axon terminals in the IPL. E: Recoverin immunoreactivity is also expressed in bipolar cell perikarya and their axons. F: Superimposition of D and E shows that approximatelyonly half of the NK3R-labeled perikarya are also immunoreactive for recoverin. Scale bar = 25 μm.
Casini and colleagues (2000) have shown that in the rat retina recoverin-immunoreactive OFF-CB cells are a subgroup of the NK3R-labeled OFF-CB cells. Hence, in the rat retina, NK3R labels at least two OFF-CB cell types.
Figure 3D–F shows a section of the mouse retina that was doubly labeled for NK3R (Fig. 3D) and for recoverin (Fig. 3E). Recoverin immunoreactivity is confined to only one layer of axon terminals in the IPL. This is in contrast to the case in the rat retina, where two such layers (type 2 and type 8) have been found (Milam et al., 1993; Euler and Wässle, 1995; Casini et al., 2000), but conicides with the case in the macaque monkey retina, where OFF-midget bipolar cells are immunoreactive for recoverin (Grünert et al., 1994). Once again, the NK3R and recoverin antibodies have been raised in the same species (rabbit), which makes double labeling more difficult; however, because NK3R labeling is restricted to the cell membrane and recoverin labeling is found throughout the cytoplasm, single- and double-labeled bipolar cells can be distinguished when images are superimposed (Fig. 3F). Comparison of Figure 3E and F shows that only approximately half of the NK3R-immunoreactive bipolar cells also expresses recoverin. This suggests that, rather than labeling a single type of OFF-CB cell, NK3R labels at least two types of bipolar cell, both with axon terminals stratifying close to the INL/IPL border.
CaB5-immunoreactive bipolar cells
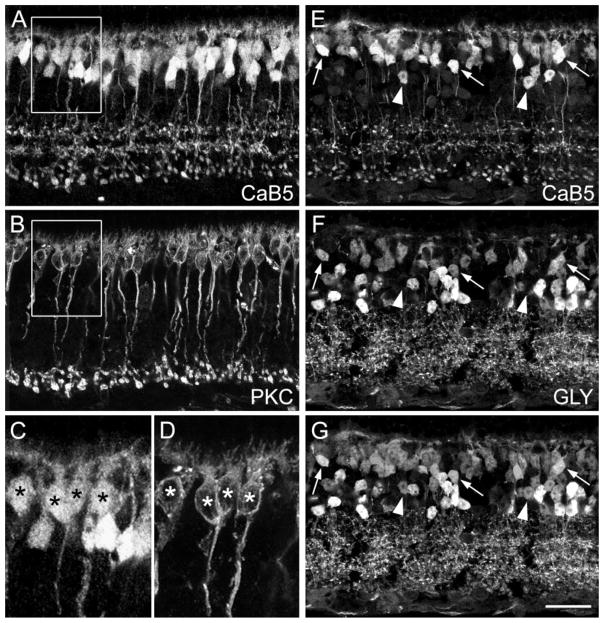
Three distinct layers of bipolar cell axon terminals can be seen in sections labeled with CaB5 antibodies (Fig. 1B). A section doubly labeled for CaB5 and the rod bipolar cell marker PKCα is shown in Figure 4A,B. Close inspection of the innermost (lowest) band of axon terminals in Figure 4A and of the RB cell axon terminals in Figure 4B demonstrates that they are in perfect register (Haeseleer et al., 2000). Comparison of the cell bodies (the boxed areas in Fig. 4A,B are shown at higher magnification in Fig. 4C,D) shows that all PKC-labeled cell bodies are also immunoreactive for CaB5. They are preferentially found at the outer margin of the INL. The remaining CaB5-labeled cell bodies occupy a more central position in the INL and are typically more intensely labeled. They most likely represent the parent cell bodies of CB cells whose axons give rise to the two outer (upper) layers of terminals in Figure 4A. Whether these are ON- or OFF-CB cells was investigated by doubly labeling sections for CaB5 (Fig. 4E) and glycine (Fig. 4F). Figure 4G shows their superimposition and, as indicated by the arrows, there are clear examples of double-labeled bipolar cell perikarya, which probably represent ON-CB cells. They are most likely the parent cell bodies of the middle layer of axon terminals in Figure 4E. However, as indicated by the arrowheads in Figure 4E–G, there are also bipolar cell bodies in the center of the INL, which are not glycine immunoreactive. We interpret these as OFF-CB cells serving the outermost (upper) band of axon terminals in Figure 4E.
Fig. 4.
Confocal micrographs of vertical frozen sections through mouse retina that were doubly labeled for the calcium-binding protein CaB5 and protein kinase Cα (PKCα; A–D) or for CaB5 and glycine (E–G). A: CaB5 immunoreactivity is found in many bipolar cell perikarya, in their descending axons, and in three distinct bands of axon terminals. B: PKCα labels rod bipolar (RB) cells, their descending axons, and their axon terminals in the innermost IPL. Comparison with A shows that RB axon terminals also express CaB5. C: High-power micrograph showing the CaB5-immunolabeled cell bodies from the boxed area in A. D: PKCα-immunolabeled cell bodies from the corresponding area in B. The asterisks indicate the double-labeled RB cell perikarya. E: CaB5-labeled section. F: Same section as in E labeled for glycine (GLY). The three arrows indicate bipolar cells expressing both CaB5 and glycine. The arrowheads indicate bipolar cells that express CaB5 but not glycine. G: Superimposition of E and F. Scale bar = 25 μm for A,B,E,F,G, 11.4 μm for C,D.
In conclusion, three distinct types of bipolar cells—rod bipolar cells, ON-CB cells, and OFF-CB cells—are immunoreactive for CaB5. We present further evidence below for this distinction by showing their cone contacts.
PMCA1- and CaB5-immunoreactive bipolar cells
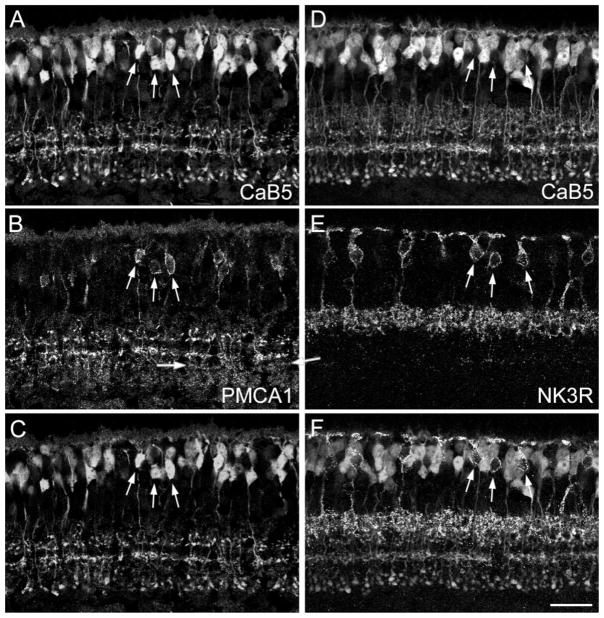
As shown in Figure 1D, PMCA1 immunoreactivity is present in two types of CB cells (Krizaj et al., 2002). Their axons are narrowly stratified in the middle of the IPL. We doubly labeled sections for CaB5 and for PMCA1 (Fig. 5A–C). The two PMCA1-immunoreactive bands of axon terminals (Fig. 5B) correspond precisely to the OFF- and ON-CB cell axon terminals revealed by CaB5 labeling (cf. Fig. 5A–C). Moreover, the bipolar cell perikarya expressing PMCA1 (arrows in Fig. 5B) are also immunoreactive for CaB5 (arrows in Fig. 5A,C). Therefore, we conclude that PMCA1 labels the same two CB cell types as CaB5. As mentioned above, there is a low expression of PMCA1 throughout the IPL, and, as indicated by the two horizontal arrows in Figure 5B, there is a faint band of axon terminals labeled by PMCA1 but not by CaB5. This might represent a further ON-CB cell type. Unfortunately, this band is too faintly labeled to be analyzed in more detail.
Fig. 5.
Confocal micrographs of vertical frozen sections through mouse retina that were doubly labeled for CaB5 and for PMCA1 (A–C) or for CaB5 and NK3R (D–F). A: CaB5 immunoreactivity is found in many bipolar cell perikarya, in their descending axons, and in three distinct bands of axon terminals. B: PMCA1 immunoreactivity decorates the perikaryal membrane of a few bipolar cells in the INL and their axons, which form two bands in the IPL. Weaker immunoreactivity is also present in additional bands of putative axon terminals in the IPL (horizontal arrows). C: Superimposition of A and B shows that the PMCA1-immunolabeled bipolar cells and their axon terminals represent a subgroup of the CaB5-labeled bipolar cells (vertical arrows). D: CaB5-immunolabeled section. E: Same section labeled for NK3R. F: Superimposition of D and E. The arrows show that NK3R-immunoreactive bipolar cells are not immunoreactive for CaB5. Scale bar = 25 μm.
NK3R- and CaB5-immunoreactive bipolar cells
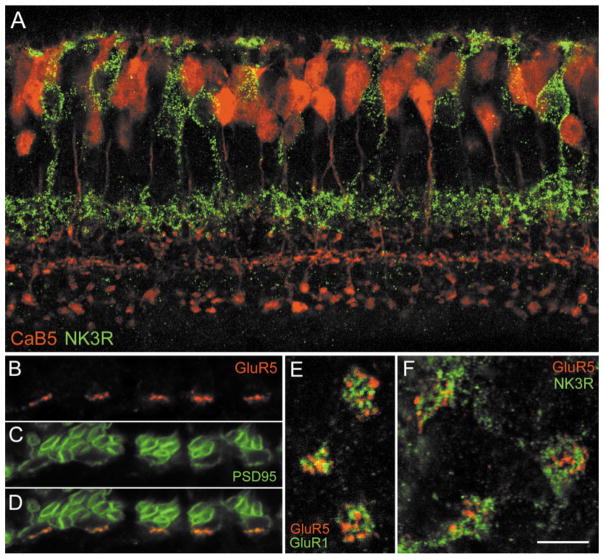
Next, we wanted to know whether NK3R and CaB5 reveal different types of bipolar cells; to determine this, we doubly labeled a section with the respective antibodies (Fig. 5D–F). NK3R immunoreactivity is confined to bipolar cell axons terminating in the outer (upper) IPL and to a few bipolar cell perikarya in the INL (Fig. 5E). CaB5 immunofluorescence shows the trilaminar pattern of axon terminals in the IPL. However, the outer IPL shows a more prominent background labeling (cf. Fig. 5A and D), because both the CaB5 and the NK3R antibodies were raised in the same species (rabbit). However, the superimposition in Figure 5F shows that the NK3R-labeled band of axon terminals does not coincide with any of the three CaB5-labeled bands but occupies a more outward (upper) position within the IPL. The NK3R-labeled bipolar cell perikarya (arrows in Fig. 5E) do not express CaB5 immunoreactivity (arrows in Fig. 5D,F). Taken together, these data indicate that the NK3R-labeled OFF-CB cells are not the same bipolar cells as the CaB5-immunoreactive cells. This is also demonstrated by the color micrograph shown in Figure 8A. The NK3-immunoreactive bipolar cells (green) and the CaB5-immunoreactive bipolar cells (red) represent different types.
Fig. 8.
A: Confocal fluorescence micrograph of a vertical section through the mouse retina doubly labeled for CaB5 (shown in red) and the NK3 receptor (shown in green). B–F: Confocal fluorescence micrographs showing the expression of the GluR5 subunit at cone pedicles of the mouse retina. B–D: Vertical section through the OPL doubly labeled for GluR5 and PSD95. B: GluR5 immunofluorescence (red) decorates the base of 5 cone pedicles. C: PSD95 immunofluorecence (green) reveals many rod spherules and the five cone pedicles. D: Superimposition of B and C shows the coincidence of GluR5 and PSD95 at the cone pedicle bases. E: Horizontal confocal section through three cone pedicles of the mouse retina doubly labeled for GluR5 (red) and GluR1 (green). The GluR1 and GluR5 clusters are not in register. F: Horizontal confocal section through three cone pedicles of the mouse retina doubly labeled for GluR5 (red) and NK3R (green). The GluR5 clusters and the NK3R-labeled bipolar cell dendritic tips are not in register. Scale bar = 15 μm for A, 6 μm for B–D, 5 μm for E,F.
So far, we have presented evidence for five bipolar cell types: the recoverin-immunoreactive putative OFF-CB cell, this and another putative OFF-CB cell being revealed by NK3R, caldendrin, and CaB1. A third putative OFF-CB cell, a putative ON-CB cell, and the RB bipolar cell were revealed by CaB5 immunofluorescence.
Cone contacts of NK3R- and CaB5-immunoreactive bipolar cells
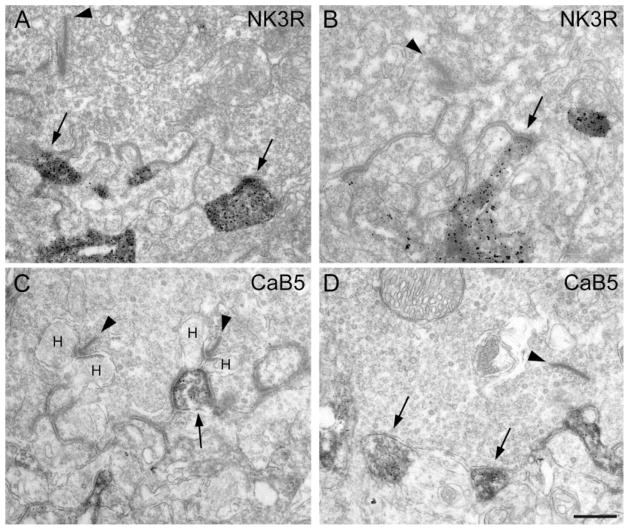
The stratification of the bipolar cell axons in the IPL, whether inner or outer, is one anatomical sign of their functional dichotomy into ON- or OFF-bipolar cells (Euler et al., 1996). The types of their contacts with cone pedicles, invaginating vs. flat contacts, are another distinguishing anatomical feature (Kolb and Nelson, 1995). Therefore, we studied the cone contacts of the labeled bipolar cells using EM. In the case of NK3R-immunoreative bipolar cells, we observed only flat contacts at the cone pedicle base (Fig. 6A,B) and never found a labeled bipolar cell process to form the central element of a triad. These findings support the identification as OFF-CB cells. In the case of CaB5-immunoreactive bipolar cells, we observed clear examples of invaginating processes (Fig. 6C) as well as flat contacts at the cone pedicle base (Fig. 6D). The EM results corroborate our conclusion that, in addition to RB cells, both ON-and OFF-CB cells express CaB5 immunoreactivity.
Fig. 6.
Electron micrograph showing the cone contacts of bipolar cell dendrites. A: Cone pedicle base containing one presynaptic ribbon (arrowhead). Two NK3R-immunoreactive processes (arrows) make flat contacts at the cone pedicle base. B: Another cone pedicle base, containing a presynaptic ribbon (arrowhead) engaged with a NK3R-labeled flat bipolar cell contact (arrow). C: Cone pedicle base containing two presynaptic ribbons (arrowheads) and their lateral horizontal cell dendrites (H). The CaB5-immunoreactive invaginating process (arrow) represents the central element opposed to the ribbon. D: Cone pedicle base containing one presynaptic ribbon (arrowhead). Two CaB5-immunoreactive processes (arrows) make flat contacts at the cone pedicle base. Scale bar = 0.57 μm for A, 0.37 μm for B, 0.5 μm for C,D.
Expression of the glutamate receptor subunit GluR5 at flat bipolar cell contacts
The contacts of bipolar cells with the cone pedicle also differ in the molecular composition of the glutamate receptor (GluRs) expressed there. Invaginating contacts express the metabotropic GluR, mGluR6 (Nomura et al., 1994; Vardi et al., 2000). This receptor causes a sign inversion and is responsible for the light responses of ON-bipolar cells. At the flat bipolar cell contacts with the cone pedicle base, usually ionotropic, sign-conserving GluRs are expressed, and they mediate the light responses of OFF-CB cells (Brandstätter et al., 1997; Qin and Pourcho, 1999; Morigiwa and Vardi, 1999; Hack et al., 1999, 2001; Haverkamp et al., 2000, 2001). Electrophysiological studies in the ground squirrel retina have shown that the signal transfer between cones and OFF-CB cells is preferentially mediated through kainate receptors (DeVries and Schwartz, 1999; DeVries, 2000). Therefore, we wanted to know whether the kainate receptor subunit GluR5 is involved at these synapses.
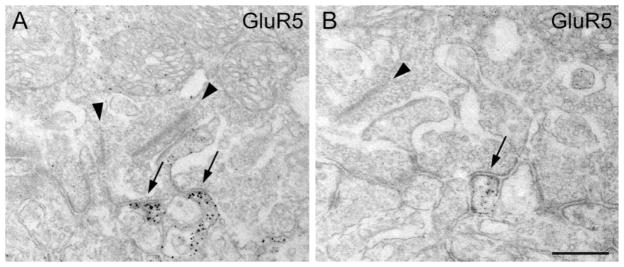
The electron micrographs in Figure 7 show that GluR5 is expressed at the flat contacts made by putative OFF-CB cells at the cone pedicle base. This is in agreement with our previous results showing the localization of GluR5 in the macaque monkey retina (Haverkamp et al., 2000, 2001). The question that then arises is: which types of OFF-bipolar cell express the GluR5 subunit at its cone contacts? Because it is difficult to study double-immunolabeled sections by EM, we studied the GluR5 expression at cone pedicles by confocal LM. First, we showed that GluR5 is aggregated in postsynaptic “hot spots” at the cone pedicle base. A section that was doubly labeled for GluR5 and PSD95 is shown in Figure 8B–D. PSD95 labels the outline of numerous rod spherules and of five cone pedicles (Fig. 8C; Koulen et al., 1998). GluR5 immunofluorescence is concentrated in hot spots (Fig. 8B) that are aligned with the cone pedicle base (Fig. 8D). We interpret these GluR5 hot spots as aggregates of GluR5 at flat bipolar cell contacts, based on our EM results.
Fig. 7.
Electron micrographs showing the expression of the glutamate receptor subunit GluR5 at the cone pedicle base. A: Cone pedicle base containing two presynaptic ribbons. Two GluR5-immunoreactive processes make flat contacts at the cone pedicle base (arrows). B: Another cone pedicle containing a presynaptic ribbon (arrowhead) is engaged with a flat bipolar cell contact expressing GluR5. Scale bar = 0.5 μm for A, 0.4 μm for B.
Next, we wanted to know whether the horizontal resolution of the confocal micrographs is sufficient to resolve individual flat contacts at the cone pedicle base of the mouse retina. This was tested by doubly labeling whole-mount preparations for GluR5 and GluR1, which have both been shown by EM to be expressed at flat contacts at the cone pedicle base (GluR1: Hack et al., 2000; GluR5: present study, Fig. 7). These two subunits have been shown in monkey cone pedicles not to coincide (Haverkamp et al., 2000, 2001). Figure 8E shows that, at mouse cone pedicles, GluR5 and GluR1 clusters are also not in register but are aggregated in different postsynaptic hot spots. This shows that the horizontal resolution of confocal LM is sufficient to localize individual GluR clusters. Finally, we doubly labeled a mouse retinal whole mount for GluR5 and NK3R (Fig. 8F). The dendritic tips of NK3R-immunoreactive bipolar cells make multiple contacts with the three cone pedicles illustrated in Figure 8F, and many GluR5 clusters are found at these three cone pedicles. However, the GluR5 hot spots are not in register with the dendritic tips of the NK3R-labeled bipolar cells, suggesting that these bipolar cells do not receive their light signals through GluRs involving the GluR5 subunits. We, therefore, postulate that other OFF-bipolar cells are involved with the GluR5-immunoreactive hot spots and that different OFF-CB cells express different sets of GluRs at their contacts with the cone pedicles.
DISCUSSION
Bipolar cell types in the mouse retina
There is no detailed study available of the different bipolar cell types of the mouse retina. Tsukamoto et al. (2001) have recently described two OFF-CB cell types (B1 and B2), which they reconstructed by EM from serial sections. They differ in their photoreceptor contacts: B1 cells synapse with cones only, whereas B2 cells contact both cones and rods. The axon terminals of B1 cells are more stout and restricted to the outermost 35% of the IPL; those of B2 cells are more delicate and terminate at a more central position. From this description, B1 cells probably represent one of the two NK3R-immunoreactive OFF-CB cells, and B2 cells may be identical to the CaB5-immunoreactive OFF-CB cell.
To distinguish mouse bipolar cells further, we have to rely on the classification scheme proposed for the rat retina by Euler and Wässle (1995). This scheme uses numbers that were chosen according to the stratification level of the axon terminals: Type 1 axons terminate close to the INL, type 9 axons close to the ganglion cell layer. This scheme is simple and is open to new types, which can be added by subdividing, for instance, type 3 into type 3a and type 3b.
Type 2 bipolar cells of the rat retina have been shown to be immunoreactive for recoverin (Milam et al., 1993; Euler and Wässle, 1995). The recoverin-immunoreactive bipolar cell of the mouse retina also matches the type 2 morphology. This holds true for the antibody against recoverin (Milam et al., 1993) applied in the present study. In our preceding study, which used another antibody against recoverin (Lambrecht and Koch, 1992), a more diffuse labeling of possibly three bipolar cell types was observed (Fig. 5; Haverkamp and Wässle, 2000). In both the rat and the mouse retinae, recoverin-immunoreactive bipolar cells form a subgroup of NK3R-labeled bipolar cells (Casini et al., 2000). NK3R-immunoreactive bipolar cells thus comprise two types, and the nonrecoverin-labeled type matches the type 1 cell of the rat. It is more difficult to identify the CaB5-immunoreactive OFF-CB cell from the rat catalogue. It does not correspond to type 4 of the rat, because type 4 has a diffusely terminating axon, whereas the CaB5 cell axon terminal is more narrowly stratified. It may correspond to type 3 of the rat retina. All bipolar cells discussed so far are OFF-CB cells, as demonstrated in the present study by the absence of glycine immunoreactivity.
At present, we have only one candidate for an ON-CB cell as revealed by CaB5 and PMCA1 immunolabeling. It may represent type 5 or type 6 of the rat retina, because its axon terminates more toward the center of the IPL. Moreover, PMCA1 immunoreactivity faintly stains another putative ON-CB cell (Fig. 5B) that terminates more toward the GCL, comparable to type 7 or type 8 of the rat retina.
Finally, it has been shown in previous studies of the mouse retina by PKCα immunostaining (Berrebri et al., 1991; Masu et al., 1995; Soucy et al., 1998) and in the present study by CaB5 immunolabeling that the morphology of mouse rod bipolar cells matches the descriptions of rod bipolar cells from other mammals.
However, we must emphasize that this preliminary immunocytochemical characterization should be verified by a careful morphological classification of mouse bipolar cells by intracellular injections with markers such as neurobiotin. The compact and space-filling distribution of bipolar cell axon terminals throughout the IPL, as revealed by immunostaining for the vesicular glutamate transporter VGlut1 (Fig. 1A), predicts that more than the five bipolar cells described in the present study must be present in the mouse retina. As has been shown in a recent study of the rabbit retina (McGillem and Dacheux, 2001), in which 12 CB cells have been identified, even the nine types of bipolar cell described for the rat might be an underestimate.
Glutamate receptors of mouse OFF-CB cells in the OPL
Hack et al. (2001) have recently studied the localization of several GluR subunits of the AMPA type at the synapses between photoreceptors and bipolar cells of the mouse retina. They described the expression of the subunits GluR1, GluR2/3, and GluR4 at flat contacts at the cone pedicle base. Hack et al. also confirmed and extended their earlier observation (Hack et al., 1999) that OFF-CB cell dendrites contact rod spherules through AMPA receptors. This novel pathway for rod signals has also been described by Tsukamoto et al. (2001). It is not yet known whether the AMPA receptors are specifically expressed by certain types of bipolar cells or whether all OFF-CB cells express AMPA receptors at some of their contacts with cone pedicles. It has recently been shown by electrophysiological recordings from the ground squirrel retina that different types of OFF-CB cells express different GluR subunits (DeVries, 2000). The b2 cells received light signals through AMPA receptors, the b7 and b3 cells through kainate receptors. It has been suggested by DeVries (2000) that the b7 CB cell might express a kainate receptor assembled from the GluR5 and GluR6 subunits. The electrophysiological results of DeVries (2000) show that specific types of bipolar cells receive cone input through AMPA receptors, whereas others receive that input via kainate receptors, suggesting a cell-type-specific expression of GluR subunits.
Brandstätter et al. (1997) studied the localization of the kainate receptor subunits GluR6/7 and KA2 in the OPL of the rat retina. They observed the KA2 subunit at flat contacts at the cone pedicle base and did not find the GluR6/7 subunits at flat contacts. However, we have recently observed the GluR6/7 subunit at flat contacts of the primate retina (Haverkamp et al., 2001). We have also studied the localization of the GluR5 subunit at cone pedicles of the primate retina and found it at flat contacts (Haverkamp et al., 2001). In the present study, we also looked at the localization of the GluR5 subunit in the mouse OPL and observed the subunit at flat contacts. However, we failed to show an expression of GluR5 at the cone contacts of the NK3R-immunoreactive bipolar cells. We also doubly labeled sections for CaB5 and for GluR5 (not shown) and did not observe GluR5 immunoreactivity in the dendritic tips of CaB5-labeled bipolar cells. It is possible that GluR5 is expressed by another OFF-bipolar cell type that is yet to be discovered. Be that as it may, this predicts a specific expression of GluR subunits by certain types of bipolar cells comparable to the results from the ground squirrel retina (DeVries, 2000).
Comparison of immunocytochemical markers of mouse bipolar cells
Rod bipolar cells of the mouse retina have been selectively immunolabeled with antibodies against PKCα and with antibodies against the cerebellar protein L7 (Berrebi et al., 1991; Masu et al., 1995; Soucy et al., 1998). However, some commercial antibodies against PKCα also recognize blue cones (Wikler et al., 1998; Hirano et al., 2000) and a small number of amacrine cells (Negishi et al., 1988; Greferath et al., 1990). Rod bipolar cells are also labeled, nonselectively, by a variety of markers, such as antibodies against the cerebellar protein PEP-19 (Ziai et al., 1986), antibodies against the G-protein Goα (Vardi, 1998), antibody mAb115A10 (Onoda and Fujita, 1987) directed against rabbit olfactory bulb (Mori et al., 1985), and antibodies against CaB5 (Haeseleer et al., 2000).
ON-CB cells in the mouse were found to be immunoreactive for Goα (Vardi, 1998; Haverkamp and Wässle, 2000) and for reelin (Rice et al., 2002). They were also immunoreactive for glycine (Vaney et al., 1998; Haverkamp and Wässle, 2000) and mAb115A10 (Onoda and Fujita, 1987). Unfortunately, none of these antibodies selected only ON-CB cells. Either rod bipolar cells (Goα, mAb115A10) or amacrine cells (glycine, reelin) were also labeled. In the present study, we were able to show that one type of ON-CB cell was immunoreactive for CaB5 and PMCA1. We also immunostained mouse retina for CD15, which is an excellent marker for ON-bipolar cells in other species (Andressen and Mai, 1997; Brown and Masland, 1999; Chan et al., 2001a,b); however, no staining of bipolar cells was observed.
The glutamate transporter GLT-1 is preferentially expressed in OFF-CB cells of the mouse retina; however, their axon terminals are not well stained, and a more detailed classification was not possible (Haverkamp and Wässle, 2000). In the present study, we were able to define three OFF-CB cells by their differential expression of NK3R, recoverin, and CaB5. Additional markers of OFF-CB cells are antibodies against hyperpolarization-activated, cyclic nucleotide-regulated cation (HCN) channels (Hirano and Brecha, 2001; Müller et al., 2001). Some OFF-CB were also immunoreactive for PEP-19 (Haverkamp and Wässle, 2000).
A completely different approach to identifying bipolar cells of the mouse retina has been established by Chiu and Nathans (1994). They used transgenic mice to define those sequences within the human blue visual pigment gene with expression directed specifically to blue cones. Unexpectedly, the blue pigment transgenes were expressed in both blue cones and a subset of CB cells. Different types of OFF- and ON-CB cells expressed the transgene (see Fig. 3 of Chiu and Nathans, 1994). More recently, Hickmann et al. (2002) produced transgenic mice expressing human aldose reductase (HAR) under control of the smooth muscle alpha actin promoter. They observed the expression of HAR in several different CB cell types. These two examples show that the transgenic approach can be used in the future as an additional tool with which to identify and characterize bipolar cells of the mouse retina.
Acknowledgments
Deutsche Forschungsgemeinschaft; Grant number: SFB269/B4; Grant sponsor: A.v. Humboldt Foundation; Grant sponsor: National Eye Institute; Grant number: EY 07026; Grant sponsor: Fight for Sight/Research to Prevent Blindness; Grant number: GA01048.
We thank M. Dumbsky, W. Hofer, and G.S. Nam for excellent technical assistance and I. Odenthal for typing the article.
LITERATURE CITED
- Andressen C, Mai JK. Localization of the CD15 carbohydrate epitope in the vertebrate retina. Vis Neurosci. 1997;14:253–262. doi: 10.1017/s0952523800011391. [DOI] [PubMed] [Google Scholar]
- Berrebi AS, Oberdick J, Sangameswaran L, Christakos S, Morgan JI, Mugnaini E. Cerebellar Purkinje cell markers are expressed in retinal bipolar neurons. J Comp Neurol. 1991;308:630–649. doi: 10.1002/cne.903080409. [DOI] [PubMed] [Google Scholar]
- Blumauer N, Brecha NC. Off-type of cone bipolar cells express neurokinin-3 (NK-3) receptors in the rat retina. Soc Neurosci Abstr. 1998:2091. [Google Scholar]
- Boycott BB, Wässle H. Morphological classification of bipolar cells in the macaque monkey retina. Eur J Neurosci. 1991;3:1069–1088. doi: 10.1111/j.1460-9568.1991.tb00043.x. [DOI] [PubMed] [Google Scholar]
- Brandstätter JH, Koulen P, Wässle H. Selective synaptic distribution of kainate receptor subunits in the two plexiform layers of the rat retina. J Neurosci. 1997;17:9298–9307. doi: 10.1523/JNEUROSCI.17-23-09298.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SP, Masland RH. Costratification of a population of bipolar cells with the direction-selective circuitry of the rabbit retina. J Comp Neurol. 1999;408:97–106. [PubMed] [Google Scholar]
- Casini G, Brecha NC, Bosco L, Rickman DW. Developmental expression of neurokinin-1 and neurokinin-3 receptors in the rat retina. J Comp Neurol. 2000;421:275–287. [PubMed] [Google Scholar]
- Chan TL, Martin PR, Grünert U. Immunocytochemical identification and analysis of the diffuse bipolar cell type DB6 in macaque monkey retina. Eur J Neurosci. 2001a;13:829–832. doi: 10.1046/j.0953-816x.2000.01449.x. [DOI] [PubMed] [Google Scholar]
- Chan TL, Martin PR, Clunas N, Grünert U. Bipolar cell diversity in the primate retina. Morphologic and immunocytochemical analysis of a new world monkey, the marmoset Callithrix jacchus. J Comp Neurol. 2001b;437:219–239. doi: 10.1002/cne.1280. [DOI] [PubMed] [Google Scholar]
- Chiu MI, Nathans J. Blue cones and cone bipolar cells share transcriptional specificity as determined by expression of human blue visual pigment-derived transgenes. J Neurosci. 1994;14:3426–3436. doi: 10.1523/JNEUROSCI.14-06-03426.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen E, Sterling P. Demonstration of cell types among cone bipolar neurons of cat retina. Philos Trans R Soc Lond B Biol Sci. 1990a;330:305–321. doi: 10.1098/rstb.1990.0201. [DOI] [PubMed] [Google Scholar]
- Cohen E, Sterling P. Convergence and divergence of cones onto bipolar cells in the central area of the cat retina. Philos Trans R Soc Lond B Biol Sci. 1990b;330:323–328. doi: 10.1098/rstb.1990.0202. [DOI] [PubMed] [Google Scholar]
- DeVries SH. Bipolar cells use kainate and AMPA receptors to filter visual information into separate channels. Neuron. 2000;28:847–856. doi: 10.1016/s0896-6273(00)00158-6. [DOI] [PubMed] [Google Scholar]
- DeVries SH, Schwartz EA. Kainate receptors mediate synaptic transmission between cones and ‘Off’ bipolar cells in a mammalian retina. Nature. 1999;397:157–160. doi: 10.1038/16462. [DOI] [PubMed] [Google Scholar]
- Ding YQ, Shigemoto R, Takada M, Ohishi H, Nakanishi S, Mizuno N. Localization of the neuromedin K receptor (NK3) in the central nervous system of the rat. J Comp Neurol. 1996;364:290–310. doi: 10.1002/(SICI)1096-9861(19960108)364:2<290::AID-CNE8>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Euler T, Wässle H. Immunocytochemical identification of cone bipolar cells in the rat retina. J Comp Neurol. 1995;361:461–478. doi: 10.1002/cne.903610310. [DOI] [PubMed] [Google Scholar]
- Euler T, Schneider H, Wässle H. Glutamate responses of bipolar cells in a slice preparation of the rat retina. J Neurosci. 1996;16:2934–2944. doi: 10.1523/JNEUROSCI.16-09-02934.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famiglietti EV. Functional architecture of cone bipolar cells in mammalian retina. Vis Res. 1981;21:1559–1563. doi: 10.1016/0042-6989(81)90032-8. [DOI] [PubMed] [Google Scholar]
- Filoteo G, Elwess NL, Enyedi A, Caride A, Aung HH, Penniston JT. Plasma membrane Ca2+ pump in rat brain. Patterns of alternative splices seen by isoform-specific antibodies. J Biol Chem. 1997;272:23741–23747. doi: 10.1074/jbc.272.38.23741. [DOI] [PubMed] [Google Scholar]
- Greferath U, Grünert U, Wässle H. Rod bipolar cells in the mammalian retina show protein kinase C-like immunoreactivity. J Comp Neurol. 1990;301:433–442. doi: 10.1002/cne.903010308. [DOI] [PubMed] [Google Scholar]
- Grünert U, Martin PR, Wässle H. Immunocytochemical analysis of bipolar cells in the macaque monkey retina. J Comp Neurol. 1994;348:607–627. doi: 10.1002/cne.903480410. [DOI] [PubMed] [Google Scholar]
- Hack I, Peichl L, Brandstätter JH. An alternative pathway for rod signals in the rodent retina: rod photoreceptors, cone bipolar cells, and the localization of glutamate receptors. Proc Natl Acad Sci USA. 1999;96:14130–14135. doi: 10.1073/pnas.96.24.14130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hack I, Frech M, Dick O, Peichl L, Brandstätter JH. Heterogeneous distribution of AMPA glutamate receptor subunits at the photoreceptor synapses of rodent retina. Eur J Neurosci. 2001;13:15–24. [PubMed] [Google Scholar]
- Haeseleer F, Sokal I, Verlinde CLMJ, Erdjument-Bromage H, Tempst P, Pronin AN, Benovic JL, Fariss RN, Palczewski K. Five members of a novel Ca2+-binding protein (CABP) subfamily with similarity to calmodulin. J Biol Chem. 2000;275:1247–1260. doi: 10.1074/jbc.275.2.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartveit E. Functional organization of cone bipolar cells in the rat retina. J Neurophysiol. 1997;77:1716–1730. doi: 10.1152/jn.1997.77.4.1716. [DOI] [PubMed] [Google Scholar]
- Haverkamp S, Wässle H. Immunocytochemical analysis of the mouse retina. J Comp Neurol. 2000;424:1–23. [PubMed] [Google Scholar]
- Haverkamp S, Grünert U, Wässle H. The cone pedicle, a complex synapse in the retina. Neuron. 2000;27:85–95. doi: 10.1016/s0896-6273(00)00011-8. [DOI] [PubMed] [Google Scholar]
- Haverkamp S, Grünert U, Wässle H. Localization of kainate receptors at the cone pedicles of the primate retina. J Comp Neurol. 2001;436:471–486. doi: 10.1002/cne.1081. [DOI] [PubMed] [Google Scholar]
- Hickmann FI, Fariss RN, Pagan-Mercado G, Yamamoto T, Wawrousek EF, Yabe-Nishimura C, Tsai JY. Characterization of retinal neurons over-expressing human aldose reductase in Smaahar mice. ARVO Abstract No. 742 2002 [Google Scholar]
- Hirano AA, Brecha NC. HCN4 is localized to type 2 cone bipolar cell terminals in rat retina. Soc Neurosci Abstr. 2001:284.7. [Google Scholar]
- Hirano AA, Brecha NC. Expression of the neurokinin-3 (NK3) receptor in off-type cone bipolar cells in mouse retina. ARVO Abstract No. 736 2002 [Google Scholar]
- Hirano AA, Hack I, Wässle H, Duvoisin RM. Cloning and immunocytochemical localization of a cyclic nucleotidegated channel α-subunit to all cone photoreceptors in the mouse retina. J Comp Neurol. 2000;421:80–94. doi: 10.1002/(sici)1096-9861(20000522)421:1<80::aid-cne5>3.0.co;2-o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby RA, Marshak DW. Synaptic connections of DB3 diffuse bipolar cell axons in macaque retina. J Comp Neurol. 2000;416:19–29. doi: 10.1002/(sici)1096-9861(20000103)416:1<19::aid-cne3>3.0.co;2-h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon C-J, Strettoi E, Masland RH. The major cell populations of the mouse retina. J Neurosci. 1998;18:8936–8946. doi: 10.1523/JNEUROSCI.18-21-08936.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb H, Nelson R. The organization of photoreceptor to bipolar synapses in the outer plexiform layer. In: Djamgoz MBA, Archer SN, Vallerga S, editors. Neurobiology and clinical aspects of the outer retina. London: Chapman and Hall; 1995. pp. 273–296. [Google Scholar]
- Koulen P, Fletcher EL, Craven SE, Bredt DS, Wässle H. Immunocytochemical localization of the postsynaptic density protein PSD-95 in the mammalian retina. J Neurosci. 1998;18:136–149. doi: 10.1523/JNEUROSCI.18-23-10136.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizaj D, DeMarco SJ, Johnson J, Strehler EE, Copenhagen DR. Cell-specific expression of plasma membrane calcium ATPase isoforms in retinal neurons. J Comp Neurol. 2002;451:1–21. doi: 10.1002/cne.10281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrecht HG, Koch KW. Recoverin, a novel calcium-binding protein from vertebrate photoreceptors. Biochim Biophys Acta. 1992;1160:63–66. doi: 10.1016/0167-4838(92)90038-f. [DOI] [PubMed] [Google Scholar]
- Martin PR, Grünert U. Spatial density and immunoreactivity of bipolar cells in the macaque monkey retina. J Comp Neurol. 1992;323:269–287. doi: 10.1002/cne.903230210. [DOI] [PubMed] [Google Scholar]
- Massey SC, Mills SL. A calbindin-immunoreactive cone bipolar cell type in the rabbit retina. J Comp Neurol. 1996;366:15–33. doi: 10.1002/(SICI)1096-9861(19960226)366:1<15::AID-CNE2>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Masu M, Iwakabe H, Tagawa Y, Miyoshi T, Yamashita M, Fukuda Y, Sasaki H, Hiroi K, Nakamura Y, Shigemoto R, Takada M, Nakamura K, Nakao K, Katsuki M, Nakanishi S. Specific deficit of the ON response in visual transmission by targeted disruption of the mGluR6 gene. Cell. 1995;80:757–765. doi: 10.1016/0092-8674(95)90354-2. [DOI] [PubMed] [Google Scholar]
- McGillem GS, Dacheux RF. Rabbit cone bipolar cells: correlation of their morphologies with whole-cell recordings. Vis Neurosci. 2001;18:675–685. doi: 10.1017/s0952523801185019. [DOI] [PubMed] [Google Scholar]
- Milam AH, Dacey DM, Dizhoor AM. Recoverin immunoreactivity in mammalian cone bipolar cells. Vis Neurosci. 1993;10:1–12. doi: 10.1017/s0952523800003175. [DOI] [PubMed] [Google Scholar]
- Mills SL, Massey SC. A series of biotinylated tracers distinguishes three types of gap junction in retina. J Neurosci. 2000;20:8629–8636. doi: 10.1523/JNEUROSCI.20-22-08629.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori K, Fujita SC, Imamura K, Obata K. Immunohistochemical study of subclasses of olfactory nerve fibers and their projection to the olfactory bulb in the rabbit. J Comp Neurol. 1985;242:214–229. doi: 10.1002/cne.902420205. [DOI] [PubMed] [Google Scholar]
- Morigiwa K, Vardi N. Differential expression of ionotropic glutamate receptor subunits in the outer retina. J Comp Neurol. 1999;405:173–184. doi: 10.1002/(sici)1096-9861(19990308)405:2<173::aid-cne3>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Müller F, Scholten A, Ivanova E, Haverkamp S, Kremmer E, Grünert, Kaupp UB. HCN channels in the mammalian retina. Soc Neurosci Abstr. 2001:284.6. [Google Scholar]
- Negishi K, Kato S, Teranishi T. Dopamine cells and rod bipolar cells contain protein kinase C-like immunoreactivity in some vertebrate retinas. Neurosci Lett. 1988;94:247–252. doi: 10.1016/0304-3940(88)90025-0. [DOI] [PubMed] [Google Scholar]
- Nomura A, Shigemoto R, Nakamura Y, Okamoto N, Mizuno N, Nakanishi S. Developmentally regulated postsynaptic localization of a metabotropic glutamate receptor in rat rod bipolar cells. Cell. 1994;77:361–369. doi: 10.1016/0092-8674(94)90151-1. [DOI] [PubMed] [Google Scholar]
- Onoda N, Fujita SC. A monoclonal antibody specific for a subpopulation of retinal bipolar cells in the frog and other vertebrates. Brain Res. 1987;416:359–363. doi: 10.1016/0006-8993(87)90919-x. [DOI] [PubMed] [Google Scholar]
- Oyamada H, Takatsuji K, Senba E, Mantyh PW, Tohyama M. Postnatal development of NK1, NK2, and NK3 neurokinin receptors expression in the rat retina. Brain Res Dev Brain Res. 1999;117:59–70. doi: 10.1016/s0165-3806(99)00099-1. [DOI] [PubMed] [Google Scholar]
- Pasteels B, Robers J, Blachier F, Pochet R. Calbindin and calretinin localization in retina from different species. Vis Neurosci. 1990;5:1–16. doi: 10.1017/s0952523800000031. [DOI] [PubMed] [Google Scholar]
- Pow DV, Wright LL, Vaney DL. The immunocytochemical detection of amino-acid neurotransmitters in paraformaldehyde-fixed tissues. J Neurosci Methods. 1995;56:115–123. doi: 10.1016/0165-0270(94)00113-u. [DOI] [PubMed] [Google Scholar]
- Qin P, Pourcho RG. Localization of AMPA-selective glutamate receptor subunits in the cat retina: a light- and electron microscopic study. Vis Neurosci. 1999;16:169–177. doi: 10.1017/s0952523899161121. [DOI] [PubMed] [Google Scholar]
- Rauen T, Kanner BI. Localization of the glutamate transporter GLT-1 in rat and macaque monkey retinae. Neurosci Lett. 1994;169:137–140. doi: 10.1016/0304-3940(94)90375-1. [DOI] [PubMed] [Google Scholar]
- Rice DS, Nusinowitz S, Azimi AM, Martinez A, Soriano E, Curran T. The reelin pathway modulates the structure and function of retinal synaptic circuitry. Neuron. 2002;31:929–941. doi: 10.1016/s0896-6273(01)00436-6. [DOI] [PubMed] [Google Scholar]
- Sassoè-Pognetto M, Wässle H, Grünert U. Glycinergic synapses in the rod pathway of the rat retina: cone bipolar cells express the α1 subunit of the glycine receptor. J Neurosci. 1994;14:5131–5146. doi: 10.1523/JNEUROSCI.14-08-05131.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidenbecher CI, Langnaese K, Sanmarti-Vila L, Boeckers TM, Smalla K-H, Sabel BA, Garner CC, Gundelfinger ED, Kreutz MR. Caldendrin, a novel neuronal calcium-binding protein confined to the somato-dendritic compartment. J Biol Chem. 1998;273:21324–21331. doi: 10.1074/jbc.273.33.21324. [DOI] [PubMed] [Google Scholar]
- Soucy E, Wang Y, Nirenberg S, Nathans J, Meister M. A novel signaling pathway from rod photoreceptors to ganglion cells in mammalian retina. Neuron. 1998;21:481–493. doi: 10.1016/s0896-6273(00)80560-7. [DOI] [PubMed] [Google Scholar]
- Takamori S, Rhee JS, Rosenmund C, Jahn R. Identification of a vesicular glutamate transporter that defines a glutamatergic phenotype in neurons. Nature. 2000;407:189–194. doi: 10.1038/35025070. [DOI] [PubMed] [Google Scholar]
- Trexler EB, Li W, Mills SL, Massey SC. Coupling from AII amacrine cells to ON cone bipolar cells is bidirectional. J Comp Neurol. 2001;437:408–422. doi: 10.1002/cne.1292. [DOI] [PubMed] [Google Scholar]
- Tsukamoto Y, Morigiwa K, Ueda M, Sterling P. Microcircuits for night vision in mouse retina. J Neurosci. 2001;21:8616–8623. doi: 10.1523/JNEUROSCI.21-21-08616.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaney DI, Nelson JC, Pow DV. Neurotransmitter coupling through gap junctions in the retina. J Neurosci. 1998;18:10594–10602. doi: 10.1523/JNEUROSCI.18-24-10594.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardi N. Alpha subunit of Go localizes in the dendritic tips of ON bipolar cells. J Comp Neurol. 1998;395:43–52. [PubMed] [Google Scholar]
- Vardi N, Duvoisin R, Wu G, Sterling P. Localization of mGluR6 to dendrites of ON bipolar cells in primate retina. J Comp Neurol. 2000;423:402–412. doi: 10.1002/1096-9861(20000731)423:3<402::aid-cne4>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Wässle H, Grünert U, Martin PR, Boycott BB. Immunoctyochemical characterization and spatial distribution of midget bipolar cells in the macaque monkey retina. Vis Res. 1994;34:561–579. doi: 10.1016/0042-6989(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Wikler KC, Stull DL, Reese BE, Johnson PT, Bogenmann E. Localization of protein kinase C to UV-sensitive photoreceptors in the mouse retina. Vis Neurosci. 1998;15:87–95. doi: 10.1017/s0952523898151155. [DOI] [PubMed] [Google Scholar]
- Ziai R, Pan Y-CE, Hulmes JD, Sangameswaran L, Morgan JI. Isolation, sequences, and development profile of a brain-specific polypeptide, PEP-19. Proc Natl Acad Sci USA. 1986;83:8420–8423. doi: 10.1073/pnas.83.21.8420. [DOI] [PMC free article] [PubMed] [Google Scholar]